- Volume 59 , Number 3
- Page: 450–7
A Serologic study of naturally acquired leprosy in chimpanzees
ABSTRACT
Data f rom longitudinally obtained serum samples spanning several years has permitted us to identify two chimpanzees with leprosy and to estimate the time of Mycobacterium leprae exposure/infection. The results confirm high levels of specific anti-M. leprae phenolic glycolipid-I (PGL-I) as well as anti-lipoarabinomannan (anti-LAM) antibodies in both chimpanzees, and identify additional chimpanzees with possible M. leprae exposure. The observations are consistent with the hypothesis that leprosy exists in chimpanzees in the U.S.A. and suggest the possibility that M. leprae may be transmitted among chimpanzees. The data suggest that monitoring anti-PGL-I and anti-LAM IgG and IgM levels longitudinally in leprosy contacts may be useful in the recognition of preclinical leprosy.RÉSUMÉ
Les données obtenues à partir d'échantillons de serums récoltés au cours d'études longitudinales s'éten-dant sur plusieurs années nous ont permis d'identifier deux chimpanzés présentant une lèpre, et d'estimer l'époque de l'exposition/infection à Mycobacterium leprae. Les résultats confirment les taux élevés d'anticorps spécifiques vis-à-vis du glycolipide phénolique-1 (PGL-i) de M. leprae et vis-à-vis du lipoarabinoman-nan (anti-LAM) chez les deux chimpanzés, et identifient d'autres chimpanzés ayant peut-être été exposés à M. leprae. Les observations sont compatibles avec l'hypothèse de l'existence de la lèpre chez les chimpanzés aux États-Unis, et suggèrent la possibilité que M. leprae puisse se transmettre entre chimpanzés. Les données suggèrent que la surveillance longitudinale des taux d'IgG et d'IgM anti-PGL-I et anti-LAM chez les contacts des malades de la lèpre pourrait être utile pour établir le diagnostic de lèpre pré-clinique.RESUMEN
Los estudios serológicos longitudinales en chimpancés, nos han permitido descubrir dos chimpancés con lepra y calcular el tiempo de exposición/infección con el Mycobacterium leprae. Los resultados confirman la presencia de altos niveles de anticuerpos anti-glicoli-pido fenólico-1 del M. leprae y anti-lipoarabinomanana (anti-LAM) en ambos chimpancés, y han permitido identificar otros chimpancés con posible exposición al M. leprae. Las observaciones son consistentes con la hipótesis de que existe lepra en los chimpancés en los Estados Unidos, y sugieren la posibilidad de que cl M. leprae pueda ser transmitido entre los chimpancés. Los datos sugieren que midiendo periódicamente los niveles de anticuerpos IgM e IgG anti-PGL-I y anti-LAM en los contactos de los pacientes con lepra, se podría identificar a los casos de lepra subclínica.There have been many unsuccessful attempts to induce leprosy by the experimental inoculation of Mycobacterium leprae into a variety of nonhuman primate species (11,13). These failures and the paucity of reports of naturally acquired leprosy among nonhuman primates have led to the belief that humans are the only primate species susceptible to infection by M. leprae. However, we have observed naturally acquired leprosy in two African-born, captive, sooty mangabey monkeys (Cercocebus atys) that had been housed together (6,15). Also, we have experimentally induced leprosy in many additional sooty mangabeys by M. leprae inoculation (11,15,17). These observations led us to postulate that enzootic leprosy may exist among feral sooty mangabeys (6).
Naturally acquired leprosy was reported in 1978 in a captive, feral-born chimpanzee (Pan troglodytes) (12), and leprosy has been induced experimentally by M. leprae inoculation of a chimpanzee (8). In early 1989, a chimpanzee (Kevin), housed at the University of Texas Science Park (UTSP), Bastrop, Texas, U.S.A., was diagnosed as having borderline lepromatous (BL) leprosy; an additional chimpanzee (Brian), housed at the Southwest Foundation for Biomedical Research (SFBR), San Antonio, Texas, U.S.A., was diagnosed with leprosy (1,9,10) in the subpolar lepromatous-to-borderline lepromatous (LLs-BL) area of the Ridley-Jopling spectrum (16). Thus, leprosy may exist naturally among chimpanzees.
