- Volume 59 , Number 3
- Page: 432–40
The value of IgM antibodies to PGL-I in the diagnosis of leprosy
ABSTRACT
An ELISA has been used to measure IgM antibodies to phenolic glycolipid-I (PGL-I) in previously undiagnosed patients who were suspected of leprosy on purely clinical grounds. The certainty of clinical diagnosis was classified as either "firm" or "indefinite." Leprosy was confirmed in 133 of 161 patients on the basis of positive slit-skin smears and/or skin and/or nerve histopa-thology. All 58 patients with multibacillary leprosy (BB, BL, or LL) were correctly diagnosed clinically, as were 50 of 54 patients (93%) with a firm diagnosis of BT or TT leprosy. The firm clinical diagnoses were more accurate than either the slit-skin smear or ELISA data. However, there were 44 patients (27% of total), designated "rule out leprosy" (RO), for whom the clinical diagnosis was indefinite. The clinical suspicion of leprosy (RO) was correct in only 24 (55%) of these patients who had BT leprosy. The slit-skin smears were positive in only 20% of these patients compared to 50% for the ELISA. It was concluded that the PGL-I IgM ELISA may have its greatest diagnostic confirmatory value in paucibacillary disease because paucibacillary leprosy comprises the major source of clinical diagnostic difficulty.RÉSUMÉ
Un ELISA a été utilisé pour mesurer le taux d'anticorps IgM vis-à-vis du glycolipide phénolique-I (PGL-I) chez des patients non diagnostiqués antérieurement qui étaient suspectés de lèpre sur une base purement clinique. Le diagnostic clinique fut classé comme "certain" ou "incertain". La lèpre fut confirmée chez 131 des 161 patients sur base de frottis cutanés positifs et/ ou de l'histopathologie cutanée et/ou nerveuse. Tous les 58 patients avec une lèpre multibacillaire (BB, BL, ou LL) avaient été correctement diagnostiqués clini-quement, tout comme 50 des 54 patients (93%) avec un diagnostic "certain" de lèpre BT ou TT. Les diagnostics cliniques "certains" étaient plus précis que le frottis cutané ou l'ELISA. Cependant, il y avait 44 patients (27% du total), pour lesquels on avait mentionné "exclure la lèpre", et pour lesquels le diagnostic clinique était incertain. La suspicion clinique de lèpre était correcte pour seulement 24 (55%) de ces patients, qui avaient une lèpre BT. Les frottis cutanés étaient positifs chez seulement 20% de ces patients, comparés à 50% pour l'ELISA. Il fut conclu que l'ELISA pour la recherche d'IgM vis-à-vis du PGL-I pourrait avoir sa plus grande valeur pour la confirmation du diagnostic dans la lèpre paucibacillaire, parce que c'est celle-ci qui présente le plus de difficultés por le diagnostic clinique.RESUMEN
Se uso un ELISA para mcdir anticucrpos contra el glicolipido fenolico-I (PGL-I) del Mycobacterium leprae en pacientes no diagnosticados pero con sospecha clinica de tener lepra. La certidumbre del diagnostico clinico se clasifico como "firme" o "indefinida". La lepra se confirmo en 133 dc 161 pacientes con base en los extendidos de linfa cutanea positivos y en la his-topatologia de la piel y/o nervios. Todos los 58 pacientes con lepra multibacilar (BB, BL, o LL) fucron correctamente diagnosticados desde el punto de vista clínico, como también lo fueron 50 de 54 pacientes (93%) con un diagnóstico firme de lepra BT o TT. Los diagnósticos clínicos firmes fueron más exactos que los datos de los extendidos de linfa cutánea o de los ELISA. Sin embargo, hubieron 44 pacientes (27% del total), designados "descartar lepra" ("rule out leprosy", RO), para quienes el diagnóstico clínico fue indefinido. La sospecha clínica de lepra (RO) fue correcta sólo en 24 (55%) de estos pacientes quienes tuvieron lepra BT. Mientras que los extendidos de linfa cutánea fueron positivos sólo en el 20% de estos pacientes, los ELISA fueron positivos en el 50% de los mismos. Se concluyó que el ELISA (IgM, PGL-I) puede tener su mayor valor diagnóstico confirmatorio en la enfermedad pauciba-cilar porque la lepra paucibacilar incluye los casos de mayor dificultad diagnóstica.The discovery of a unique glycolipid antigen of Mycobacterium leprae (12), phenolic glycolipid I (PGL-I), was followed by studies in which it was shown that sera from leprosy patients often contain antibodies to this antigen (2,5,6). Since then, a number of diagnostic (1,13,14,18,19) and epidemiologic (3,4,9) studies have been published. All these studies have shown that the quantity of specific IgM antibodies, and consequently the frequency of antibody-positive sera, varies across the leprosy spectrum. There are relatively few positive sera among tuberculoid (TT) patients; whereas > 90% of leproma-tous leprosy (LL) patient sera are positive. The frequency of positive sera among borderline tuberculoid (BT) patients has varied between 25% and 50%, the latter estimate being more common (1,6,14). The inference from these studies is that the assay of IgM antibodies to PGL-I may be of value in the diagnosis of multibacillary, but not pauci-bacillary leprosy.
