- Volume 59 , Number 3
- Page: 441–9
Serum lgA1 and IgM antibodies against Mycobacterium leprae-derived phenolic Glycolipid-I: a comparative study in leprosy patients and their contacts
ABSTRACT
In order to evaluate the potentials of lgA1 versus IgM as well as of native phenolic glycolipid-I (PGL-I) versus PGL-I-disac-charide coupled to bovine serum albumin (D-BSA) as antigens in the serodiagnosis of leprosy, anti-D-BSA IgA1 and anti-PGL-I IgM were investigated and compared to anti-PGL-I IgA1 in sera f rom patients and contacts.Anti-D-BSA and anti-PGL-I IgA1 significantly correlate in patients and contacts. The higher IgA1 positivity rates obtained with D-BSA as compared to PGL-I may suggest D-BSA as the favorable antigenic material. In patients but not in contacts anti-PGL-I IgM and IgA1 correlate, IgM predominating over IgA1. In all three antibody systems, the mean values as well as the positivity rates increased f rom the tuberculoid toward the lepromatous disease pole. Also, the levels of all three antibodies significantly increased with the bacterial index (BI). However, anti-D-BSA (PGL-I) IgA1 appears to be preferable to IgM with respect to sensitivity, i.e., detection of disease activity, in paucibacillary or Bl-negative patients. A number of contacts were detected as seropositive with anti-D-BSA and/or anti-PGL-I IgA1 but not with anti-PGL-I IgM. This suggests that IgA1 is a better tool than IgM for the detection of leprosy in its subclinical stage.
RÉSUMÉ
Dans le but d'évaluer la valeur des IgAl comparés aux IgM ainsi que celle du glycolipide phénolique-I (PGL-I) naturel comparé au disaccharide-PGL-I couplé à de l'albumine sérique bovine (D-BSA) comme antigènes pour le sérodiagnostic de la lèpre, on a recherché les anticorps IgAl anti-D-BSA et les IgM anti-PGL-I, et on les a comparés aux IgAl anti-PGL-I dans le sérum de patients et de contacts.Il y a une corrélation significative entre les IgA1 vis-à-vis du D-BSA et celles dirigées contre le PGL-I chez les patients et les contacts. Les taux de positivité d'IgA1 plus élevés vis-à-vis du PGL-I suggèrent une meilleure antigénicité du D-BSA. Chez les patients, mais non chez les contacts, il y a une corrélation entre les IgA1 et les IgM vis-à-visdu PGL-I, les IgM étant plusélevécs que les IgA1. Pour les trois types d'anticorps, les valeurs moyennes ainsi que les taux de positivité augmentaient du pôle tuberculoide vers le pôle lépromateux de la maladie. De plus, les taux des trois types d'anticorps augmentaient de manière significative avec l'indice bactériologique (IB). Cependant les anticorps IgA1 vis-à-vis du D-BSA (PGL-I) semblent être préférables aux IgM du point de vue de la sensibilité, c'est-à-dire la détection de signes d'activité de la maladie chez des patients paucibacillaires ou avec un indice bactériologique négatif. Un certain nombre de contacts ont été détectés comme séropositifs pour les IgA1 vis-à-vis du D-BSA et/ou du PGL-I mais non pour les IgM anti-PGL-I. Ceci suggère que les IgA1 sont préférables aux IgM pour la détection de la lèpre subclinique.
RESUMEN
Para poder evaluar la utilidad potencial de IgAl vs IgM y del glicolipido fenólico-I nativo (PGL-I) vs el disacárido del PGL-I acoplado a la albúmina sérica bovina (D-BSA) como antígenos para el serodiagnós-tico de la lepra, se investigaron los anticuerpos IgAl anti-D-BSA e IgM anti-PGL-I y se compararon con los anticuerpos IgAl anti-PGL-I en los sueros de pacientes y contactos.Los anticuerpos IgA1 anti-PGL-I y anti-D-BSA correlacionaron apropiadamente en los pacientes y contactos. Las mayores frecuencias de positividad se encontraron con D-BSA y ésto lo señala como el material antigénico más favorable. En los pacientes, pero no en los contactos, se encontró una buena correlación entre los anticuerpos IgM anti-PGL-I e IgA1, predominando los IgM sobre los IgA1. En los tres sistemas de anticuerpo, los valores medios y las frecuencias de positividad aumentaron del extremo tuberculoide al lepromatoso. Los niveles de los tres anticuerpos también aumentaron proporcionalmente al Índice bacteriano (IB). Sin embargo, los anticuerpos anti-D-BSA (PGL-I) IgA1 parecen ser preferibles a los IgM con respecto a sensibilidad, sobre todo en la detección de la enfermedad en los casos paucibacilares o en los pacientes con IB negativos. Un gran numero de contactos resultaron seropositivos con anti-D-BSA y/o anti-PGL-I IgA1 pero no con IgM anti-PGL-I. Esto sugiere que IgA1 es una mejor herramienta que IgM para la detección de la lepra en su etapa subclínica.