This report describes: a) a longitudinal serologic study of antibodies to the M. lep-rac-specific phenolic glycolipid-I (PGL-I) antigen and to the mycobacterial common lipoarabinomannan (LAM) antigen in two of the chimpanzees with naturally acquired leprosy, Brian (PTT229) and Kevin (2038), and b) a cross-sectional serologic survey for anti(A)-LAM and A-PGL-I in 160 chimpanzees housed in two primate centers in the U.S.A.
MATERIALS AND METHODS
ELISAs. Details of the ELISA methods employed were reported previously (5,7). Optimal dilutions of reagents and sera utilized previously in mangabey monkey serological studies were found to be similar to those chosen for chimpanzee sera. LAM and PGL-I antigens were purified and provided under NIAID contract #l-AI-52582 by Dr. Patrick J.Brennan, Colorado State University, College of Veterinary Sciences, Fort Collins, Colorado, U.S.A. Similar methods have been used to detect PGL-I antibodies in sera from various forms of leprosy (18) and contacts of leprosy patients (2), and to follow changes in antibody levels in leprosy patients under therapy (3,14). In some experiments, we utilized the synthetic BSA-disaccharide (ND-O-BSA) containing the specific PGL-I disaccharide epitope in ELISA, rather than the natural PGL-I, in order to confirm serological specificity. This epitope is the specific serological recognition unit identifying M. leprae PGL-I, and is not known to crossrcact with other mycobacteria (4). ND-O-BSA was also kindly provided by Dr. Brennan.
Briefly, chimpanzee sera were diluted 1:100 for IgM and 1:300 for IgG A-PGL-I and ND-O-BSA determinations and 1:200 for IgM and 1:100 for IgG A-LAM determinations. Peroxidase conjugated A-IgM and A-IgG were diluted 1:2000 and 1:1000, respectively, for A-PGL-I studies and 1:500 and 1:1000 respectively, for A-LAM determination. These dilutions were experimentally determined by "checkerboard" titrations of all reagents. Optimal combinations were chosen so that the mean optical density (OD) values for A-PGL-I and A-LAM fell below 0.5; OD values for known negative control sera fell below 0.1. ND-O-BSA was used to coat the ELISA plates in amounts such that stoichiometric equivalents of di-saccharide were adhered relative to native PGL-I. The ELISA conjugates were A-Fc piece, μ or γ-specific affinity-purified reagents from Cooper Biomedical, Malvern, Pennsylvania, U.S.A. Each serum sample was assayed in duplicate experimental wells (PGL-I, ND-O-BSA or LAM antigen) and control wells (buffer only). Final OD values represent the mean OD values of experimental wells minus the mean OD values of control wells. All sera were assayed simultaneously on multiple occasions, and the data presented were from one of these repetitive experiments. Consistent relative results were reproducibly obtained with minor variations in absolute OD values in the different experiments.
Chimpanzees. We studied a total of 160 chimpanzees housed at either SFBR or UTSP. Of the 122 animals from UTSP, 65 were wild-born adults, 15 captive-born adults or adolescents received from other institutions, and 42 UTSP colony-born infants, juveniles, or adolescents. Between 1977 and 1984, UTSP had received apes from 14 different sources for resocialization and integration into breeding groups. The UTSP breeding groups of 6 to 11 adults and varying numbers of offspring were housed in eight outdoor corrals with indoor quarters heated and ventilated with nonrecir-culated air exceeding 15 air changes per hr. There was no contact between apes in adjacent corrals and no relocation of animals between groups in the corrals, except for the transfer of a few females from their natal groups as they reached reproductive age. Chimpanzees not housed in the large breeding groups were housed in pairs or small groups in indoor-outdoor runs in one of five quarantine buildings, or in one of ten adjacent and interconnectable indoor-outdoor runs in a holding/socialization building. Some apes could contact others in adjacent runs through wire panels; other runs were separated by solid walls. Heating and ventilation air flow was nonrecirculated in these buildings. Relocation of apes in the socialization building was more frequent than in the corral groups. Records were kept of all ape movements in quarantine, in the socialization building, and in the corral groups.
