- Volume 59 , Number 3
- Page: 426–31
Seroepidemiological study of leprosy in a highly endemic population of south india based on an ELISA using synthetic PGL-I
ABSTRACT
As part of a continuing longitudinal im-munoepidemiological study, blood samples were collected by finger prick f rom 4243 individuals living in a highly endemic area for leprosy in South India. The samples were tested for IgM antibodies against phenolic glycolipid-I using an ELISA. Seropositivity defined as optical density > 0.2000 was marginally higher in the age group 10-30 years and in females. There was no evidence for a higher level in contacts than in non-contacts. The future prospect for the large scale use of this ELISA in high-endemic populations in special epidemiological investigations or routine control programs as a serological tool to detect leprosy infection appears questionable.RÉSUMÉ
Dans le cadre d'une étude immunoépidémiologique longitudinale, des échantillons sanguins ont été prélevés par piqûre au doigt chez 4243 personnes vivant dans une région à forte endémicité de lèpre dans le Sud de l'Inde. Les échantillons ont été testés par un ELISA pour rechercher la présence d'anticorps IgM vis-à-vis du glycolipide phénoliquc-I. La séropositivité définie comme une densité optique > 0.2000, était légèrement supérieure pour le groupe d'âge 10-30 ans et pour les femmes. Il n'y avait pas d'évidence de taux plus élevés pour les contacts que pour les non-contacts. La perspective d'une utilisation à grande échelle de cet ELISA comme un outil sérologiquc pour détecter l'infection lépreuse dans les populations à haute endémicité pour des recherches épidémiologiques particulières ou pour des programmes de routine de lutte contre la lèpre, apparaît discutable.RESUMEN
Como parte de un estudio inmunoepidemiológico, se colectaron muestras de sangre por punción digital de 4243 individuos habitantes de un área altamente endémica del Sur de la India. Las muestras se probaron para la búsqueda de anticuerpos IgM in contra del glicolípido fenólico-I usando un ELISA. La seroposi-tividad, definida como una densidad óptica igual o mayor a 0.2000, fue marginalmente mayor en el grupo de edad entre 10 y 30 años y en mujeres. No hubieron evidencias de Que los niveles de anticuerpos fueran mayores en los contactos que en los no contactos. Por lo tanto, la utilidad de este tipo de ELISA como herramienta para detectar la infección leprosa en los estudios a gran escala en poblaciones altamente endémicas, parece ser muy cuestionable.Leprosy is an enigmatic disease. Persistent efforts to unravel the various facets of the disease have met with limited success. The phase of infection still remains unrecognized. A reasonably valid marker, serological or otherwise, of past or current infection with Mycobacterium leprae would certainly help in understanding the natural history of the disease and would answer many epidemiological questions, such as the probable major course of transmission, the incubation period, the risk factors for infection and disease, etc., answers that remain elusive to this day (9). The recognition of infection, which is one of the principal goals of leprosy research (17), will also provide us with credible support for the control of the disease.
Several serological tests have been developed over the past 80 years to detect leprosy infection (15). The results, however, are not gratifying. Interest in the past few years in serodiagnosis has been heightened by the development of serological tests based on supposedly M. leprae-specific antigenic determinants (4,7,13,18,19). The enzyme-linked immunosorbent assay (ELISA) based on phenolic glycolipid-I (PGL-I) (12) is now widely used in seroepidemiological studies. The availability of the synthetic disaccha-ride portion of PGL-I as an antigen in ELISA, its relative specificity to leprosy, and the fact that it is the only assay thus far standardized among laboratories, may be the reasons for its widespread acceptance.
The majority of the serological studies that have been carried out so far have been confined to small, selected, nonrandom populations of cases and controls. These studies have reported widely varying results, e.g., from 6% to 92% (1-3,5,16) seropositivity among contacts of leprosy cases. There have been only a few large-scale seroepidemiological investigations using an ELISA based on PGL-I reported to date (6,10).
This paper presents the results of a seroepidemiological study conducted in a highly endemic area of leprosy in South India using a synthetic ND-O-BSA ELISA.
MATERIALS AND METHODS
The 54 villages in the field area of the Central Leprosy Teaching and Research Institute (CLTRI) located about 10 kms from Madras, with a total population of about 100,000, were divided into 135 population clusters varying in size from 400 to 1000. Each cluster, which was formed keeping in mind the natural or administrative boundary, was weighted. Cluster weighting was done using the criteria of relative risk derived from available data of the study area to obtain the incremental gain(s) in prospective case detection. Each household in a cluster was given a risk factor depending on the presence or absence of a leprosy case (paucibacillary or multibacillary) in the household. If there was one or more paucibacillary (PB) cases in a household, a risk factor of 2 was given and this was multiplied by the number of household members minus the case to arrive at the weightage score for the household. For instance, a PB case in a household of five constitutes a weight-age of 8 = [2 x (5 - 1)]. A single multi-bacillary (MB) case in a household would get a score of 5, and multiple MB cases, a score of 8.
