- Volume 59 , Number 3
- Page: 458–78
Impact of multidrug therapy on the treatment and control of leprosy*
Editorial opinions expressed are I hose of the writers.
Leprosy (Greek lepros scaly, scabby, rough) is a condition which results from infection with Mycobacterium leprae, also called Hansen's disease after the Norwegian physician who discovered the responsible bacillus in 1874.1 Records of leprosy date back much earlier than this, however, to India circa 600 B.C., and even these probably embody earlier oral traditions. 2 Nevertheless, the disease was unknown in Western Europe until the return from the Indian campaign of Alexander's troops in 327-326 B.C.
Some of the earliest references to the treatment of leprosy can be found in the Nei Ching and other ancient Chinese texts. Treatment followed preconceived notions of the time ("prick the swollen parts with a sharp needle to let the foul air out"), and was generally ineffective. Later, in the Middle Ages, writings refer to chaulmoogra oil, a pressing from seeds of an East Indian tree, as a specific for leprosy, and "drug" treatment in subsequent centuries followed similar lines until the introduction of the sul-fone derivatives in the early 1940s.3
In the five decades since, more progress has probably taken place in the treatment of leprosy than occurred in all preceding centuries put together, and the recent introduction of multiple drug therapy (MDT) has played an important part in this.
Scope of the essay
In this essay, a brief outline of the principles, development, and nature of MDT shall be given, followed by a more detailed discussion of the impact of MDT on various aspects of leprosy treatment and control. In particular, improvements in the efficacy and acceptability of leprosy treatment shall be considered, together with the associated operational and technical changes which have occurred within leprosy control programs.
It is hoped to show how the efficacy of MDT, both apparent and actual, is dependent on the nature and extent of the changes in leprosy control programs. Indeed, minimal operational standards of leprosy control have been defined, below which MDT should not be introduced, on the basis that any therapeutic value of MDT would be nullified if it were inefficiently implemented. Any evaluation of the impact of MDT should therefore ask the following questions: a) Does MDT reach the patients who need it? b) Is MDT administered properly? and c) Is MDT taken correctly? Finally, the possible directions in which MDT may evolve will be discussed.
GLOBAL LEPROSY SITUATION
Estimated and registered cases
Estimates by the World Health Organization (WHO) of the number of leprosy cases worldwide have remained fairly constant over the last 20 years, at 10.8 million in 1966, 10.6 million in 1976, and 10-12 million in 1988.4-7 The number of registered cases, on the other hand, has steadily increased over this period, from 2.85 million in 1966, to 3.6 million in 1976, and 5.4 million in 1985.5 -6 Moreover, despite underestimating the world leprosy problem, registered cases are more reliable than estimated cases.
Interestingly, over the period 1985-1990 returns have suggested the beginnings of a decline in the number of registered cases, from 5.4 million in 1985 to 5.1 million in 1987,6 -8 and the possible association between this reduction and the introduction of MDT in the early 1980s has not gone unnoticed.6 The reduction could equally, however, reflect an increased detection or reporting of leprosy. Nevertheless, regardless of the explanation these findings give encouragement to those working in the field of leprosy.
Leprosy - a major public health problem
From the above figures, leprosy clearly represents a significant worldwide health problem but its true scale is perhaps not appreciated until one considers the overall population at risk of 1.6 billion, which provides a vast reservoir of potential sufferers.5 - 6 It is, therefore, not surprising that the WHO has put leprosy on its top six priority list of tropical diseases demanding attention.
PATHOLOGICAL CONSIDERATIONS
Leprosy results from infection with Mycobacterium leprae, an acid-fast bacillus which closely resembles the tubercle bacillus in appearance. M. leprae is believed to have high infectivity but low pathogenicity, and its predilection for the cooler regions of the body (skin, mucous membranes, and peripheral nerves) is well-known. Leprosy has a prolonged incubation period (1-30 years), and progresses slowly.
Classification of leprosy patients
Leprosy patients can be classified on a spectrum according to the degree of host immunity (Ridley-Jopling classification).9 In so-called tuberculoid leprosy (TT), for instance, the patient's immunological resistance is high, with an intense inflammatory response; thus, few leprosy bacilli are present. Lesions tend to be localized to the skin or peripheral nerves, and spontaneous recovery may occur.
In contrast, lepromatous leprosy (LL) is associated with a low immunological resistance and numerous bacilli, which results in a generalized multisystem infection that, if untreated, is likely to persist.
A borderline (dimorphous) type of leprosy is also recognized, with features of both TT and LL, and this can be subclassified into borderline-tuberculoid (BT), midbor-derline (BB), and borderline-lepromatous (BL), depending on the relative numbers of bacilli, lymphocytes, epithelioid cells, and macrophages. Finally, an indeterminate, mild form of leprosy exists which is difficult to diagnose.
Before commencing MDT, it is necessary to divide patients into two groups, pauci-bacillary (PB) and multibacillary (MB), according to the scheme: 10,11 PB patients = TT, BT and indeterminate leprosy in the Ridley-Jopling classification, with a bacterial index (BI) of < 2; MB patients = BB, BL and LL leprosy, with a BI > 2.