The 38 serologically tested SFBR chimpanzees were members of a breeding colony kept in small social groups. Each breeding group consisted of 1 male and 3 or 4 females. The animals were housed in large, indoor-outdoor runs adjacent to each other. Some of the females were occasionally moved from one group to another; otherwise the only other contact between the groups was separation by wire mesh or solid walls between runs. Central heating and ventilation of the indoor section of the runs provided nonrecirculating air with over 15 air changes per hr. Of the 38 animals tested, 17 were wild-born, 4 were captive-born animals received from other institutions, and 17 were captive-born at SFBR. Two of these animals were obtained from the University of Iowa, Ames, Iowa, U.S.A., in 1979 and had been experimentally inoculated with M. leprae in 1977. Both of these animals were serologically negative for antibodies to PGL-I and LAM, based on a screen of a single serum sample, and had no direct contact with PTT229, the infected SFBR chimpanzee.
Serum samples were taken from the chimpanzees at both SFBR and UTSP periodically, as part of routine clinical examinations, and were stored at - 70ºC. Serum samples were also obtained from nine human contacts of the SFBR ape for comparison. Aliquots of serum samples were shipped overnight to the Delta Regional Primate Research Center, Covington, Louisiana, U.S.A., on dry ice for ELISA.
Statistics. Data were analyzed on a Macintosh II CX computer with a Statview II statistical package (Abacus Concepts, Inc.). Scattergrams and frequency distribution plots were generated from which cut-off points were determined.
RESULTS
A screen of recent serum samples from chimpanzees 2038 and PTT229 revealed high ELISA levels of antibodies to both the M. leprae-specific PGL-I and to the mycobacterial common LAM antigens. This observation prompted an examination of serially obtained sera from both 2038 and PTT229 and a survey of their cagemates and other chimpanzees that might have had indirect contact with them.
A total of 160 chimpanzees were surveyed by ELISA to generate data sufficient to define statistically the mean values and cut-off points. Four separate sets of confirming observations were experimentally obtained utilizing native PGL-I and LAM antigens; frequency distributions of one set are shown in Figure 1. The following mean OD values were observed (Fig. 1): 0.012, A-PGL-I IgG; 0.025, A-PGL-I IgM; 0.142, A-LAM IgG; and 0.049, A-LAM IgM. The sum of the mean + 2 standard deviations (S.D.) of the mean for A-PGL-I and mean + 3 S.D. for A-LAM were chosen as the cut-off points below which ELISA OD values were considered negative. These cut-off values are as follows: A-PGL-I IgG, 0.102; A-PGL-I IgM, 0.125; A-LAM IgG, 0.530; and A-LAM IgM, 0.199. A higher cut-off point was chosen for A-LAM because it is a common mycobacterial antigen and because of the large number of animals with high OD levels of A-LAM relative to those with A-PGL-I levels.
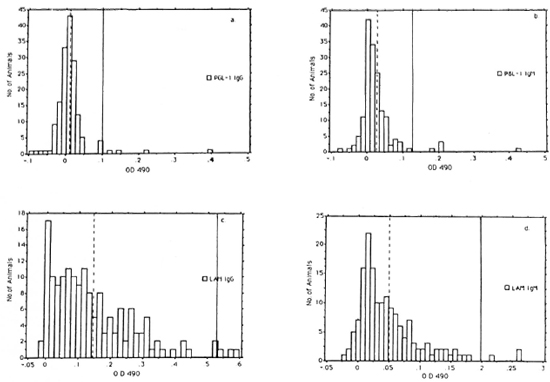
Fig. 1. Frequency distributions of scrum ELISA od 490 levels of A-PGL-I IgG (a.) and IgM (b.), and A-LAM IgG (c.), and IgM (d.) in 160 chimpanzees. (  ) = mean values; ( | ) = cut-off points. Cut-off points are mean + 2 S.D. for A-PGL-I and mean + 3 S.D. for A-LAM.