It is hypothesized that a case in a household would contribute to a neighborhood effect in terms of transmission of infection, especially since the households and houses in a rural setting are closely crowded. Risk factor scoring ranging from 1.5 to 2.5 was therefore resorted to for neighboring houses and households, and for one house all around the house in which the case was located, keeping in mind the proximity of the inhabited structures. A house was defined as a structure, tent, shelter, etc., used for residential or nonresidential purposes, or both. A household was defined as a group of persons, who may or may not be related to one another by blood, living together and taking food from a common kitchen.
All of the 135 clusters were weighted and a random sample of 20 clusters was selected after stratification for size and weightage. The population sampled was 14,633, with a ratio of cases to household contacts, neighborhood contacts, and noncontacts of 1:5: 10:30, respectively.
Examination of the population for leprosy was done by research officers, and confirmation of the diagnosis of leprosy was based on clinical examination alone.
Finger-prick blood samples were collected from individuals in the study area on Whatman no. 2 chromatography paper. Four spots (each at least 15 mm in diameter) were collected from each person; two spots each on a single 2.0 x 7.5-cm sheet of paper on which the identification particulars were written. The papers were air dried, placed in zip-seal plastic bags, brought immediately to the laboratory at CLTRI and stored at -20ºC until tested.
At the time of testing, the blood specimens were taken from the freezer and eluted in phosphate-buffered saline (PBS) containing 1% bovine serum albumin (BSA) and 0.05% Tween-20 (PBST). A 7-mm-diame-ter disc (containing approximately 7 µl of blood) was punched out from each dried blood spot and treated with 140 µl of PBST separately in plastic vials for 2 hr. The eluates were considered equivalent to a 1:40 dilution of the sera.
Synthetic glycoconjugate antigen (ND-O-BSA), supplied by IMMLEP, World Health Organization (WHO), was coated on one half of flat-bottom, microtiter plates (Dy-natech, Germany). The other half was coated with BSA alone in order to measure the nonspecific binding of the eluates. The coated plates were incubated overnight at 37ºC and then washed with PBST. The eluates of the blood specimens were then applied in duplicate antigen and control wells. After incubation and washing, peroxidase-con-jugated, antihuman IgM (Dakko, Denmark) was added, and the plates were incubated and washed again. Following washing, chromogenic substrate O-phenylene diamine and hydrogen peroxide in citrate buffer, pH 5.0, were added to develop color. The color was then read as absorbance at 490 nm using a Dynatech ELISA reader.
The serological data were linked to information in the household schedules and analyzed by computer at CLTRI. The standardized seropositivity ratio for contacts and noncontacts was calculated using the indirect standardization method.
A contact was defined as a person who had stayed for more than 6 months with and was found in the same household as the case at the time of blood collection.
RESULTS
Sera from 4243 individuals (including 132 leprosy cases) from seven clusters have been analyzed. The demographic particulars of this population arc given in Table 1. Out of the 132 cases examined, there were 10 MB cases and 122 PB cases. The disease prevalence rate among females was 24.15 per 1000 and it was 38.19 per 1000 among males; 36% of the PB cases and 63.6% of the MB cases were females. Seropositivity (defined as OD > 0.200) was 12.7% in PB and 28.6% in MB cases (Fig. 1). All of the MB cases were under treatment for varying periods (3 months to more than 1 year).
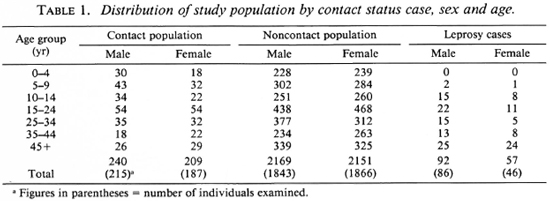
Fig. 1. Antibody level against PGL-I (Absorbance 490) among paucibacillary and multibacillary leprosy cases.
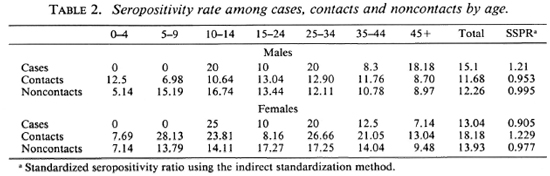
Figure 2 shows that nearly 80%-90% of the population in the different age groups had low antibody levels. Seropositivity in the general (noncase = contact + noncontact) population was 10.5%. Although the peak of seropositivity is seen at 10 years, it is sustained until the age of 30 years and then shows a gradual decline (Fig. 3). Among contacts the seropositivity was 14.5%; in noncontacts it was 13%. It was higher among female contacts than male contacts (Table 2). The marginally higher rates are borne out by the standardized rates for the two groups of both sexes. (Standardization was done in order to even out the differences in the age structure between the contact group and the noncontact group.) This difference, however, was not significant.