The discovery of phenolic glycolipid-I (PGL-I) as the major species-specific antigen of Mycobacterium leprae has resulted in significant progress in the serology of leprosy. Based on the antigenic moiety of PGL-I, a semi-synthetic antigen, D-BSA, consisting of the terminal PGL-I-disaccharide conjugated to bovine serum albumin (BSA), has been synthesized (10,11) and extensively employed in serologic studies of leprosy (4,7,22).
Anti-PGL-I antibodies, specifically directed against the trisaccharide epitope, have been demonstrated in sera from leprosy patients in levels increasing from the tuberculoid to the lepromatous pole of the disease (3,8,23) as well as increasing with an increasing bacterial index (BI) (9,13,21). Antibody titers have been shown to decrease the antibacterial treatment of leprosy (3,8,9). With respect to immunoglobulin classes, serum anti-PGL-I IgM, owing to its predominance over anti-PGL-I IgG and IgA1 has received the most attention (4,7,8,24). Nevertheless, since determination of anti-PGL-I IgM antibodies primarily detects multibacillary (MB) as opposed to paucibacillary (PB) leprosy (3,4,7,8), another effective tool for monitoring disease activity in the PB forms as well as for the early detection of infection with M. leprae is needed.
A major role in immunological defense against microorganisms or viruses that infect mucosae is attributed to IgA (16). It has been suggested that antigen-specific IgA in serum may be of mucosal origin and may be an early sign of infection (5,17). In view of the possible mode of transmission of leprosy via dermal amd mucosal (respiratory tract) body surfaces (12), a closer study of anti-PGL-I IgA is justifiably necessary. The results of our previous study revealed that serum anti-PGL-I IgA in leprosy patients and their contacts is predominantly of the IgA1 subclass (20). In the present work, we have studied anti-D-BSA IgA1 and anti-PGL-I IgM in leprosy and have compared the results to our data on anti-PGL-I IgA1 (20) in order to evaluate the relative sérodiagnostic potentials of these parameters.
MATERIALS AND METHODS
Sera. Sera from 88 leprosy patients, clinically and histologically classified according to the Ridley-Jopling scale (19) as lepromatous (LL, N = 44), borderline lepromatous (BL, N = 9), midborderline (BB, N = 9), borderline tuberculoid (BT, N = 17), and tuberculoid (TT, N = 9) leprosy, as well as sera from 28 household or family contacts of leprosy patients, were obtained from Bayley Seton Hospital, Staten Island, New York, U.S.A. The bacterial index (BI) was measured on a semilogarithmic scale (0-6+) approximating that of Ridley (18). Histology, including the BI, from skin biopsies was reported from the Gillis W. Long Hansen's Disease Center, Carville, Louisiana, U.S.A. Sera from 31 healthy subjects (laboratory personnel) were used as normal controls. Serum samples were stored in aliquots below -70ºC.