) = mean values; ( | ) = cut-off points. Cut-off points are mean + 2 S.D. for A-PGL-I and mean + 3 S.D. for A-LAM.
According to this procedure, the following numbers of chimpanzees were positive (Fig. 1): IgG A-PGL-I, 4 (2.5%); IgM A-PGL-I, 5 (3.1%); IgG A-LAM, 3 (1.8%); and IgM A-LAM, 3 (1.8%). Two of the A-PGL-I-positive apes, 2038 and PTT229, were positive for both IgG and IgM antibody isotypes and 5 were positive for either the IgG or IgM isotype for a total of 7 of 160 (4.4%) positive for antibody to the specific M. lepraePGL-l antigen. One A-LAM-positive animal, 2038, was positive for both IgG and IgM isotypes and 4 were positive for either the IgG or IgM isotype for a total of 5 of 160 (3.1%) A-LAM-positive chimpanzees.
Frequency distributions suggested that all four antibody types were normally distributed but skewed to differing degrees (Fig. 1). IgG A-PGL-1 showed the largest skew (4.559) and IgM A-PGL-I was next (4.133), followed by IgM A-LAM (1.724) and IgG A-LAM (1.257).
The earliest sample from 2038 (September 1983 or 9/83) had highly elevated positive levels of both IgG A-PGL-I and IgG A-LAM (Fig. 2). At the same time, there was a negligible OD for IgM A-LAM but a positive level of IgM A-PGL-I (Fig. 2). Thereafter there was a decline in the IgG A-PGL-I titer and a steep increase in both the IgM A-PGL-I and IgM A-LAM OD levels, both peaking in March 1988 (3/88). The IgG and IgM antibody profiles obtained by utilizing the native PGL-I antigen in ELISA were similar to those obtained by utilizing the synthetic ND-O-BSA as antigen, confirming specificity of the assay (Fig. 2). Linear correlation coefficients (R) between serial OD values for A-PGL-I and A-ND-O-BSA were 0.98 for IgG and 0.84 for IgM. BL leprosy was diagnosed histopathologi-cally in 2038, and noncultivable acid-fast bacteria (AFB) were found in his nasal secretions in 1989 (1).
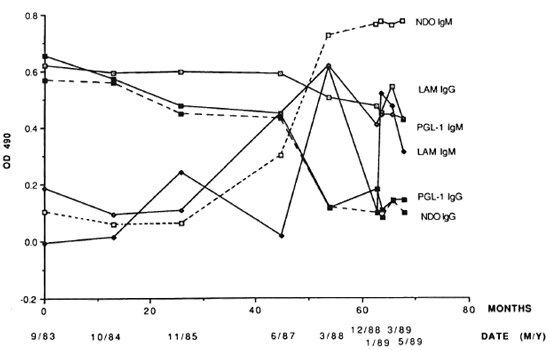
Fig. 2. Serum ELISA OD 490 levels of IgG and IgM A-PGL-I, A-ND-O-BSA and A-LAM at intervals in the leprosy-positive chimpanzee 2038.