Following this system of classification, PB leprosy is typically found to account for 70% or more of registered leprosy cases.12 -13 The classification has remained unchanged since 1982, except for a modification in 1988 to "include all skin-smear positive cases in the multibacillary group."5,6 This followed digressions from the classification system by some control programs.14
Classification of the type of leprosy is not always clear cut and downgrading from borderline PB to MB leprosy, for instance, is often difficult to recognize, with clinical and histological appearances remaining unchanged for several months.15
Finally, a number of control programs have failed to follow the WHO system of classification and some, for instance, define all cases who are BB, BL or LL as MB irrespective of skin-smear status, and also all those who are BT with 10 or more skin lesions.16 Other programs classify patients with more than five lesions as MB, irrespective of smear status.14
Slit-skin smear examination
The examination of slit-skin smears is used to assess the bacteriological status of leprosy patients by means of the bacterial index (BI), and the morphological index (MI).17 The BI represents the number of bacilli seen under an average microscopic field, using an oil-immersion objective, and is quantified as follows: 6+ = Many clumps of bacilli in an average field (over 1000); 5 + = 100-1000 bacilli in an average field; 4 + = 10-100 bacilli in an average field; 3+ = 1-10 bacilli in an average field; 2 + = 1-10 bacilli in 10 fields; and 1 + = 1-10 bacilli in 100 fields. The MI represents the percentage of solid-staining bacilli seen under the microscope.
Leprosy reactions
Leprosy reactions are the consequence of inflammation occurring secondary to an immunological response to M. leprae,™ and can be divided into two types. Type 1 reactions are a phenomenon of cell-mediated immunity (CMI) and occur only in borderline leprosy in which CMI is unstable. Thus, borderline leprosy may regress toward TT ("reversal reaction") or progress toward LL ("downgrading reaction") due to an increase or decrease in CMI, respectively.
Type 2 or erythema nodosum leprosum (ENL) reactions, on the other hand, result from changes in the status of humoral (antibody-mediated) immunity, and are confined to MB leprosy.
DRUG THERAPY OF LEPROSY AND THE EVOLUTION OF MDT
Single-drug therapy (monotherapy)
Effective chemotherapy of leprosy began with the sulfone group of drugs in 1941,19 the most important of which is dapsone (DDS; 4,4'-diaminodiphenyl sulfone), which acts by inhibiting microbial folate synthesis.
Dapsone monotherapy has been the predominant form of leprosy chemotherapy for over 30 years and, prior to the problems outlined in the following section, was highly successful. Other types of antileprosy drugs, including clofazimine, ethionamide and prothionamide, have all been used in monotherapy, but here the term "monotherapy" will refer to dapsone therapy unless otherwise stated.
Reasons for shift from monotherapy to MDT
Three main factors have necessitated the development of multiple drug therapy (MDT): drug resistance, bacterial persistence, and defaulting, each of which shall briefly be considered. In addition, the following have been mooted as other reasons in favor of the introduction of MDT: a) reduced incidence of post-treatment relapse compared with monotherapy; b) reduced side effects; and c) increased cost-effectiveness by a shorter duration of treatment leading to an earlier release of patients from control.
Drug resistance. Two types of drug resistance are recognized -secondary and primary.20 Secondary drug resistance results from the multiplication of drug-insensitive strains of M. leprae, and is most often seen in MB leprosy in which the number of bacilli is high (10 11 compared with 106 in PB leprosy).
Secondary resistance to dapsone was first suspected in 195319 and confirmed in 1964 using the mouse foot pad model.21 Its reported prevalence varies between 1%22 and 12%,23 factors purported to influence the prevalence including selection bias, duration of therapy, and dapsone dose. 23, 24
One long-running Malaysian program, for instance, has reported a consistent increase with time of the prevalence of dapsone resistance from 1/1000 in 1966, to 35/1000 in 1973, and 100/1000 in 1975. 25, 27 Evidence such as this has been used to question the long duration of dapsone monotherapy (3-5 years in PB and lifelong in MB leprosy) and to prompt the switch to shorter duration MDT.
In addition to dapsone, secondary resistance has been reported with rifampin, 28, 29 clofazimine, 30 ethionamide, 31 and prothionamide. 32
Primary drug resistance occurs on infection of nonresistant patients with bacilli from resistant patients (usually MB) and, thus, unlike secondary resistance can occur in both MB and PB leprosy. As discussed later, this may have important therapeutic implications.
Primary dapsone resistance was first conclusively documented in 19 7 7,33 following a number of earlier prima facie cases.34, 35
Its reported prevalence has been highly variable, at anything between 3.5%3h and 73%,24 but most studies seem to record levels in the middle of this range.17 These reports are likely to overestimate prevalence, however, since the assumption of no prior antileprosy medication is often mistaken.
To date, primary resistance has also been reported with rifampin,38 among other drugs.