ELISA for anti-D-BSA IgA1. Both BSA and PGL-I-disaccharidc-BSA (D-BSA), synthesized according to Gigg (11), were kind donations from Dr. H. D. Engcrs of the IMMLEP Program of the World Health Organization. Flat-bottom, polystyrene microliter plates (Nunc) were coated by incubation with 100 µI/well of D-BSA or of BSA, respectively, diluted to 100 ng/ml in carbonate-bicarbonate buffer (15 mM Na2CO3, 35 mM NaHCO3, pH 9.6) for 18 hr at 37ºC. After washing three times with phosphate-buffered saline (PBS: 8.1 mM Na2HPO4-2H2O2 1.5 mM KH2PO4, 2.9 mM KC1, 136.9 mM NaC1, pH 7.4) containing 0.1% v/v Tween 20 (PBST), the plates were incubated for 1 hr at 37ºC with 200 µI/well of 1% w/v BSA (Behring) in PBST (BSA-PBST). BSA-PBST was then replaced by 100 µI/well of test serum (in duplicate), diluted 1/20-1/160 in PBST. An LL serum, positive for anti-PGL-I IgA1, diluted 1/2001/1600, was included as reference on each plate. After incubation with test serum for 1 hr at 37ºC, the plates were washed three times with PBST and incubated for 1 hr at 37ºC with 100 µI/well of mouse monoclonal (IgG1) anti-human IgA1 (Nordic, NI69-11), diluted 1/2000 in PBST. Washing three times with PBST was followed by incubation (for 1 hr at 37ºC) with 100 µI/well of peroxidase-conjugated goat (affinity-purified antibody) anti-mouse IgG (Zymed) diluted 1/500 in BPST. After again washing three times with PBST, 100 /d/well of substrate solution, containing 1.8 mM 2, 2'-azino-di(3-ethyl-benzthiazoline-6-sulfonic acid) (ABTS; Boehringer) and 2.9 mM H2O2 in citrate-phosphate buffer (60 mM C6H8O7 H2O, 80 mM Na2 HPO4- 2H2O, pH 4.0) were added for 1 hr at room temperature in the dark. The reaction was stopped by addition of 100 µI/well of 0.32% w/v NaF. Extinction (E) was read with a double wavelength micro-ELISA autoreader MR 580 (Dynatech) at 405 nm (versus 490 nm) against a blank made up of substrate and stop solutions.
ELISA for anti-PGL-I IgM. Armadillo-derived PGL-I was kindly provided under a National Institutes of Health contract by Dr. P. J. Brennan, Colorado State University, Fort Collins, Colorado, U.S.A. The ELISA procedure was the same as for anti-D-BSA IgA1 except for the following differences: Coating was performed with 100 µI/well of PGL-I-liposomes, prepared as described elsewhere (20), diluted to 2.5 µgPGL-I/ml in Tris-buffered saline (20 mM Tris, 150 mM NaCl, pH 8.0), as well as with control-liposomes (without PGL-I). After washing with PBS (70 mM Na2HPO42H2O, 30 mM KH2PO4, 150 mM NaCl, pH 7.2), blocking was performed for 1.5 hr with 2.5% w/v human serum albumin (HSA; kindly donated by Dr. F. Elsinger, Immuno A.G., Vienna, Austria) in PBS (HSA-PBS). Test serum was diluted 1/500-1/8000 and the positive LL serum for anti-PGL-I IgM (reference) was diluted 1/1000-1/16,000 in HSA-PBS. Incubation with peroxidase-conjugated rabbit anti-human IgM (Dako), diluted 1/500 in HSA-PBS, was for 1.5 hr at 37ºC. PBS was used for washing after serum and conjugate incubations.
ELISA results were expressed as ΔE = E (coat with D-BSA or PGL-I-liposomes) minus E (coat with BSA or control-liposomes, respectively). In order to minimize the influence of plate-to-plate as well as day-today variations on serum screening, ΔE values obtained for test sera were corrected by relating them to the reference serum ΔE on each plate. Serum samples were considered seropositive when their antibody titers exceeded the cut-off point determined as the mean ΔE + 2 standard deviations (S.D.) of the control group for that particular antibody.
Statistical analysis. Statistical analysis of the data was done by comparison between groups using analysis of variance with follow-up t tests. In addition, data were also analyzed by the nonparametric Kruskal-Wallis test with follow-up Mann-Whitney U tests to confirm the analysis of variance results. The relationships between antibody titers as well as between titers and parameters of disease activity were assessed by calculation of Pearson correlation coefficients.
RESULTS
Anti-D-BSA IgA1, anti-PGL-I IgA1, and anti-PGL-I IgM antibody titers in leprosy patients
Correlation between antibody titers. Comparison of anti-PGL-I IgA1 titers (20) with anti-D-BSA IgA1 and anti-PGL-I IgM titers in leprosy patients showed significant correlations between anti-D-BSA and anti-PGL-I IgA1 (r = 0.501, p < 0.001, N = 42) (Fig. 1) as well as between anti-PGL-I IgA1 and IgM (r = 0.460, p < 0.001, N = 85) (not shown).