The early studies used banked sera from leprosy patients (2,5,6,16), and frequently the criteria for the diagnosis were unstated or unclear. Moreover, no distinction was made between treated and untreated patients, and the nonleprosy controls were drawn from leprosy nonendemic areas rather than the areas from which the patients were often drawn. The more recent studies have concentrated on untreated patients, but the diagnostic criteria are variable. Some are derived from clinical evaluation and slit-skin smears (18,19), but most have also utilized histopathology.
Only Fine and his co-workers have addressed the issue of the degree of certainty in diagnosis (9,16). Consequently, it is virtually impossible to determine from the published data how useful the antibody assays might be in supplementing the clinical diagnosis alone. This is an important issue, because in countries in which leprosy is endemic, other than in research centers, the clinical diagnosis is either the sole criterion of leprosy or is supplemented only by slit-skin smear examination. Moreover, the accuracy of clinical diagnosis is variable (16), and the performance of skin-smear examination leaves much to be desired (11).
In light of these problems, we have conducted a study of serum IgM antibodies to PGL-I in previously undiagnosed patients who presented at a diagnostic dermatology clinic at a leprosy hospital. The diagnostic value of the antibody assays has been evaluated in relation to the certainty of the provisional clinical diagnosis (PCD) that was made before any laboratory tests were performed.
MATERIALS AND METHODS
Human subjects. Patients were drawn from subjects who attended the Diagnostic Clinic at the All Africa Leprosy Rehabilitation and Training Centre (ALERT), Addis Ababa, Ethiopia. The Diagnostic Clinic is a walk-in clinic where patients are seen without charge. The patients are self-referred, and only approximately 1% of them are found to have leprosy. A clinical examination is made, and any patient who is suspected of having leprosy is assigned a provisional diagnosis and routinely investigated by examination of slit-skin smears for acid-fast bacilli (AFB) and histopathology of skin and/or nerve biopsy. For this study, venous blood was also drawn for a serum PGL-I antibody assay. The clinicians, of whom there were at least eight, used the standard Ridley and Jopling classification (17): tuberculoid (TT), borderline tuberculoid (BT), borderline (BB), borderline lepromatous (BL), and lepromatous (LL). A few patients were designated "neural leprosy" (NL), and no patient was designated indeterminate leprosy. If a more complex diagnosis was assigned to a patient (e.g., BT/BL), the first designation (in this case BT) was adopted for purposes of analysis. The preceding types of clinical diagnosis were designated as "firm," but a substantial number of patients were given a more indefinite diagnosis, such as "leprosy" or "rule out leprosy." Such diagnoses have been consolidated under the rubric of "rule out" (RO) leprosy. Other subsidiary diagnoses, such as reversal reaction or erythema nodosum leprosum, have been ignored for purposes of analysis.
Three populations of control subjects were also evaluated: a) a group of healthy Wayne State University (U.S.A.) medical students (N-WSU); b) a group of apparently healthy Ethiopian laboratory personnel (N-ETH); c) a nonleprosy cohort patient group of Ethiopians who were initially suspected of having leprosy but who were found not have the disease (N-NL).