Bacterial persistence. Bacterial persistence refers to the ability of some leprosy bacilli to survive treatment by effective dosages of antileprosy drugs, despite being fully susceptible to their effects. The term "persistence" was first used by Bigger in 1944 to describe the response of some staphylococci to penicillin,39 and in leprosy the phenomenon has been widely attributed to some form of bacillary nascency.40
M. leprae persistence proved a major problem during dapsone monotherapy,41-43 and in one study was observed in 7 out of 12 patients treated for 10-12 years.44 Qther drugs with which persistence has been observed include rifampin 45 -46 and clofazimine.11
Defaulting. Defaulting (or noncompliance) is the term applied to the failure of leprosy patients to complete treatment, either by not attending treatment centers, or by failing to take their drug tablets regularly. Poor compliance to dapsone monotherapy, principally because of its long duration, has been the third major argument in favor of the implementation of MDT,47-49 and reports of compliance have included 42%,50 56%51 and 68%.52 The impact of MDT on compliance is considered in detail later.
WHO recommendations for MDT
In light of the above problems, the WHO Expert Committee on Leprosy recommended in 1977 the use of two-drug therapy for MB (but not PB) leprosy.53 At a further meeting in 1982, the WHO Expert Committee recommended MDT in its current form, with two regimens for MB and PB leprosy, respectively.
A number of bodies, including the Indian Association of Leprologists (IAL), 54 also recommended MDT regimens at about the same time as WHO, but none has been as widely accepted or implemented, and here the term "MDT" will refer to the WHO regimens.
Regimen for MB leprosy. The three drugs recommended for the treatment of MB leprosy were the potent bactericidal rifampin, which acts by actively killing bacilli, and the less potent bacteriostatics dapsone and clofazimine, which act by preventing the growth of bacilli (elimination of bacilli depending on the body's immune system).
The proposed regimen was as follows: Rifampin, 600 mg (> 35 kg body weight) once monthly, supervised; dapsone, 100 mg (12 mg/kg) daily, self-administered; and clofazimine. 300 mg once monthly, supervised, and 50 mg daily, self-administered.
The WHO recommended continuing this regimen for at least 2 years, and up to skin-smear negativity if possible, followed by clinical and bacteriological follow-up at least once per year for a minimum of 5 years on the completion of treatment. Provision was also made for the use of the weak bacteriostatic agent ethionamide in patients who found the reddish-brown skin pigmentation of clofazimine unacceptable.
Regimen for PB leprosy. Drug resistance in PB patients was considered a small enough problem to justify short-term two-drug therapy with rifampin and dapsone,55 according to the regimen: Rifampin, 600 mg once monthly for 6 months, supervised; and dapsone, 100 mg (1-2 mg/kg) daily for 6 months, supervised. On completion of treatment, the WHO recommended clinical follow-up of PB patients at least once per year for 2 years.
IMPACT OF MDT ON LEPROSY CONTROL
At the time of its recommendation in 1982, MDT was heralded by an editorial in The Lancet at "one of the most important and stimulating contributions to leprosy control for well over a decade."56 Its main objectives were to: a) prevent drug resistance; b) eliminate M. leprae persistence; c) improve the acceptability of treatment (and hence patient compliance); d) increase the efficacy of leprosy treatment; and e) prevent leprosy.
To date, M. leprae resistance to MDT has thankfully not been observed, and the degree to which the other objectives have been met shall now be discussed. The value of the relatively untried drugs clofazimine, ethionamide and prothionamide shall also be considered, as shall factors which can limit the impact of MDT on leprosy control.
Impact of MDT on bacterial persistence
When MDT was introduced, it was hoped that it would prevent the emergence of persistent M. leprae by the different mechanisms of action of its component drugs. This has not been observed, however, and the WHO-sponsored THELEP trials in Bamako and Chingleput, for instance, have detected persistence in 9% of MB patients receiving MDT.57 Another study has reported persistence in 7% of MB patients.58 Little difference in the frequency of persistence has been observed between MDT and monotherapy,57 -59 and no identifiable characteristic, such as duration of therapy or bacterial resistance, has yet been related to the occurrence of persistence.
These findings are a cause for concern, and in a review of the topic Toman 40 concluded that "there is little reason to believe that, in the near future, a new drug or combination of drugs will be found that is capable of eradicating persisting M. leprae." Further research is clearly required into this important area which could prove in the future to be a major limiting factor for the efficacy of MDT.
Acceptability of MDT and patient compliance
There is good evidence to show that MDT is more acceptable to patients than was the case with dapsone monotherapy, based on the findings of improved patient compliance, compliance being defined as the adequacy and regularity of both drug intake and attendance at leprosy clinics.
PB leprosy. Regularity of attendance has little value in respect to PB leprosy, and studies of compliance in these patients are more concerned with the proportion of patients completing therapy. Reports of compliance have ranged between 72% and 920 /o,60_63 and one Indian study, for instance, reported a compliance of 91% compared with 64% seen with dapsone monotherapy.62 On the basis of this and other reports of compliance to dapsone monotherapy, including 42%,50 5 6%,51 and 68%,52 MDT would appear to offer some considerable advantage over monotherapy in terms of compliance.