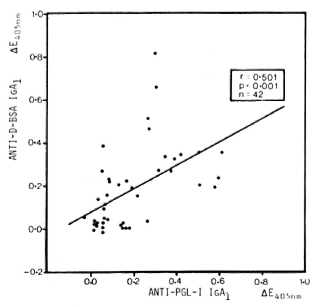
Fig. 1. Correlation between anti-D-BSA and anti-PGL-I IgA1 in sera (diluted 1/20) from leprosy patients. Each point represents one individual; line is best fitting regression line.
Antibody titers in relation to disease classification. Similar to anti-PGL-I IgA1 (20), anti-D-BSA IgA1 and anti-PGL-I IgM mean levels as well as seropositivity rates generally increased from the tuberculoid to the lepromatous pole of the disease spectrum (The Table). Statistical analysis revealed that for anti-D-BSA IgA1 the LL, BL, and BB groups (p < 0.001) as well as the TT group (p < 0.05) were significantly different compared with the control group; the BT group did not differ from the controls. With respect to anti-PGL-I IgA1, the LL and BL groups (p < 0.001) were significantly different from the control group; the other leprosy groups did not differ from controls (20). Similarly, for anti-PGL-I IgM only the LL (p < 0.001) and BL (p < 0.01) groups were significantly different as compared to the control group.
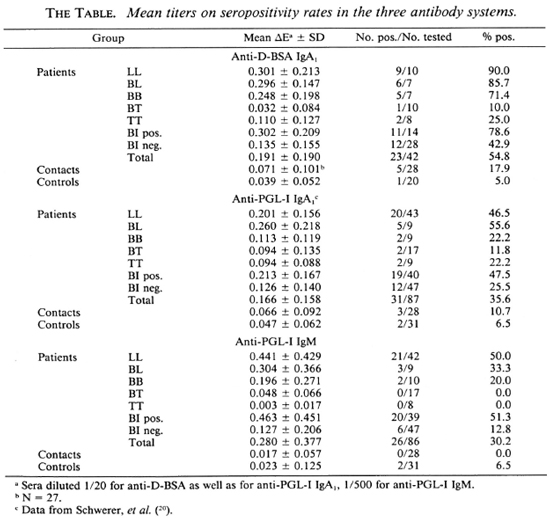
Antibody titers in relation to BI. Significant correlations between antibody levels and the BI were obtained for anti-D-BSA IgA1 and for anti-PGL-I IgM as has been demonstrated for anti-PGL-I IgA1 (20), with anti-PGL-I IgM being highly responsive to the BI (Fig. 2). However, there is a wide scatter of antibody values in each of the three systems. In particular, 12/28 (43%), 12/47 (26%), and 6/47 (13%) patients with a BI of zero were seropositive for anti-D-BSA IgA1, anti-PGL-I IgA1 (20) and anti-PGL-I IgM, respectively (The Table). A very similar pattern was obtained when a group of 27 Bl-negative patients were used for the calculation of percentages of seropositivity in all three systems (44% for anti-D-BSA IgA1, 30% for anti-PGL-I IgA1, and 19% for anti-PGL-I IgM). Regardless of the antibody system, a high percentage (around 80%) of seropositive patients with a BI of zero were found to have moderate-to-severe neuropathy, as assessed by clinical examination and nerve conduction velocity tests.
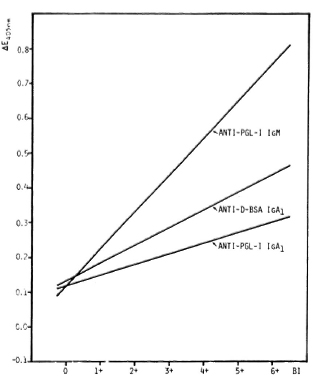
Fig. 2. Serum anti-PGL-I IgM, anti-D-BSA IgA1, and anti-PGL-I IgA1 titers in relation to BI in leprosy patients as represented by best fitting regression lines. Anti-PGL-I IgM: Y = 0.107X + 0.114 (r = 0.575, p < 0.001, N = 86); anti-D-BSA IgA1: Y = 0.051X + 0.131 (r = 0.502, p < 0.001, N = 42); anti-PGL-I IgA1 (20): Y = 0.031X + 0.117 (r = 0.400, p < 0.001, N = 87); sera diluted 1/20 for anti-D-BSA as well as for anti-PGL-I IgA1; 1/500 for anti-PGL-I IgM.