Slit-skin smear examination. Smears were prepared from six sites: earlobe, eyebrow, both elbows, and both knees. The smears were stained by the Ziehl-Neelsen method and examined by bright field microscopy. The bacterial index (BI) was graded from 0 (no bacilli per 100 high-power fields) through 6+ (> 1000 per field), and the mean BI was estimated.
Serum antibodies to PGL-I. Venous blood was drawn, and the serum was separated, distributed into vials, and stored at - 20ºC. The assay method was similar in principle to that described by Cho, et al. (5),the main difference being the use of alkaline phosphatase-conjugated instead of peroxi-dase-conjugated secondary antibodies. The antigen was a semisynthetic glycoconjugate analog of PGL-I, the natural terminal di-saccharide linked to bovine serum albumin through an octyl-methyl linker arm (10), and was obtained through NIAID from Dr. P. J. Brennan, Colorado State University, Fort Collins, Colorado, U.S.A. Flat-bottom, 96-well, polystyrene plates (Immulon 2; Dy-natech Labs., Inc., Chantilly, Virginia, U.S.A.) were coated overnight with antigen at a concentration of 250 ng/ml, 2.5 ng/well, in carbonate buffer, pH 9.6. The plates were blocked with 0.5% gelatin and 0.05% Tween 20 in phosphate-buffered saline, pH 7.2 (PBS). Normal or control serum (100 μ1) was added to sets of four wells and incubated for 2 hr at 37ºC. After washing, 100 μl of alkaline phosphatase-conjugated goat anti-human IgM (Sigma A-3275; Sigma Chemical Co., St. Louis, Missouri, U.S.A.), diluted 1:1000, was added to each well, and the plates were reincubated for 2.5 hr. After further washing, disodium p-nitrophenyl phosphate substrate tablets (Sigma 104; Sigma) were dissolved in diethanolamine buffer and added to each well. The color was allowed to develop for 30 min at room temperature. The reaction was stopped with 3 N NaOH, and the optical density (OD) was measured with a Titertek Multiskan Plus ELISA reader using a 405-nm filter. Each plate comprised 24 sets of four wells that were used for reagent controls, several negative (N-WSU) sera, one standard positive control LL pooled serum, and test sera from the patients, among which the N-ETH sera were blindly scattered. The reagent control wells were used to blank the ELISA reader before measuring the OD of the other wells. The mean OD of each serum was calculated from the four replicate wells. The OD data was also expressed as a percentage of the OD of the LL pooled serum that was tested in the same plate (relative OD).
Histopathology. Skin and/or nerve biopsies were processed for routine histopathology. Sections were stained by the Fite-Faraco method, followed by hematoxylin and safranin, to stain AFB and tissue cells in the same section. The histopathologic reports were scrutinized for whether or not the sections were diagnostic of leprosy. For the purpose of this report, the sole concern was the distinction between leprosy and nonleprosy; the histopathologic classification of disease was ignored.
RESULTS
Patient population. The sample of patients who were included in the study was smaller than anticipated because not all patients suspected of leprosy were fully investigated by slit-skin smears, skin biopsy, and/ or optional nerve biopsy as the protocol required. Scrutiny of the laboratory log book revealed no evident bias in the lack of compliance with the agreed procedures, so the patient sample that was studied appears to be representative of the larger hospital population. In practice, consecutive serum samples, collected between September 1986 and November 1987, were taken. Patients who were later discovered to have previously received treatment for leprosy, no matter how brief, were omitted. At the end of this selection process, 161 patients remained. The following criterion of leprosy was adopted: a PCD of leprosy (firm or indefinite) that was confirmed by either a positive slit-skin smear or a histopathologic diagnosis of leprosy (skin or nerve).
Based on negative skin smears and his-topathology, 28 patients were classified as "nonleprosy" (N-LEP); the diagnosis of leprosy was confirmed in the remaining 133 subjects. Skin biopsies were obtained from 127 of the 133 confirmed leprosy patients, of which 104 samples were diagnostic of leprosy; in 52 of these patients, the slit-skin smears were also positive. Of 23 patients whose skin biopsies were histopathologi-cally negative for leprosy, two had positive skin smears, and were therefore included in the leprosy category.