The second chimpanzee with diagnosed leprosy (PTT229) was a feral-caught, 28-year-old male with clinical signs first recognized in early 1987. PTT229 had no known contact with M. leprae or leprosy. Retrospective serological studies were conducted by ELISA using serum samples from PTT229 (Fig. 3). The earliest serum sample from PTT229 (September 1981 or 9/81) was negative for all but A-PGL-I IgG (0.13 OD) (Fig. 3). Thereafter, the levels of each of the four antibodies rose concomitantly to a peak in November 1985 (1 1/85) after which the IgG A-PGL-I level (> 0.6 OD) began a rapid, sustained decrease and the IgG A-LAM level also decreased, whereas IgM A-PGL-I and IgM A-LAM fell slightly (Fig. 3). As with chimpanzee 2038, the specificity of these serial observations was confirmed by utilizing synthetic ND-O-BSA as antigen (Fig. 3). R values were 0.81 for IgG and 0.72 for IgM.
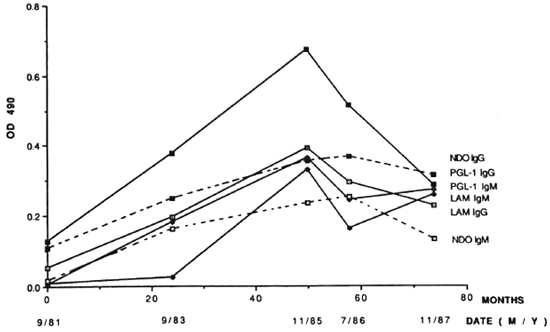
Fig. 3. Serum ELISA OD 490 levels of IgG and IgM A-PGL-I, A-ND-O-USA and A-LAM at intervals in the leprosy-positive chimpanzee PTT229.
An ELISA screen on two separate occasions using a single, recent, serum sample from nine humans who had been in contact with PTT229 failed to detect a positive ELISA level of IgG or IgM A-PGL-I or A-LAM (data not shown).
DISCUSSION
Previously we have reported an apparent relationship between the likelihood of developing clinically recognizable leprosy and IgG and IgM antibody responses to PGL-I and LAM cell-wall antigens of M. leprae in experimentally inoculated sooty mangabey monkeys (5,7). The previous data suggested that it may be possible to identify animals with subclinical leprosy by observing the ELISA IgG and IgM antibody levels to PGL-I and LAM at intervals in at-risk individuals (5,7). These observations form the basis for the present study in which sequentially obtained serum samples were available from two chimpanzees with leprosy. Such temporal serologic studies in untreated leprosy are rarely possible in humans.
High levels of specific A-PGL-I antibodies were observed in both chimpanzees 2038 and PTT229 consistent with the histopathologic diagnosis of leprosy (1,9,10). Chimpanzee 2038 was feral born and was imported at age 18-24 months in November 1972 to Walter Reed Army Institute of Research (WRAIR) in Washington, D.C., U.S.A., where he remained individually caged indoors until August 1980, when he was shipped to UTSP. He began self-mutilating his hands and feet in 1978 while still housed at WRAIR. Thirteen partial or total amputations of digits were required between 1978 and 1985 to control osteomyelitis, now presumed to have been complications of leprous neuritis. Retrospective evaluations of tissue sections of a finger amputated in 1983 revealed leprosy with neural involvement (1,10). The longitudinal serological assays revealed antibodies to PGL-I in the earliest serum sample from 2038 in September 1983. There were highly elevated levels of both IgG A-PGL-I and IgG A-LAM and a positive level of IgM A-PGL-I at that time. That these A-PGL-I antibodies are specific for PGL-I was confirmed by an essentially identical longitudinal profile for both IgG and IgM A-PGL-I utilizing the ND-O-BSA antigen containing the specific PGL-I disaccharide epitope O-(3, 6-di-o-methyl-β-D-glucopyranosyl)-(1-4)-O-(2,3-di-o-methyl-α-L-rhamnopyranose). Between 1985 and 1988, the IgM A-PGL-I ELISA level rose steeply and the IgG A-PGL-1 dropped. In our opinion, this is prognostic for advancing leprosy and a tendency toward lepromatous disease in experimental leprosy in sooty mangabey monkeys (5). These serologic changes preceded the deteriorating health and clinical recognition of leprosy in chimpanzee 2038 in 1989 (1).