In leprosy patients with a positive BI, seropositivity rates were higher in anti-D-BSA than in anti-PGL-I IgA1, while they were similar in anti-PGL-I IgA1 and IgM (The Table).
Antibody titers in relation to antibacterial treatment, ENL, and neuropathy. Anti-PGL-I IgM seemed to be unrelated to the duration of any antibacterial treatment in all patients (r = -0.025, N = 82) as well as in multibacillary (LL, BL) patients (r = - 0.221, N = 50), thus resembling anti-PGL-I IgA1 (20). Apparently the selection of our patient population did not favor the detection of a treatment effect: there were only 6 untreated patients and 22 patients on short-term treatment (< 2 years); most patients (56) had received long-term treatment ranging from 2 up to 38 years.
Occurrence of erythema nodosum lepro-sum (ENL) in 29 multibacillary (LL, BL) patients was without significant effect upon anti-PGL-I IgM (r = -0.075, N = 51) or anti-PGL-I IgA1 (20) values.
Although there was a high incidence of neuropathy among Bl-negative patients with elevated antibody levels, statistical analysis of all patients showed that the presence of neuropathy in 55 leprosy patients, as assessed by clinical examination and nerve conduction velocity tests, appeared not to be related to anti-PGL-I IgM (r = -0.081, N = 78) or to anti-PGL-I IgA1 (20) levels.
Anti-D-BSA IgA1, anti-PGL-I IgA1, and anti-PGL-I IgM antibody titers in contacts vs controls
Comparison of anti-PGL-I IgA1 titers (20) with anti-D-BSA IgA1 and anti-PGL-I IgM titers in contacts and controls gave the following relationships: In contacts, anti-D-BSA IgA1 significantly (r = 0.532, p < 0.01,N = 27) correlated with anti-PGL-I IgA1; whereas there was no correlation (r = -0.091, N = 28) between anti-PGL-I IgA1 and IgM (not shown). In controls, no significant correlations were found either between anti-D-BSA and anti-PGL-I IgA1 (r = 0.308, N = 20), or between anti-PGL-I IgA1 and IgM (r = 0.256, N = 31).
Although statistical analysis gave no significant differences between contacts and controls in all three antibody systems, three (3/28, 10.7%) seropositive contacts were detected with anti-PGL-I IgA1 (20) but none (0/28, 0.0%) with anti-PGL-I IgM (Fig. 3). However, with anti-D-BSA IgA1, an even higher number of seropositive contacts, namely, five (5/28, 17.9%) cases, were detected, two of which were also detected as seropositive with anti-PGL-I IgA1. Nevertheless, in all three systems false-positive controls, i.e., 1/20 (5.0%) for anti-D-BSA IgA1, 2/31 (6.5%) for anti-PGL-I IgA1 (20) and 2/31 (6.5%) for anti-PGL-I IgM, were recorded. The high titers of anti-PGL-I IgM in three controls, which resulted in a high seropositivity cut-off point, appear to be responsible for the inability of the anti-PGL-I IgM system to detect any of the contacts as seropositive.
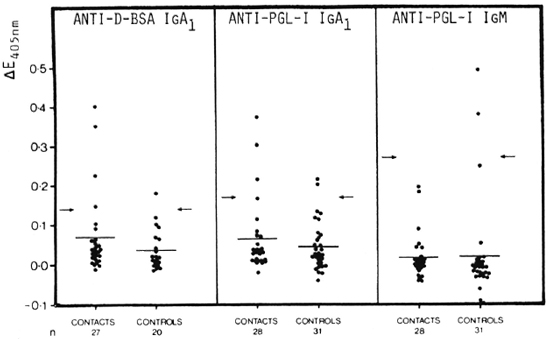
Fig. 3. Comparison of anti-D-BSA IgA1 (left panel), anti-PGL-I IgA1 (data from Schwerer, et al. (20), middle panel), and anti-PGL-I IgM (right panel) in contacts of leprosy patients and in normal controls. Sera diluted 1/20 for anti-D-BSA as well as for anti-PGL-I IgA1; 1/500 for anti-PGL-I IgM. Each point = one individual; horizontal bars = mean AE values; arrows = corresponding mean ΔE + 2 S.D. of the control group.