There were 24 patients for whom nerve biopsies but no skin biopsies were done: 14 biopsies revealed evidence of leprosy, of which 9 showed paucibacillary neural leprosy (PBNL), and 5 showed multibacillary neural leprosy (MBNL). None of the PBNL, but four of the MBNL, patients had positive skin smears. There remained six patients in whom the confirmation of the diagnosis of leprosy rested on the nerve biopsy alone.
No histopathologic data were available from 10 leprosy patients. In one of these patients the BI was 1, but in the remainder the Bis were > 1, with a mean of 3.6, leaving little doubt of the diagnosis. As mentioned above, there were two patients whose skin biopsies were negative but whose skin smears were positive, with Bis of 1 and 3, respectively. Thus, there was a total of 12 leprosy patients whose diagnosis was confirmed by skin smears only.
PGL-I antibody assay. The arithmetic means of the absolute and relative OD data for the sera from 133 patients with confirmed leprosy are shown in Figure 1 in relation to the PCD, As in other similar studies, there was a strong association between high antibody levels and the multibacillary forms of the disease. The antibody levels in the three types of nonleprosy subjects are shown in Figure 2. The mean absolute OD values were 0.12, 0.17, and 0.16 for the N-WSU, N-ETH and N-LEP subjects, respectively; these three means did not differ significantly from each other, using analysis of variance. The relative OD values for these groups were correspondingly similar. Criteria of serum positivity were based on the data derived from the N-WSU sera: any result which exceeded 3 S.D. above the mean values of the N-WSU samples was considered "positive." In practice, these critical values were an absolute OD > 0.30 or a relative OD > 25%.
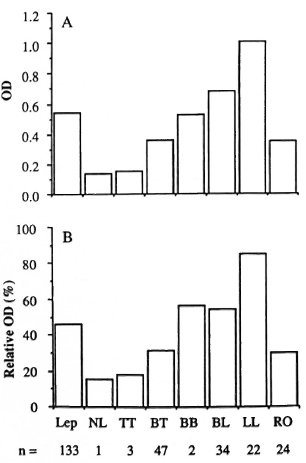
Fig. 1. ELISA results of leprosy patient sera assayed for IgM antibodies to PGL-I. A = Results expressed as absolute OD at 405 nm; B = results expressed as percentage of positive control scrum (relative OD).
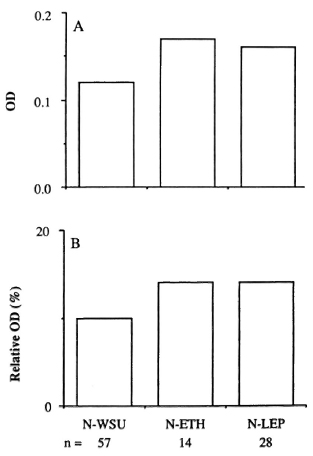
Fig. 2. ELISA results of sera from healthy controls and patients who did not have leprosy. A = Results expressed as absolute OD at 405 nm; B = results expressed as percentage of positive control serum (relative OD).
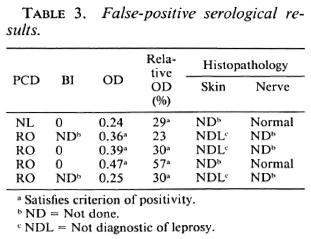
Applying these criteria first to the non-leprosy sera, a single N-WSU serum was positive but barely exceeded the criterion of positivity, having an absolute OD of 0.31 and a relative OD < 25%. However, one N-ETH serum was unequivocally positive, with an absolute OD of 0.55 and relative OD of 39%. If the latter serum were excluded from the N-ETH group, the mean absolute OD would be 0.14 (N = 13), even more closely similar to the N-WSU value. The details of the five N-LEP sera that gave an absolute OD > 0.30 and/or a relative OD > 25% are described later (Table 3).
The positivity of the leprosy patient sera as a function of the PCD is shown in Figure 3. The absolute and relative OD results were discordant in only 10% of the leprosy sera, the discrepancies arising from sera with barely positive levels of antibody. The data in Figure 3 are similar to those observed in other studies. Either criterion of serum positivity detected approximately 60% of the proven leprosy patients, a low overall level of sensitivity, but in LL patients the serum positivity exceeded 90%. The serum positivity was only about 50% in BT patients, but it is notable that a similar percentage of RO patients was also positive. It was anticipated that the relative OD (%) would be a more useful parameter than the absolute OD, in that the former would compensate for inter-experimental variation. This proved not to be the case, possibly because the ELISA tests were completed in a relatively short time frame, using single lots of antigen, secondary antibodies, and other reagents, and unchanging technical personnel. It may well transpire that studies conducted over a much longer period might show greater consistency in terms of the relative OD than the absolute OD.