The serologic evidence of leprosy in chimpanzee 2038 in September 1983 is consistent with longstanding disease, possibly predating shipment from Africa. It is unlikely that he contracted the disease while individually caged at WRAIR. This possibility is strengthened by the clinical evidence of neuritis beginning in 1978, and by the histopathological confirmation of leprosy in the digit amputated in 1983 (1). AFB were observed in nasal secretions from chimpanzee 2038, providing a source of exposure of additional chimpanzees to M. leprae between his arrival at UTSP in 1980 and the diagnosis and initiation of treatment of his leprosy in 1989. We are now closely monitoring two of this animal's female cagemates and other contacts for evidence of M. leprae infection.
In 1989, subsequent to the diagnosis of leprosy in chimpanzee 2038 at UTSP, subpolar lepromatous leprosy was diagnosed in a 28-year-old, wild-born chimpanzee at the SFBR (1,10). This chimpanzee, PTT229, was imported from Africa to another institution at age 3 and had been housed at SFBR for 20 years. There was no history of experimental exposure to M. leprae. Nodular skin lesions had been noted approximately 2 years prior to diagnosis (10). Serologic studies showed that in September 1981 PTT229 was negative for IgM and IgG A-LAM and for IgM A-PGL-I, but had positive A-PGL-I and A-ND-O-BSA IgG levels. Thereafter, OD levels of each of the antibodies, including A-PGL-I and A-ND-O-BSA antibodies, rose concomitantly to a peak in excess of 0.3 OD in November 1985. The A-PGL-I IgG peak was in excess of 0.65 OD, an exceedingly high ELISA level. These results suggest that PTT229 most likely became infected with M. leprae in captivity prior to or near September 1981 and that the disease progressed rapidly thereafter. We cannot, however, rule out the possibility that PTT229 harbored M. leprae without gen-crating a high antibody response and developing clinically observable leprosy prior to September 1981.
We are presently closely observing the cagemates of PTT229 and other contacts serologically and clinically for evidence of M. leprae infection. Aside from chimpanzees 2038 and PTT228, a screen of serum samples from each of 158 chimpanzees showed that 5 (3.2%) were positive for IgG or IgM A-PGL-I OD levels. We do not know the significance of this observation, since these percentages are near those expected statistically for false-positives within a normal distribution using a mean + 2 S.D. cutoff point. We will continue to serologically monitor any chimpanzee with possible positive, specific, M. leprae antibody levels for longitudinal increases in A-PGL-I together with A-LAM antibody levels.
We do not know the significance of high mean levels of A-LAM antibodies in chimpanzees. Since LAM antigen is ubiquitous among mycobacteria, A-LAM might arise from contact with "environmental" or other mycobacteria. Captive chimpanzees in the U.S.A. are repeatedly skin tested for tuberculosis, and this might be related to A-LAM serological responses.
Regardless of the possible significance of A-LAM in the "normal" captive chimpanzee population, similarities in longitudinal profiles between A-LAM and A-PGL-I IgG and IgM in M. leprae-infected chimpanzees appear noteworthy. We previously have reported that parallels exist between A-LAM and A-PGL-I IgG and IgM longitudinal ELISA OD profiles in M. leprae-infected sooty mangabeys (5,7). In both chimpanzees PTT229 and 2038 there were similar trends in the direction of increasing and decreasing levels of IgG A-LAM and IgG A-PGL-I and of IgM A-LAM and IgM A-PGL-I. Insufficient numbers of chimpanzees with leprosy are presently available to confirm these possible relationships. Meeker, et al. (14) reported, however, that parallel decreases in IgM A-PGL-I and IgM A-LAM ELISA levels took place in conjunction with a decreasing bacterial index (BI) in leprosy patients undergoing chemotherapy. Further simultaneous studies of both IgG and IgM antibody levels to PGL-I and LAM will be required to clarify possible interrelationships and to determine any possible practical significance.