DISCUSSION
In leprosy patients, anti-D-BSA IgA1 significantly correlated with anti-PGL-I IgA1, similar to what has been shown for IgM (7-22). This is consistent with the fact that both PGL-I and D-BSA carry the same antigenic terminal disaccharide (10,11). Although anti-PGL-I IgM predominates over IgA1 as has been shown before (4,7,24), this study demonstrates a significant correlation between anti-PGL-I IgA1 and IgM. The observed scatter within both correlations may be due to individual differences in the polyclonal B-cell response to PGL-I with respect to both antigen specificity and Ig class (4).
In all three antibody systems mean values as well as seropositivity rates were found to increase from the tuberculoid to the lepro-matous pole of the disease spectrum, confirming earlier reports (3,4,7,8,13,22,24). Within the paucibacillary groups, however, anti-D-BSA (PGL-I) IgA1 appeared to be well demonstrable; whereas anti-PGL-I IgM seemed to be practically negative.
All three antibodies were demonstrated to increase significantly with the BI similar to what has been found with anti-PGL-I IgM (13,21); anti-PGL-I IgM appeared to be most sensitive to the BI. However, the considerable scatter of antibody values in relation to the BI in all three systems indicates that the BI may not be the only parameter of disease activity reflected in anti-PGL-I levels. In particular, a number of Bl-negative patients recorded elevated anti-D-BSA (PGL-I) IgA1 and/or anti-PGL-I IgM levels; among these patients there was a high incidence of moderate-to-severe neuropathy. This supports the view that bacillary and/ or antigenic material persisting especially in the peripheral nervous system despite antibacterial treatment may lead to a persisting immune response.
For Bl-negative patients, anti-PGL-I se-ropositivity was higher in IgA1 than in IgM, while for Bl-positive patients seropositivity was similar in IgA1 and IgM. One report comparing positivity rates in different Ig classes (') showed the anti-PGL-I IgA rate to be higher than the IgM rate in leprosy patients. Looking at anti-D-BSA versus anti-PGL-I seropositivity rates, all patients' rates were higher in anti-D-BSA than in anti-PGL-I IgA1. Although these results may be due to the lower number of patients tested against D-BSA, they seem to support the view that D-BSA possesses higher sensitivity than native PGL-I (4,7). On the other hand, authors comparing IgM positivity rates with the two antigens have obtained very similar overall rates (4,7,22).
As has been observed for anti-PGL-I IgA1 (20), anti-PGL-I IgM values appeared not to depend upon the duration of antibacterial treatment. This is at variance with earlier data (3,8,9) and may be due to the small number of short-term-treated patients in our study. Similarly, in another study little effect of the disease duration upon anti-PGL-I levels was observed (M). Surprisingly, anti-PGL-I IgM levels did not seem to depend upon the occurrence of ENL in MB leprosy patients; whereas earlier reports have shown anti-PGL-I IgM to decrease in ENL patients (2,7,13,21). However, the detection of the ENL effect has been shown to depend on the ELISA method used (13,15). Thus, the present result may be related to minor differences in methodology and/or an unfavorable selection of our patient population.
In controls, neither anti-D-BSA and anti-PGL-I IgA1 nor anti-PGL-I IgA1 and IgM correlated. The three systems recorded false-positive controls with a similar incidence (around 6.0%). This figure is slightly higher than the seropositivity rates reported for controls, i.e., 1.4% for anti-PGL-I IgA (1) and around 3.0% for anti-PGL-I (D-BSA) IgM (7), and may be due to the relatively low number of controls tested.
In household or family contacts of leprosy patients there was a sigificant correlation between anti-D-BSA and anti-PGL-I IgA1; however, there was no correlation between anti-PGL-I IgA1 and IgM. Among the 28 contacts, 5 (17.9%) were detected seropositive in the anti-D-BSA IgA1 system, 3 (10.7%) were detected seropositive in the anti-PGL-I IgA1 system (20), but none was detected in the anti-PGL-I IgM system. Three of the seropositive contacts detected with anti-D-BSA and/or anti-PGL-I IgA1 were from families with more than one case of MB leprosy and had presented with (bacillary negative) skin lesions. Extensive studies have used the determination of anti-D-BSA IgM to screen contacts in endemic areas giving positivity rates of around 20% (6). Our results, however, agree very well with a previous report (') showing that anti-PGL-I IgA rates exceed IgM rates in contacts.