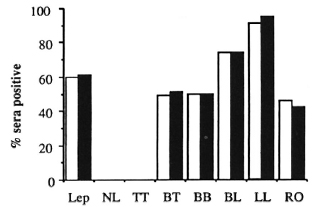
Fig. 3. PGL-I antibody scropositivily across the leprosy spectrum.  = OD > 0.30;
= OD > 0.30;  = % > 25.
= % > 25.
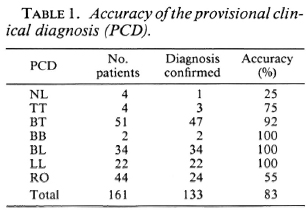
Diagnostic power of clinical evaluation, slit-skin smears, and PGL-I antibody assays. It might be inferred from Figure 3 that the diagnostic value of the scrum antibody assay might be limited to those patients with multibacillary leprosy, but no account has yet been taken of the accuracy of the PCD alone, nor of the diagnostic value of the slit-skin smears. The PCD is compared with the confirmed diagnosis in Table 1. For the purpose of these analyses, the accuracy of the PCD is based only on the distinction between leprosy and nonleprosy. It is apparent that the accuracy of the PCD is generally high and, with respect to multibacillary leprosy (BB, BL and LL), the PCD was infallible. The clinicians were only slightly less accurate with respect to "firm" diagnoses of BT. The main clinical problem lay in the diagnosis of the "rule out leprosy" (RO) patients, in whom the accuracy of the rule out leprosy diagnosis was only slightly better than a coin toss with regard to the final diagnosis. The accuracy of the diagnosis of neural leprosy was low, but this condition is notoriously difficult to diagnose due to the absence of skin lesions, and few patients fall into this category. As can be determined from Table 1 and Figure 3, the PGL-I antibody positivity, although high in multibacillary leprosy, does not surpass the accuracy of the PCD, and the firm PCD of BT is much more reliable than the serological test.
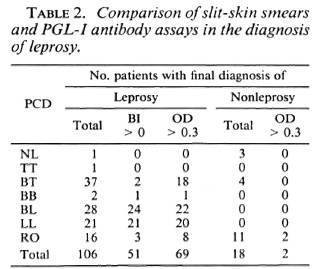
The value of slit-skin smears was similarly compared with the value of the PGL-I antibody assay (Table 2) in a subgroup of 124 patients for whom both sets of data were available. In multibacillary leprosy, both laboratory procedures were highly efficient in detecting leprosy, but slit-skin smears were marginally better. In pauci-bacillary leprosy, notably the BT patients, both procedures were relatively insensitive but the serological test was substantially superior to the skin smear. Of most interest were the RO patients, who represented the greatest clinical diagnostic difficulty, in whom the antibody assay was again appreciably more sensitive than the skin smears.
Validity of confirmed diagnosis. In this study, no false-positive results could arise from positive slit-skin smears or skin/nerve histopathology because such findings were, by definition, criteria of leprosy. In the entire population of 161 patients, there was a total of five false-positive serological results (absolute OD > 0.30 and/or relative OD > 25%; Table 3). Three sera (patients 1, 2 and 5) were positive by only one serological criterion; one scrum (#3) was weakly positive by both criteria and only one scrum (#4) was unequivocally positive. The nonleprosy status of these patients was supported by histopathologic evidence in all five cases, in three of whom slit-skin smears were also obtained and were negative.
The low sensitivity of the skin smear and antibody assay, especially in paucibacillary leprosy, yielded many false-negative results. More interesting was the observation that two skin biopsies were falsely negative, suggesting the possibility that some of the "nonleprosy" patients, particularly those with positive serology, may be incorrectly diagnosed.