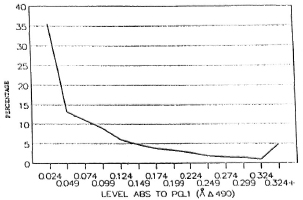
Fig. 2. Distribution of IgM against PGL-I in the general population.
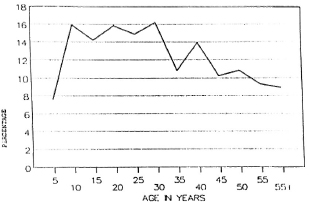
Fig. 3. Seropositivity among the general population.
BCG scar status as a variable was not studied because the BCG scar rate in this population was less than 10%.
DISCUSSION
This study is part of a longitudinal im-munoepidemiological study being carried out in a highly endemic district (prevalence of > 10 per 1000) of South India. One of the objectives of the study was to see if ELISA using PGL-I could be used as a specific marker of leprosy infection in endemic populations. The determinate criterion for appreciating the difference between infected and noninfected populations should be based on disease endemicity. In an endemic area the criterion value is set at a decidedly high level to achieve reasonably unequivocal specificity. Positivity criterion of OD > 0.200 was adapted in the study because the area is highly endemic, and the value was found to represent three standard deviations above the mean (+ 3 S.D.) of a sample of sera from healthy persons in this area. The finger-prick spots on Whatman paper withstood the rigors of the field conditions, and the results obtained were comparable with those of venipuncture sera (8).
Even though a higher positivity is seen in MB cases, which could be explained on the basis of a higher antigen load seen in these cases, overall it appears surprising that in both MB and PB cases the seropositivity is low. The very low seropositivity (11%) among cases could be due to the fact that a majority of the cases were PB and most of the cases were under treatment. The prevalence of seropositivity of 14.5% among contacts compares well with the 14.4% obtained in an earlier study (4) carried out among contacts in the same area using the FLA-ABS test. The manifest default of distinction between contacts and noncontacts by this test appears disappointing. This possibly could be due to the fact that in an endemic area the intensity of exposure to M. leprae is likely to be high and, at the same time, the risk of exposure may be uniform in the population.
The reason for a marginally higher sero-positivity among females is not clear. Higher seroprevalcnce rates among females were reported in other studies as well (6,10). Case rates among the female population in this region are not higher than in males. An attempt has been made to explain this phenomenon on the basis of relative IgM glob-ulinemia reported among females (11,15) and/ or to a higher probability of seroconversion among males (10).
The reason for the higher positivity rates among adolescents and young adults, when compared to other age groups, is not clear. Seropositivity may be a transient phenomenon and is perhaps reversible. What is really interesting is that it parallels the case prevalence pattern observed in the area.
It now appears that the excitement generated a few years back by the introduction of various serological tests, especially ELISA using PGL-I, is waning because of the disappointing results that have been obtained in several studies. PGL-I based on an ELISA does not appear to be effective as a seroepi-demiological tool for diagnosing preclinical infection or of prognostic value for clinical disease, at least in high-endemic populations.
Acknowledgment. This investigation received financial support from the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR). The authors arc thankful to Mr. Muthukumar Muthukrishnan and Mr. Balakrishnan Chengalvarayan, senior computors at Central Leprosy Teaching and Research Institute, Chengalpattu, India, for their assistance in data entry and the computerization process.
REFERENCES
1. Abe, M., Minagawa, F., Yoshino, Y., Ozawa, T., Saikawa, K. and Saito, T. Fluorescent leprosy antibody absorption (FLA-ABS) test for detecting subclinical infection with Mycobacterium leprae. Int. J. Lepr. 48(1980)109-119.
2. Bharadwaj, V. P., Ramu, G. and Desikan, K. V. A preliminary report on subclinical infection in leprosy. Lepr. India 54(1982)220-227.
3. Bhatia, V. N., Dhandayuthapani, S., Ananthan, D., Rajendran, M., Vasanth, B., Jayasingh, K. and Vinod Kumar, C. H. D. Sub-clinical infection with Mycobacterium leprae in household contacts of leprosy. Indian J. Lepr. 62(1990)296304.
4. Brett, S. J., Draper, P., Payne, S. N. and Rees, R. J. W. Serological activity of a characteristic phenolic glycolipid from Mycobacterium leprae in sera from patients with leprosy and tuberculosis. Clin. Exp. Immunol. 52(1983)271-279.