It is important to note that in longitudinal types of studies, where changes in the levels of a given type of antibody relative to itself and to other types of antibodies are being observed simultaneously in a given M. leprae-infected animal, each animal becomes its own control. There is no other valid control. Thus, in such studies the mean + 2 S.D. or 3 S.D. cut-off point established in a population study does not apply. Therefore, the changes observed with time in the relative levels of IgG and IgM A-PGL-I and A-LAM reported here in chimpanzees PTT229 and 2038, which have clinical leprosy, are viewed without regard to whether or not the OD level in a given sample was above or below the cut-off point chosen to compare cross-sectional data points obtained from a population of animals.
The observations reported here are consistent with the hypothesis that leprosy exists in the chimpanzee population in the U.S.A. and may affect additional captive chimpanzees. Our studies suggest that a simultaneous longitudinal study of IgG and IgM A-PGL-I and A-LAM levels may aid in the diagnosis of preclinical leprosy. It seems prudent to advise that sera from all chimpanzees (and, perhaps, other nonhuman primates) in primate facilities, zoos and private holdings be examined for antibody to PGL-I and LAM to screen for possible subclinical leprosy.
Acknowledgment. We are indebted to Mrs. Cyndi Trygg for technical assistance, Mrs. Mary Ann Bennett and Mrs. Sharon Nastasi for typing the manuscript, and Mr. Murphy Dowouis for graphics. Financial support was provided by grants from the National Institutcs of Health (NIH) #RR-00164, the National Institute for Allergy and Infectious Diseases (NIA1D) #2R22 All9302, the American Leprosy Missions, the Da-mien-Dutton Society, and from the Xerox Corporation (via the American Leprosy Foundation) for support of a fellowship for Dr. Xu.
REFERENCES
1. Alford, P. and Mathekne, C. Leprosy in two wildborn outdoor housed adult chimpanzees. (Abstract) Annual Meeting of the American Association for Laboratory Animal Science, Little Rock, Arkansas, November 2, 1989.
2. Douglas, J. T., Cellona, R. V., Fajardo, T. T., Abalos, R. M. and Madarang, M. G. Early detection of leprosy with a semi synthetic disaccha-ride antigen (ND-O-BSA). (Abstract) Int. J. Lepr. 51Suppl.(1989)395.
3. Douglas, J. T., Steven, L. M., Fajardo, T., Cellona, R. V., Madarang, M. G., Abalos, R. M. and Steenhergen, G. J. The effects of chemotherapy on antibody levels in lcpromatous patients. Lepr. Rev. 59(1988)127-135.
4. Gaylord, H. and Brennan, P. J. Leprosy and the leprosy bacillus: recent developments in characterization of antigens and immunology of the disease. Ann. Rev. Microbiol. 41(1987)645-647.
5. Gormus, B. J., Ohashi, D. K., Ohkawa, S., Walsh, G. P., Meyers, W. M., Brennan, P. J. and Trygg, C. Serologic responses to Mycobacterium leprac-specilic phenolic glycolipid-I antigen in sooty mangabey monkeys with experimental leprosy. Int. J. Lepr. 56(1988)537-545.
6. Gormus, B. J., Wolf, R. H., Baskin, G. B., Ohkawa, S., Gerone, P. J., Walsh, G. P., Meyers, W. M., Binford, C. H. and Greer, W. E. A second sooty mangabey with naturally acquired leprosy: first reported possible monkey-to-monkey transmission. Int. J. Lepr. 56(1988)61-65.
7. Gormus, B. J., Xu, K., Meyers, W. M., Walsh, G. P., Levis, W. R. and Meeker, H. C. Antibodies to lipoarabinomannan antigen in sooty mangabey monkeys experimentally inoculated with Mycobacterium leprae. Int. J. Lepr. 58(1990)65-72.
8. Gunders, A. E. Progressive experimental infection with Mycobacterium leprae in a chimpanzee; a preliminary report. J. Trop. Med. Hyg. 61(1958)228-230.