DISCUSSION
Early studies of antibodies to PGL-I in leprosy patients utilized banked sera from a mixed bag of treated and untreated patients in whom the criteria of diagnosis were not standardized. Such studies do not lend themselves to critical evaluation of the diagnostic power of the assay. More recently, there have been several prospective studies, limited to newly diagnosed untreated patients, in which the diagnostic criteria have been clearly stated (1,3,10,13,14,18,19). Even so, no distinction has been made between the original clinical diagnosis and the final definitive diagnosis which was based on a combination of clinical, smear, and histopathologic data. In this study, an evaluation has been made of the PCD that was made by the physician at the time of the initial examination of the patient, without benefit of skin-smear, histopathologic, or serologic data. The final diagnosis of leprosy was based on the histopathologic examination of skin and/or nerve biopsies. The histopathologic diagnosis of leprosy is not infallible (8), as was evident in this study, but histopathol-ogy is probably the most reliable confirmatory tool available. For the few patients without histopathologic data, positive slit skin smears confirmed the presence of leprosy. It must be emphasized that the histopathologic diagnosis was not used to classify patients within the disease spectrum; the only issue of interest was whether or not the patient was thought to have leprosy. The classification of leprosy within the disease spectrum was based solely on the PCD.
The importance of the PCD is that it is the sole criterion of leprosy in many leprosy-endemic areas, or is supplemented only by slit-skin smear examination. In this study, the diagnostic accuracy of the physicians was remarkable among patients in whom an unqualified, "firm" PCD was made. It is notable that the physicians were a heterogeneous group, including residents in training and very experienced dermatologists. No attempt was made to compare the physicians with respect to accuracy of diagnosis.
The physicians were infallible in the diagnosis of multibacillary leprosy (BB, BL or LL), and almost as accurate with respect to a firm diagnosis of BT leprosy. For these patients, the clinical diagnosis was more accurate than the laboratory data furnished by the antibody assays or smears. However, there were a substantial number of patients, the group suspected of having the disease and placed in the RO group, in whom the PCD was only 55% correct. Clearly, there is a pressing need for laboratory help in correctly diagnosing this substantial group of patients who comprised 27% of all the patients studied. Histopathologic examination does not entirely satisfy this need because of the impracticality of securing biopsies from all such patients under field conditions and the shortage of trained pathologists. The other choices are examination of slit-skin smears or serological assays.
The routine use of slit-skin smears for the diagnosis of leprosy is a hallowed tradition, but the practice has recently been questioned (11). The examination of smears is labor-intensive and subject to observer variation. In the present study, the smears were invariably positive in multibacillary leprosy but, since such disease presented no diagnostic difficulty, the smear results were almost superfluous. As others have found (16), the smears were not very helpful in pau-cibacillary leprosy generally, being positive in only 2 of 38 proven leprosy patients with a firm clinical diagnosis of TT or BT. In the RO group the smears were positive in 3 out of 16, a more encouraging but hardly impressive rate of success.
It has been observed in all of the studies of IgM antibodies to PGL-I, including the present one, that mean antibody levels increase across the disease spectrum from TT to LL, as does the proportion of patients who are considered seropositive (1,6,14). Another feature common to this and several other studies is that, with few exceptions, the PGL-I antibody levels in nonleprosy subjects from the same leprosy-endemic area as the patients were closely similar to the levels in subjects who had never been exposed to leprosy (4,9). It is inferred that casual exposure to M. leprae, or subclinical infection, is insufficient to induce a detectable humoral immune response to PGL-I.
The consensus emerging from the studies of antibodies to PGL-I is that their diagnostic value is limited to multibacillary disease, in which the absolute concentrations of antibodies are high, permitting the adoption of a stringent criterion of positivity that ensures high levels of specificity and sensitivity. By contrast, in paucibacillary leprosy the sensitivity of the assay is approximately 50% at best, and the antibody concentrations are relatively low. Accordingly, an acceptable level of sensitivity is obtained only if the criterion of positivity is less stringent, with the consequent loss of specificity. It has been argued that criteria of positivity should favor specificity over sensitivity (7,9), and that position is valid for population surveys in which the prevalence of disease is very low. However, in clinical situations, the prevalence of disease may be much higher, as in the present study in which approximately 1% of new patients were found to have leprosy. In such situations, it may be acceptable or even desirable to adopt criteria of positivity that favor sensitivity relative to specificity (7).