5. Buchanan, T., Dissanayake, S., Young, D. B., Miller, R. A., Acedo, J. R., Harnisch, J. P., Khanolkar, S. R. and Estrada-Parra, S. Evaluation of the significance of antibodies to phenolic glycolipid of Mycobacterium leprae in leprosy patients and their contacts. (Abstract) Int. J. Lepr. 51(1983)658-659.
6. Cartel, J.-L., Chanteau, S., Boutin, J.-P., Plichart, R., Richez, P., Roux, J.-F. and Grosset, J.-H. Assessment of anti-phenolic glycolipid-I IgM levels using an ELISA for detection of M. leprae infection in populations of the South Pacific Islands. Int. J. Lepr. 58(1990)512-517.
7. Cho, S.-N., Yanagihara, D. L., Hunter, S. W., Geluer, R. H. and Brennan, P. J. Serological specificity of phenolic glycolipid I from Mycobacterium leprae and use in scrodiagnosis of leprosy. Infect. Immun. 41(1983)1077-1083.
8. Dhandayuthapani, S., Anandan, D., Vasanthi, B. and Bhatia, V.N. Use of cluates of filter paper blood spots in ELISA for the scrodiagnosis of leprosy. Indian J. Med. Res. 89(1989)150-157.
9. Fine, P. E. M. Leprosy - the epidemiology of a slow bacterium. Epidemiol. Rev. 4(1982)161188.
10. Fine, P. E. M., Ponnighaus, J. M., Burgess, P., Clarkson, J. A. and Draper, C. C. Seroepide-miological studies of leprosy in northern Malawi based on an ELISA using synthetic glycoconjugate antigen. Int. J. Lepr. 56(1988)243-254.
11. Grundbacher, E. J. Human X chromosome carries quantitative genes for immunoglobulin M. Science 176(1972)311-312.
12. Hunter, S. W. and Brennan, P. J. A novel phenolic glycolipid from Mycobacterium leprae possibly involved in immunogenicity and pathogenicity. J. Bactcriol. 147(1987)728-735.
13. Klatser, P. R., de Wit, M. Y. L. and Kolk, A. H. An ELISA-inhibition test using monoclonal antibody for the serology of leprosy. Clin. Exp. Immunol. 62(1985)468-473.
14. Maddison, S. E., Stewart, C. C, Farshy, C. E. and Reimer, C. The relationship of race, sex, and age to concentrations of serum immunoglobulins expressed in international units in healthy adults in the U.S.A. Bull. WHO 52(1975)179-185.
15. Melsom, R. Scrodiagnosis of leprosy: the past, the present, and some prospects for the future. Int. J. Lepr. 51(1983)235-252.
16. Menzel, S., Harboe, M., Bergsvik. H. and Brennan, P. J. Antibodies to a synthetic analog of phenolic glycolipid-I of Mycobacterium leprae in healthy household contacts of patients with leprosy. Int. J. Lepr. 55(1987)617-625.
17. Serological Test for Leprosy. (Editorial) Lancet 1(1986)533-535.
18. Sinha, S., Sengupta, U., Ramu, G. and Ivanyi, J. A serological test for leprosy based on competitive inhibition of monoclonal antibody binding to the MY2a determinant of Mycobacterium leprae. Trans. R. Soc. Med. Hyg. 77(1983)869-871.
19. Young, D. B. and Buchanan, T. M. A serological test for leprosy with a glycolipid specific for Mycobacterium leprae. Science 221(1983)1057-1059.
1. M.D. (PSM), Assistant Director (Epid.); Central Leprosy Teaching & Research Institute, Chengalpattu 603001, India.
2. D.P.H., D.T.M.&H., Deputy Director (Epid.); Central Leprosy Teaching & Research Institute, Chengalpattu 603001, India.
3. M.D. (PSM), Assistant Director (Epid.); Central Leprosy Teaching & Research Institute, Chengalpattu 603001, India.
4. M.Sc. (Stat.), Statistical Assistant; Central Leprosy Teaching & Research Institute, Chengalpattu 603001, India.
5. M.Sc, Ph.D., Senior Research Officer; Central Leprosy Teaching & Research Institute, Chengalpattu 603001, India.
6. M.D. (Micro.), Former Director (Micro.); Central Leprosy Teaching & Research Institute, Chengalpattu 603001, India.
7. D.P.H., M.P.H., Former Director; Central Leprosy Teaching & Research Institute, Chengalpattu 603001, India.
8. D.P.H., D.E.C.D., Director, Central Leprosy Teaching & Research Institute, Chengalpattu 603001, India.
Reprint requests to Dr. Krishnamurthy. Present address for Dr. Bhatia: Serologist and Chemical Examiner, Calcutta, India.
Received for publication on 2 January 1991.
Accepted for publication in revised form on 16 May 1991.