9. Hubbard, G. li.. Lee, D. R. and Eichberg, J. W. Diseases and pathology of chimpanzees at the Southwest Foundation for Biomedical Research. Am. J. Primatol. 23(1991)1-10.
10. Hubbard, G. B., Lee, D. R., Eichberg, J. W., Gormus, B. J.. Xu, K., Meyers, W. M. and Had-field, T. L. Leprosy in a chimpanzee. (Abstract) In: 40th Annual Meeting of the American College of Veterinary Pathologists, November2, 1989, Baltimore, Maryland.
11. Johnstone, P. A. S., Meyers, W. M., Binford, C. H., Walsh, G. P., Gormus, B. J. and Baskin, G. B. Recent advances in the development of nonhuman primates as animal models for leprosy. Scand. J. Lab. Anim. Sci. 16Suppl.1(1989)102105.
12. Leininger, J. R., Donham, K. J. and Rubino, M. Leprosy in a chimpanzee: morphology of the skin lesions and characterization of the organism. Vet. Pathol. 15(1978)339-346.
13. Martin, L. N., Gormus, B. J. and Wolf, R. H. Experimental leprosy in nonhuman primates. Adv. Vet. Sci. Corap. Med. 28(1984)201-236.
14. Meeker, H. C, Schuller-Levis. G., Fusco, F., Giardina-Becket, M.-A., Sersen, E. and Levis, W. R. Sequential monitoring of leprosy patients with serum antibody levels to phenolic glycolipid-I and mycobacterial lipoarabinomannan. Int. J. Lepr. 58(1990)503-511.
15. Meyers, W. M., Walsh, G. P.. Brown, H. L., Binford, C. H., Imes, G. D., Jr., Hadfield, T. L., Schlagel, C. J., Fukunishi, Y., Gerone, P. J., Wolf, R. H., Gormus, B. J., Martin, L. N., Harboe, M. and Imaeda. T. Leprosy in a mangabey monkey -naturally acquired infection. Int. J. Lepr. 53(1985)1-14.
16. Ridley, D. S. and Jofling, W. H. Classification of leprosy according to immunity: a five-group system. Int. J. Lepr. 34(1966)255-273.
17. Wolf, R. H., Gormus, B. J., Martin, L. N., Baskin, G. B., Walsh, G. P.. Meyers, W. M. and Binford, C. H. Experimental leprosy in three species of monkeys. Science 227(1985)529-531.
18. Young, R. A. and Buchanan, T. M. A serological test for leprosy with a glycolipid specific for Mycobacterium leprae. Science 221(1983)10571059.
1. Ph.D.; Delta Regional Primate Research Center, Tulane University Department of Microbiology, 18703 Three Rivers Road, Covington, Louisiana 70433.
2. M.D., Delta Regional Primate Research Center, Tulane University Department of Microbiology, 18703 Three Rivers Road, Covington, Louisiana 70433.
3. D.V.M.; University ofTexas M.D. Anderson Cancer Center, Science Park, Department of Resources, Rt. 2, Box 151-B, Bastrop, Texas 78602.
4. D.V.M., University ofTexas M.D. Anderson Cancer Center, Science Park, Department of Resources, Rt. 2, Box 151-B, Bastrop, Texas 78602.
5. D.V.M.; Departments of Laboratory Animal Medicine and Virology and Immunology, Southwest Foundation for Biomedical Research, P.O. Box 28147, San Antonio, TX 78284.
6. D.V.M., Ph.D., Departments of Laboratory Animal Medicine and Virology and Immunology, Southwest Foundation for Biomedical Research, P.O. Box 28147, San Antonio, TX 78284.
7. M.D., Ph.D., Armed Forces Institute of Pathology, Washington, D.C. 20306-6000, U.S.A.
Received for publication on 7 March 1990.
Accepted for publication in revised form on 12 March 1991.