MB leprosy. In a recent study which assessed drug compliance by means of the urine paper spot test for dapsone, and pill-counting for clofazimine (rifampin is given supervised), 94.3% of patients were found to take clofazimine regularly, and 88.1% to take dapsone regularly (regularity defined as > 75% drug intake).64 This regularity of intake was significantly greater than the 60%-65% previously observed by the same workers with monotherapy, and the better compliance seen with clofazimine than with dapsone was partly attributed to "new drug" popularity.
Postulated reasons for the improved patient compliance observed with MDT in both PB and MB patients include:47 -64 a) reduced duration of therapy compared with monotherapy; b) lower relapse rate; c) reduction in reversal reactions; d) reduction in toxicity and side effects; and c) enthusiasm for "new" treatments.
Of these, the shorter duration of MDT compared with monotherapy is likely to be the most important factor. In addition to these "drug" factors, however, the favorable impact of the operational changes associated with MDT should not be forgotten. These include improved health education, leading to increased self-reporting by patients of problems at an early stage, and better surveillance of patients by the health staff.
Other favorable operational changes include the convenient location of clinic sites and a more efficient system of defaulter retrieval, including home visits to patients aimed at contacting and dissuading treatment refusers where possible. Finally, attempts should be made to discourage the practice of giving proxy treatment without discretion, which is detrimental to compliance.
Importance of compliance to efficacy of MDT. Mathematical models of M. leprae population dynamics during MDT predict that the high initial rate of killing by the bactericidal agent rifampin would soon diminish due to the selection of resistant organisms.65 For MDT to be successful, therefore, it is essential that these bacteria are contained by the relatively weak bacteriostatic drugs while being removed by the body's immune system. This places great importance on good patient compliance to these drugs, the administration of which, unlike rifampin, is not supervised.
Therefore, despite the undoubted improvement which has occurred in compliance in association with MDT, it is essential to consider ways in which compliance can be further improved. In addition to the operational changes already considered, proposals have included rational prescription, correct dispensing, and suitable packaging of drugs, improved accuracy of diagnosis, and clear instructions to the patient.66
A working group of the WHO in 1985 drew particular attention to the importance of health education,66 and concentration on the following aspects has been recommended:67 a) the causation of leprosy, since traditional beliefs often underlie lack of faith in modern treatment; b) medication, particularly when and how much to take; c) the shorter duration, better expected results, and reduced likelihood of side effects with MDT; and d) the early recognition and prevention of deformity and disability.
In practice, difficulty is often experienced in instructing patients how to take MDT.68 Such innovations as blister calendar packs69 and a combined preparation of dapsone and clofazimine may help to overcome this.
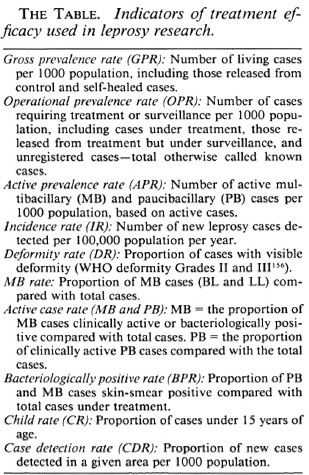
Efficacy of MDT as measured by selected indicators
The efficacy of MDT can be assessed using a number of selected indicators, some of which are summarized in The Table. In this section, the following indicators shall be considered (in order): case-load, workload, MB case rate, child rate, deformity rate, prevalence rate, case detection rate, incidence rate, disease inactivity, leprosy reactions, relapse rate, and drug toxicity. Where possible, comparison of MDT with dapsone monotherapy will be made.
Case-load. The preparatory screening of leprosy patients prior to the introduction of MDT leads to the release from control of many patients who are skin-smear negative, clinically inactive, or have completed treatment.70 Typically, this reduces case-load by about 25%,71 most of whom are PB patients.
Following the introduction of MDT, the reduction in case-load continues due to the shorter duration of MDT compared with monotherapy70 and in the Tanzania National Leprosy Program, for instance, the number of registered cases decreased by 59.5% between 1979 and 1985, representing some 20,500 cases.72 Similarly, in an Indian program in the North Arcot District of Tamil Nadu, a reduction of 87% (85,000 cases) occurred between 1983 and 1988.73
As a result of this case-load trend [due to the new definitions of a case-RCH], over a million or more patients worldwide have so far been discharged as cured since the introduction of MDT.74
Workload. One consequence of a reduction in case-load might be expected to be a decrease in the workload of health workers. A balance exists, however, with increased workload associated with preparatory screening of patients and closer patient supervision, as necessitated by the relative potency and potential for toxicity of MDT compared with monotherapy.
In a recent study which quantified workload in terms of clinic attendance, an initial increase of attendance of 120% was observed in the year prior to MDT introduction (1982), using 1981 as a base-line, subsequently followed by a consistent decline to 75% above the 1981 level in 1983, 40% in 1984, 25% in 1985, and 12% in 1986.75
Provided that program managers are required to make human and material investments prior to the implementation of MDT, later dividends in terms of diminished health worker workload, among others, are likely. In practice, this could enable more time to be devoted to such activities as the treatment of MB cases, contact tracing, and disability prevention.