Discussion of the relative merits of sensitivity versus specificity (7,9) is incomplete unless the relative difficulty of diagnosing different types of leprosy clinically is taken into account. For example, if the clinical diagnosis of all types of leprosy were 100% accurate, any laboratory diagnostic test would be redundant, regardless of its sensitivity and specificity. Conversely, as the difficulty of clinical diagnosis increases, so the need for supplementary laboratory data also rises. In the latter circumstance, even tests of suboptimal sensitivity and specificity may prove useful. In practice, the accuracy of clinical diagnosis exceeded 90% for those patients in whom a firm diagnosis was made, 70% of all the patients. Consequently, serologic confirmation of disease was required only for the remaining 30% of patients suspected of leprosy, for whom a relatively low stringency criterion of serological positivity was acceptable.
In light of these considerations, the RO group of patients merits further analysis. Leprosy was confirmed in 24 of 44 of these patients whose skin-smear and ELISA data were closely similar to those patients with a firm PCD of BT leprosy. In effect, all of the leprosy patients in whom the clinical diagnosis was doubtful had BT disease, and of all the BT leprosy patients, 1 out of 3 fell into the RO category. In this study, the leprosy patients were divided equally between multibacillary and paucibacillary disease, but in areas where paucibacillary disease predominates, as in Malawi, the clinical diagnosis of BT leprosy may be an even larger problem (3,9). The ELISA was positive in 12 of 24 RO patients with leprosy compared to 3 of 20 RO patients without leprosy; so if an RO patient were serum positive, the odds were 4:1 that the patient had leprosy.
Considering the diagnosis of leprosy in its totality, it appears that, paradoxically, the greatest potential for the PGL-I antibody assay lies in the diagnosis of paucibacillary leprosy, even though the sensitivity of the assay is much higher in multibacillary disease. The ELISA is more sensitive than skin-smear examination in paucibacillary disease, and the reproducible ELISA results obtained in numerous studies from widely dispersed geographic regions attest to the robust nature of the test (16).
Acknowledgment. We thank the physicians of the ALERT hospital for their collaboration, and Sister Genet and Tadelle Gebre Tsion for collecting and maintaining the serum samples. This research was approved by the joint ALERT/AHRI Research Committee, which oversees the ethics of studies performed at these institutions, and was supported by grant POl AI-20198 from the National Institute of Allergy and Infectious Diseases, Microbial and Infectious Disease Program, National Institutes of Health, Bethesda, Maryland, U.S.A. AHRI is also supported by agencies for development of the Norwegian and Swedish governments (NORAD and SIDA).
REFERENCES
1. Bach, M.-A., Wallach, D., Flageul, B., Hoffenbach, A. and Cottenot, F. Antibodies to phenolic glycolipid I and to whole Mycobacterium leprae in leprosy patients: evolution during therapy. Int. J. Lepr. 54(1986)256-267.
2. Brett, S. J., Draper, P., Payne, S. N. and Rees, R. J. W. Serological activity of a characteristic phenolic glycolipid from Mycobacterium leprae in sera from patients with leprosy and tuberculosis. Clin. Exp. Immunol. 52(1983)271-279.
3. Burgess, P. J., Fine, P. E. M., Ponnighaus, J. M. and Draper, C. Serological tests in leprosy. The sensitivity, specificity and predictive value of ELISA tests based on phenolic glycolipid antigens, and the implications for their use in epidemiological studies. Epidemiol. Infect. 101(1988)159-171.
4. Cartel, J., Chanteau, S., Boutin, J., Plichart, R., Richez, P., Roux, J. and Grosset, J. Assessment of anti-phenolic glycolipid-I IgM levels using an ELISA for detection o(M. leprae infection in populations of the South Pacific Islands. Int. J. Lepr. 58(1990)512-517.
5. Cho, S.-N., Fujiwara, T., Hunter, S. W., Rea, T. H., Gelber, R. H., Aspinall, G. O. and Brennan, P. J. Use of an artificial antigen containing the 3, 6-di-0-methyl-j3-d-glucopyranosyl epitope for the scrodiagnosis of leprosy. J. Infect. Dis. 150(1984)311-322.