On the whole, this study confirms the usefulness of anti-PGL-I antibodies for monitoring disease activity throughout the leprosy spectrum, and favors D-BSA as the better antigenic material than native PGL-I with respect to sensitivity. While anti-PGL-I IgM appears to be well suited, especially in MB or Bl-positive patients, anti-D-BSA (PGL-I) IgA1 seems to be more sensitive, particularly for PB or Bl-negative patients. For the contacts of leprosy patients, our study favors anti-D-BSA (PGL-I) IgA1 as a better sérodiagnostic tool than IgM in the detection of subclinical infection with M. leprae.
Acknowledgment. Our work was supported by the Austrian Science Research Fund (Project P 6438M) and by the Medical-Scientific Fund of the Lord Mayor of the Federal Capital of Vienna. We would like to thank Dr. P. Fischer and Dr. G. Sersen for help with statistical analyses, as well as Dr. G. F. Suchanck and Dr. J. Radl for valuable discussions.
REFERENCES
1. Abe, M., Miyaji, I., Okushita, T., Minagawa, F., Yoshino, Y., Sakamoto, Y. and Saikawa, K. Anti-mycobacterial antibodies in saliva. Lepr. Rev. 57 Suppl. 2(1986)213-223.
2. Andreoli, A., Brett, S. J., Draper, P., Payne, S. N. and Rook, G. A. W. Changes in circulating antibody level to the major phenolic glycolipid during erythema nodosum leprosum in leprosy patients. Int. J. Lepr. 53(1985)211-217.
3. Brett, S. J., Draper, P., Payne, S. N. and Rees, R. J. W. Serological activity of a characteristic phenolic glycolipid from Mycobacterium leprae in sera from patients with leprosy and tuberculosis. Clin. Exp. Immunol. 52(1983)271-279.
4. Brett, S. J., Payne, S. N., Gigg, J., Burgess, P. and Gigg, R. Use of synthetic glycoconjugates containing the Mycobacterium leprae specific and immunodominant epitope of phenolic glycolipid I in the serology of leprosy. Clin. Exp. Immunol. 64(1986)476-483.
5. Brown, T. A., Murphy, B. R., Radl, J., Haau-man, J. J. and Mestecky, J. Subclass distribution and molecular form of immunoglobulin A hemagglutinin antibodies in sera and nasal secretions after experimental secondary infection with influenza A virus in humans. J. Clin. Microbiol. 22(1985)259-264.
6. Chanteau, S., Cartel, J.-L., Roux, J., Plichart, R. and Bach, M.-A. Comparison of synthetic antigens for detecting antibodies to phenolic glycolipid I in patients with leprosy and their household contacts. J. Infect. Dis. 157(1988)770-776.
7. Cho, S. N., Fujiwara, T., Hunter, S. W., Rea, T. H., Geluer, R. H. and Brennan, P. J. Use of an artificial antigen containing the 3, 6-di-O-mcthyl-/j-d-glucopyranosyl epitope for the scro-diagnosis of leprosy. J. Infect. Dis. 150(1984)311-322.
8. Cho, S.-N., Yanagihara, D. L., Hunter, S. W., Gelber, R. H. and Brennan, P. J. Serological specificity of phenolic glycolipid I from Mycobacterium leprae and use in scrodiagnosis of leprosy. Infect. Immun. 41(1983)1077-1083.
9. Douglas, J. T., Steven, L. M., Fajardo, T., Ce-llona, R. V., Madarang, M. G., Abalos, R. M. and Steenbergen, G. J. The effects of chemotherapy on antibody levels in lepromatous patients. Lepr. Rev. 59(1988)127-135.
10. Fujiwara, T., Hunter, S. W., Cho, S.-N., Aspi-nall, G. O. and Brennan, P. J. Chemical synthesis and serology of disaccharides and trisac-charides of phenolic glycolipid antigens from leprosy bacillus and preparation of a disaccharide protein conjugate for scrodiagnosis of leprosy. Infect. Immun. 43(1984)245-252.