MB case rate. The MB case rate is a ratio of MB cases/total cases. With the release from control of a sizable number of particularly PB leprosy cases, the numerator (MB cases) decreases less than the denominator (total cases) in the early stages of MDT, and the MB case rate thus increases.70 Subsequently, the level of the MB rate will depend on the nature of new cases, and may decrease. Such a decrease was observed from 5.81 /1000 to 1.01 /1000 over 3 or more years of MDT in the Baroda District of India.76
Child rate. Like the MB case rate, the child rate is a ratio of child leprosy cases/ total cases, and since most child cases are PB, the child rate decreases in the early years of MDT.70 Moreover, a number of control programs have observed a continuing decline of the child rate among new cases,73, 76 in one study from 2.1 to 1.7/1000 between 1983 and 19 8 7.73 MDT perse would appear to be responsible for at least some of this decline.
Deformity rate. The deformity rate is a ratio of cases with visible deformity/total cases. It typically increases following the introduction of MDT, due to a statistical effect of the release from control of many PB patients in whom deformities are usually absent.70 Subsequently, successful MDT would be expected to reduce the deformity rate, and a reduction from 6.15% to 1.50% over 3 or more years has been observed in one program in India.76
Nevertheless, findings have been inconsistent and in the LEPRA Control Project in Malawi, for instance, the percentage of patients on MDT who developed new disabilities or experienced a deterioration (5.7%) was little different from the rate of 6.1% previously observed with dapsone monotherapy.77 Nevertheless, a greater percentage of potentially reversible disabilities did improve with MDT.
Prevalence rate. In India, after 3-5 years of MDT, the following reductions in the active prevalence rate have been observed: 11.1/1000 to 1.8/1000 in Wardha, 19.2/ 1000 to 7.7/1000 in Purulia, and 18/1000 to 2.6/1000 in Srikakulam.78
A more detailed analysis in Guyana observed an annual reduction in prevalence of 0.1/1000 for 3 years prior to the introduction of MDT, reaching a level of 0.6/1000 in 1981,75 This was followed by a consistent decrease in prevalence of 0.2/1000 per year, reaching a level of 0.3/1000 in 1984. In the last 3 years reported (1985-1987) prevalence remained stable at 0.2/1000.
The stabilization of leprosy prevalence after a number of years of MDT is interesting, and elsewhere levels of 1-3/1000 have been reported. Stability is likely to result from an altered composition of the pool of patients, made up more of MB patients (70% in the Guyana study), who are more long-term and less easily cured than PB patients. Moreover, no further fall in prevalence is likely unless primary prevention methods, such as vaccination, are introduced.
There is clearly good evidence now to support a favorable impact of MDT on leprosy prevalence. Nevertheless, many factors other than the efficacy of MDT can influence prevalence, and these should be recognized. For example, in parallel with caseload, leprosy prevalence often falls prior to the introduction of MDT.79 Conversely, a reduction in prevalence may be delayed by an increase in the number of new cases associated with increased case detection.
The relative numbers of estimated and actual leprosy cases are also important regarding prevalence, and may determine the time required for the detection and inclusion of new patients in case data. Thus, in a low-prevalence area, MDT would be expected to have a relatively early impact on prevalence. Finally, the definition of the prevalence rate used can influence the apparent level, for example, on switching from an operational to an active prevalence rate, a "reduction" in prevalence would be likely.
It can therefore be seen that treatment targets based on the prevalence rate alone may be misleading, particularly since many cured patients are disabled or deformed and require continuing supportive care.
Case detection and incidence rates. The detection of new leprosy cases usually suffers in the first few years of MDT as a result of the greatly increased workload in other aspects of leprosy control. 70 Indeed, it is not infrequent for MDT to have no overall impact on the case detection rate. Nevertheless, consistent decreases in the case-detection rate have been observed, and in one large-scale Indian program fell from 3.56% in 1983 (the year in which MDT was introduced) to 2.82% in 1984, and 2.17% in 1985. 73 An increase in 1986 to 8.2% occurred, following a thorough survey, illustrating the dependence of the detection rate on the intensity of case detection but, subsequently, the detection rate again decreased to 1.6% in 1987. Despite the difficulty in attributing all of this reduction to MDT, it is likely to have had some impact.
In order to maintain the case-detection rate, many control programs emphasize the importance of health education, with the aim of encouraging voluntary self-reporting by patients. In the Baroda District program in India, for instance, 60% of new cases are detected by self-reporting.76 In a similar manner, health education could also encourage patients to report leprosy reactions, relapse, and drug toxicity.