6. Cho, S.-N., Yanagihara, D. L., Hunter, S. W.. Gelber, R. H. and Brennan, P. J. Serological specificity of phenolic glycolipid I from Mycobacterium leprae and use in scrodiagnosis of leprosy. Infect. Immun. 41(1983)1077-1083.
7. Daniel, T. M. and Debanne, S. M. The scrodiagnosis of tuberculosis and other mycobacterial diseases by enzyme-linked immunosorbent assay. Am. Rev. Respir. Dis. 135(1987)1137-1 151.
8. Fine, P. E. M., Job, C. K., McDougall, A. C, Meyers, W. M. and Ponnighaus, J. M. Comparability among histopathologists in the diagnosis and classification of lesions suspected of leprosy in Malawi, Int. J. Lepr. 54(1986)614-625.
9. Fine, P. E. M., Ponnighaus, J. M., Burgess, P., Clarkson, J. A. and Draper, C. C. Seroepide-miological studies of leprosy in northern Malawi based on an enzyme-linked immunosorbent assay using synthetic glycoconjugate antigen. Int. J. Lepr. 56(1988)243-254.
10. Fujiwara, T., Hunter, S. W., Cho, S.-N., Aspinall, G. O. and Brennan, P. J. Chemical synthesis and serology of disaccharides and trisaccharides of phenolic glycolipid antigens from the leprosy bacillus and preparation of a disaccharidc protein conjugate for scrodiagnosis of leprosy. Infect. Immun. 43(1984)245-252.
11. Georgiev, G. D. and McDougall, A. C. Skin smears and the bacterial index (BI) in multipledrug therapy leprosy control programs: an unsatisfactory and potentially hazardous state of alfairs. (Letter) Int. J. Lepr. 56(1988)101-104.
12. Hunter, S. W. and Brennan, P. J. A novel phenolic glycolipid from Mycobacterium leprae possibly involved in immunogenicity and pathogenicity. J. Bactcriol. 147(1981)728-735.
13. Hussain, R., Jamil, S., Kifayet, A., Firdausi, F., Dockrell, H. M., Lucas, S. and Hasan, R. Quantitation of IgM antibodies to the M. leprae synthetic dissacharide can predict early bacterial multiplication in leprosy. Int. J. Lepr. 58(1990)491-502.
14. Koster, F. T., Scollard, D. M., Umland, E. T., Fishbein, D. 15., Hanly, W. C, Hrlnnan, P. J. and Nelson, K. E. Cellular and humoral immune response to a phenolic glycolipid antigen (phcnGL-I) in patients with leprosy. J. Clin. Microbiol. 25(1987)551-556.
15. Meeker, H. C, Levis, W. R., Sersen, E., Schuller-Levis, G., Brennan, P. J. and Buchanan, T. M. ELISA detection of IgM antibodies against phenolic glycolipid-I in the management of leprosy: a comparison between laboratories. Int. J. Lepr. 54(1986)530-539.
16. Ponnighaus, J. M., Fine, P. E. M. and Bliss, L. Certainty levels in the diagnosis of leprosy. Int. J. Lepr. 55(1987)454-462.
17. Ridley, D. S. and Joi'lino, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
18. Roche, P. W., Britton, W. J., Failbus, S. S., Williams, D., Pradhan, H. M. and Theuvenet, W. J. Operational value of serological measurements in multibacillary leprosy patients: clinical and bacteriological correlates of antibody responses. Int. J. Lepr. 58(1990)480-490.
19. Roche, P. W., Britton, W. J., Warwick, J., Failbus, S. S., Ludwig, H., Theuvenet, W. J. and Adiga, R. B. Heterogeneity of serological responses in paucibacillary leprosy - dillercntial responses to protein and carbohydrate antigens and correlation with clinical parameters. Int. J. Lepr. 58(1990)319-327.
1. M. B., B. S., Department oflmmunology and Microbiology, Wayne State University School of Medicine, 540 E. Canfield Avenue, Detroit, Michigan 48201, U.S.A.
2. M. D., Department oflmmunology and Microbiology, Wayne State University School of Medicine, 540 E. Canfield Avenue, Detroit, Michigan 48201, U.S.A.
3. M. D., Armauer Hansen Research Institute, Addis Ababa, Ethiopia.
Received for publication on 2 January 1991.
Accepted for publication in revised form on 8 May 1991.