11. Gigg, J., Gigg, R., Payne, S. and Conant, R. Synthesis of propyl 4-O-(3, 6-di-O-methyl-β-d-glucopyranosyl)-2, 3-di-O-methyI-α-D-glucopyranoside. Carbohydr. Res. 141(1985)91-97.
12. Leiker, D. L. On the mode of transmission of Mycobacterium leprae. Lepr. Rev. 48(1977)9-16.
13. Levis, W. R., Meeker, H. C, Schuller-Levis, G., Sersen, E. and Schwerer, B. IgM and IgG antibodies to phenolic glycolipid I from Mycobacterium lepraein leprosy: insight into patient monitoring, erythema nodosum leprosum, and bacillary persistence. J. Invest. Dermatol. 86(1986)529534.
14. Lyons, N. F., Shannon, E. J., Ellis, B. P. B. and Naafs, B. Association of IgG and IgM antibodies to phenolic glycolipid I antigen of Mycobacterium leprae with disease parameters in multibacillary leprosy patients. Lepr. Rev. 59(1988)45-52.
15. Meeker, H. C, Levis, W. R., Sersen, E., Schuller-Levis, G., Brennan, P. J. and Buchanan, T. M. ELISA detection of IgM antibodies against phenolic glycolipid-I in the management of leprosy: a comparison between laboratories. Int. J. Lepr. 54(1986)530-539.
16. Mestecky, J. and McGhee, J. R. Immunoglobulin A (IgA): molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv. Immunol. 40(1987)153-245.
17. Persson, M. A. A., Ekwall, E., Hammarström, L., Lindberg, A. A. and Smith, C. I. E. Immunoglobulin G (IgG) and IgA subclass pattern of human antibodies to Shigella flexneri and Salmonella scrogroup B and D lipopolysaccharide O antigens. Infect. Immun. 52(1986)834-839.
18. Ridley, D. S. Bacterial indices. In: Leprosy in Theory and Practice. Cochrane, R. G. and Davcy, T. F. eds. Baltimore: Williams and Wilkins, 1964, pp. 620-622.
19. Ridley, D. S. and Jopling, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
20. Schwerer, B., Chujor, C. S. N., Bernheimer, H., Radl, J., Haaijman, J. J., Meeker, H. C, Sersen, G. and Levis, W. R. IgA antibodies against phenolic glycolipid I from Mycobacterium leprae in scrum of leprosy patients and contacts: subclass distribution and relation to disease activity. Clin. Immunol. Immunopathol. 53(1989)202-211.
21. Schwerer, B., Meeker, H. C, Sersen, G. and Levis, W. R. IgM antibodies against phenolic glycolipid I from Mycobacterium leprae in leprosy sera: relationship to bacterial index and erythema nodosum leprosum. Acta Leprol. (Geneve) 2(1984) 395-402.
22. Wu, Q., Ye, G. and Li, X. Serological activity of natural disaccharide octyl bovine serum albumin (ND-O-BSA) in sera from patients with leprosy, tuberculosis, and normal controls. Int. J. Lepr. 56(1988)50-55.
23. Young, D. B. and Buchanan, T. M. A serological test for leprosy with a glycolipid specific for Mycobacterium leprae. Science 221(1983)1057-1059.
24. Young, D. B., Dissanayake, S., Miller, R. A., Khanolkar, S. R. and Buchanan, T. M. Humans respond predominantly with IgM immunoglobulin to the species-specific glycolipid of Mycobacterium leprae. J. Infect. Dis. 149(1984)870-873.
1. Ph.D.; Neurological Institute, University of Vienna, Schwarzspanierstrasse 17, A-1090 Vienna, Austria.
2. M.D.; Neurological Institute, University of Vienna, Schwarzspanierstrasse 17, A-1090 Vienna, Austria.
3. Ph.D., Neurological Institute, University of Vienna, Schwarzspanierstrasse 17, A-1090 Vienna, Austria.
4. M.D., New York State Institute for Basic Research in Developmental Disabilities, Stat-en Island, New York, U.S.A.
Reprint requests to Dr. Schwerer.
Received for publication on 9 August 1990.
Accepted for publication in revised form on 22 February 1991.