The incidence rate is defined as the number of new leprosy cases detected in a given population (e.g., 100,000) per year. A number of hypothetical studies have predicted that MDT is unlikely to lead to a reduction in the incidence of leprosy for 5-10 years after its introduction,70 '80 since: a) newly detected cases represent only a small proportion of the total prevalence pool; b) compliance to treatment and case-holding may be poor; c) cases may be detected after disease transmission; d) a number of years may be needed for MDT to interrupt the transmission of leprosy; and e) persisters may be responsible for disease transmission. Indeed, most studies report no change in leprosy incidence even after 5 years of MDT and in some, moreover, incidence has transiently increased owing to an intensification of case-finding. A few isolated studies have been encouraging, however.
Since the definition of incidence implies an unlikely state of perfection in which all new cases are identified as they occur, the case-detection rate is often used instead. In the Guyana Leprosy Control Programme, the case-detection rate was examined closely over a number of years and was found to increase in the late 1970s, following program expansion to peak at 14/100,000 in 1982, the first year of MDT implementation.75 Subsequently, the detection rate consistently fell to reach a steady level of 7/100,000 in 1986 and 1987. What makes these findings of particular significance is the trouble that was taken to validate the case-detection rate as a measure of incidence. This was achieved by assessing three factors: a) the proportion of disabled new patients; b) the proportion of MB patients among new patients; and c) the proportion of PB patients presenting with a single macule.
The first criterion would be expected to tend toward zero with time, the second to stabilize at a lower baseline, and the third to increase, if the case detection rate approximated the incidence rate. Only the second of these criteria was not met, and the conclusion was therefore reached that a reliable estimate of leprosy incidence had been made in the study and, moreover, that MDT had been responsible at least in part for the decrease in incidence observed since 1982.
Why this study should be one of the few to date to have demonstrated a fall in leprosy incidence following the introduction of MDT is unclear, although the relatively low prevalence of leprosy in Guyana (0.2/1000 in 1987) may have helped to quickly bring "estimated" leprosy cases under MDT so that prevention of disease transmission occurred relatively soon. Whatever the reason, one would hope that as MDT begins to have an impact on leprosy transmission elsewhere, such findings will be duplicated.
Disease activity. PB patients. Because PB patients are, by definition, skin-smear negative, assessment of "cure" (disease inactivity) is made on clinical grounds alone. Figures for the percentage of clinically inactive patients following dapsonc monotherapy include 43.9% after 6 months,81 79.6% after 12 months,81 76.6% after 12 months' treatment,82 and 50% after 48 months' treatment.81 This compares with post-MDT inactivity results ranging between 25%-95%,84 including 83%,85 72.2%,12 71%,62 7 0.4%,8 '' and 45%87 after 6 months, and 91.2%88 and 65.5%89 after 12 months.
In general, therefore, MDT seems to be an improvement on monotherapy as regards the time required for disease inactivity, but studies can be quoted which found no difference between MDT and monotherapy.90
Interestingly, continued improvement in inactivity has been reported on extension of MDT to 12 months -in two recent studies from 43.9% and 62.8% at 6 months, to 69.4% and 91.2% at 12 months, respectively14 81 -and the treatment of all PB patients for 12 months rather than 6 months has therefore been proposed. Without control groups in which treatment was stopped at 6 months, however, the role of self-healing cannot be determined, which may be important given the findings of improvement to inactivity in 20% of patients in whom MDT was stopped at 6 months.62 Even treatment to inactivity has been recommended,20 but the authors do not seem to have considered the likely consequences of reduced patient compliance and poor cost-effectiveness. Moreover, one report of this practice was disappointing since the percentage of clinical inactivity improved little from 69.4% at 12 months to 72.8% after 5 years.14
When clinical activity persists beyond 12 months, some workers reclassify PB patients as MB and give them 2 years of MDT according to the MB regimen, 91 apparently based on the assumption that continuing disease activity results from inadequate chemotherapy. There is good evidence, however, that clinical activity resolves in the majority of PB patients within 1-2 years of stopping MDT.92
On balance, therefore, 6 months of MDT would appear to be inadequate and a reassessment of patients after 6 months for further treatment, if necessary, would seem advisable. Nevertheless, it is useful to consider a recent comparative study of MDT and dapsone monotherapy which was unique in being the first study to evaluate the histopathological "impact" of dapsone monotherapy.90 Interestingly, no significant difference in histological cure was observed between MDT and monotherapy, and the histological cure rate was slow: 27.1% at 1 year, 51.4% at 2 years, 70.1% at 3 years, and 85.1% at 4 years after the start of treatment. On the basis of these findings, the authors concluded that observation periods of just 6 or 12 months, as used in many studies, are inadequate to justify proposals for increasing the duration of MDT for PB leprosy.
MB patients. Disease activity in MB leprosy patients is judged both clinically and bacteriologically. Reports of bacterial negativity in MB patients given dapsone monotherapy include 28% after 2 years, and 80% at the end of 7 years' treatment.93 This compares with highly variable bacteriological cure rates at the end of 2 years of MDT, ranging between 0%94 and 100%.95 Other findings at the end of 2 years of MDT have included 25.8% and 56%96 and after 3 years, 64.7%.97
On balance, therefore, MDT would appear to be more effective than monotherapy in producing a "cure," particularly when one compares equal durations of therapy, but firm conclusions are not possible in light of such variability. Finally, extending MDT from 2 to 3 years has been shown to improve bacterial negativity from 56% to 66%96 but, as with PB leprosy, the problems of compliance and cost-effectiveness must be considered.
Leprosy reactions. PB leprosy. Reversal (type 1) reactions are peculiar to PB leprosy, and their reported frequency during 6 months of MDT has included 2.2% (2/92 patients),98 3.6% (2/55),99 and 28.6% (6/ 21).95 Reports in other studies have been in the range of 1.5-10%.59 -63 -100 These levels are low and acceptable, and have been attributed to the immunosuppressive actions of rifampin and dapsonc independent of their antibacterial actions. The reports represent only a small number of patients, however, and carefully controlled larger-scale studies are needed.
The diagnosis of relapse is straightforward in MB leprosy, but in PB leprosy relapse may be confused with reversal reaction. Indeed, clinically, reversal reaction may be the first sign of relapse in these patients, and can produce almost all of the signs of relapse. 15, 101 Reaction and relapse can also be difficult to differentiate histologically. 102, 103
One proposal to aid differentiation has been the careful observation of the timing of onset, since signs developing within 1 year of diagnosis are likely to be due to reversal reaction, while those occurring beyond 18 months suggest relapse.10 Nevertheless, early relapse may be difficult to distinguish from late reaction. To avoid this dilemma, it has been proposed that the worst hypothesis of relapse always be adopted.104
MB patients. Data are scarce regarding type 2 (ENL) reactions in MB patients, but in one study no worsening of existing reactions or development of fresh reactions after 2 years of MDT was observed.95
In the Tamil Nadu program in India, which has a coverage of 98,000 patients (MB and PB), 90 type 2 reactions were observed over the course of 5 years.73 In another study, type 2 reactions occurred in 29% of the patients (9/31 patients) over the same time period.
Relapse rate. Relapse is defined as the reappearance of active disease in patients who have completed the prescribed course of treatment,58 and the relapse rate has been proposed by some authors as the best measure of cure.105 For leprosy patients as a whole (MB and PB), relapse rates have included 11.6% after 12 months of therapy,106 14% after 18 months, and 28% for treatment lasting up to 5 years.107
PB leprosy. The unstandardized way in which relapse data were collected during dapsone monotherapy, particularly as regards the follow-up period, limits its comparability with MDT data. Nevertheless, the available data regarding monotherapy can be listed as follows: 1.8% in PB patients treated for 5-6 years (73.5% of the relapses in the first 3 years of follow-up),108 3% after 4 years,109 4.4% in borderline cases treated for 3-9 years after subsidence,110 9.4% after 6-18 months,82 and 6% in tuberculoid and 29% in indeterminate and borderline leprosy.111
A few studies have claimed to show no difference in the relapse rates between MDT and monotherapy in PB patients,112 but these are the exception and not the rule, and other reports of relapse following 6 months of MDT include 2.2% (2/92),98 2.3% (1/44),85 and 2.9% (16/555);58 in the latter study 92% of relapses occurred within 3 years of stopping MDT.
One recent study conducted in the Baroda District of India is particularly worthy of note since it confirmed all relapses histo-pathologically and was a relatively large-scale study. 113 The mean reported relapse rate was 0.19% (21/11,050), and all relapses occurred within 3 years of stopping MDT (76.2% in the first 2 years).
The above figures are clearly consistent with a superiority of MDT over monotherapy as regards relapse rate, and are perhaps the best proof of the greater efficacy of MDT. In addition, a follow-up period of 2-3 years after stopping MDT to examine for relapse would appear optimal. Nevertheless, the percentages given represent relatively small numbers of patients, and firm conclusions will not be possible until more large-scale studies have been conducted. This need was recognized by the WHO in 1982.11
MB leprosy. Even fewer follow-up studies of relapse in MB leprosy have been conducted than in PB leprosy, probably because MB patients are less numerous and less readily released from control than PB patients. Nevertheless, recent reports have included 1.6% following the completion of 2 years of MDT (6/372 patients).58 Moreover, only 50% of the relapses have been found to occur within 2 years of stopping MDT,104, 114 which suggests that the follow-up period of 2 years recommended by the WHO may be too short. As with PB leprosy, however, more large-scale data are needed before firm conclusions about the follow-up period can be made.
The relapse rate in patients released from control at the point of bacterial inactivity seems to be no greater than in patients given maintenance therapy, and it has been proposed that MDT should be stopped after 2 years even in the presence of a positive BI. 115 In this way, the absence of relapse would serve as the measure of cure. Indeed, one study which utilized a modified MDT regimen of just 3 months' duration observed no relapses out of 64 patients over a 3-year follow-up period, despite all patients being bacteriologically positive at the end of therapy. Moreover, skin biopsies over this period yielded no viable bacilli as measured in the mouse foot pad system. 115
A point peculiar to relapse in MB leprosy is the reliance on the reappearance of morphologically intact bacilli in skin smears as proof of relapse.15 Skin smears have a number of important limitations, not restricted to the evaluation of relapse, a topic which will be considered in detail later. In brief, however, problems arise from the accuracy and reliability of skin-smear results and such factors as the choice of appropriate sites for taking smears.58, 84
There is a need for standard criteria for the diagnosis of relapse and a simple tool, such as a urine dipstick capable of reliably indicating a rise in the level of M. leprae antigens, for instance, would be invaluable.
The possible causes of relapse in both PB and MB leprosy include:58 a) persistence of M. leprae; b) misclassification of patients as PB, leading to wrong treatment; c) inadequate chemotherapy, either too low a dose, irregularity of treatment, noncompliance, poor protocol, or other treatment mistakes; d) M. leprae drug resistance; and e) re-infection of patients (especially MB). Of these possible causes, persistence of M. leprae is the most likely, and patient compliance is also of importance. The other factors listed are of doubtful significance, and drug resistance, for instance, has to date not been observed.
Drug toxicity. Over 5 years in one program with a coverage of 98,000 patients (PB and MB), there were 24 cases of toxicity necessitating cessation of MDT; 15 of hepatitis who later recovered, 2 of hepatitis who died, 3 renal failures, and 4 hypersensitivity reactions.73
In another study, side effects were observed in 5.4% of MB and PB patients receiving MDT.85 There have also been isolated case reports of hypersensitivity reactions to dapsone with fatalities, 116 and these seem to have increased since the introduction of MDT. 117
In practice, however, toxicity and side effects due to MDT are not a major problem, and one is principally concerned with ensuring good patient compliance by the early detection of any difficulties. In addition, at present the potential for toxicity of MDT regimens incorporating one of the thio-amides in combination with rifampin requires further evaluation.
Value of clofazimine, ethionamide and prothionamide
Assessment of the impact of MDT on leprosy control should include the collection of data regarding the relatively untried drugs clofazimine, ethionamide and prothionamide. In the case of clofazimine, clarification is needed concerning: a) the optimal monthly dose, following reports of diminished percentage oral absorption with increasing dose; b) the minimal effective daily and monthly dose; and c) the acceptability of clofazimine-induced skin pigmentation, and implications for patient compliance.
The thioamides ethionamide and prothionamide are even less well understood than clofazimine, and it is essential that the minimum frequency of administration exerting a measurable effect be determined, particularly given the relative toxicity and expense of these drugs. In practice, therefore, the thioamides are seldom used in place of clofazimine, even in the face of patient intolerance to clofazimine-induced skin pigmentation.
Factors influencing the efficacy of MDT
The efficacy of MDT, both apparent and actual, is influenced by a number of factors, including: a) definitions of selected indicators; b) quality of health infrastructure, affecting population coverage, case detection, case-holding, service delivery and laboratory facilities, among others; c) nature of the plan of action; and d) initial prevalence and incidence rates.
More generally, leprosy control has suffered from the limited priority given to it by the health services, particularly resources and training. Moreover, even getting programs to implement MDT has historically been difficult, for reasons which shall now be discussed.
Historical considerations. The first WHO recommendations in 1977 for some form of MDT were very poorly implemented, and it is useful to consider the reasons for this: a) failure to perceive the urgency of the situation, particularly regarding dapsone resistance; b) unpopularity of the radical revision of control programs needed before the introduction of MDT; c) uncertainty regarding the value of MDT; d) uncertainty regarding the precise nature of MDT regimens, because of limited clinical experience; and e) fear of toxicity and other associated complications.
The subsequent 1982 report of the WHO Study Group on Leprosy helped to dispel these concerns, and set out in more concrete terms the rationale and protocol for MDT.10 Now, 9 years later, most health workers are aware of the basis for and expected benefits of MDT, and this educational process has been essential in ensuring the widespread implementation of MDT.
Finally, operational and technical changes within leprosy control programs have had a major influence on the actual and apparent impact of MDT, and these will now be discussed.
IMPACT OF MDT ON OPERATIONAL AND TECHNICAL ASPECTS OF LEPROSY CONTROL PROGRAMS
Implementation of MDT
For the implementation of MDT to be successful, many aspects of leprosy control programs have to be reorganized, and a number of prerequisites for starting MDT have been identified:84 '118 a) leprosy prevalence > 5/1000 (i.e., endemic region); b) > 80% of estimated cases already detected and registered; c) commitment of state government assured; d) plan of operations prepared; e) job descriptions prepared and adequate training of staff; f) laboratory attached to the control program; g) adequate referral facilities; and h) adequate financial and material resources, to enable uninterrupted treatment delivery to patients.
Considering the latter point, additional resources are particularly needed in the first few years of MDT implementation to cover the increased costs of drugs and field operations. Despite the inadequacy of many control programs in this regard, however, encouragement can be gained from the operational feasibility of MDT observed under difficult field conditions, such as those in Nepal. 119 Indeed, lack of financial resources per se is unlikely to be a major constraint to leprosy control, and should not divert attention from the identification of operational and technical factors preventing wider and faster MDT implementation.
The operational efficiency of the early stages of MDT implementation is indicated by: a) MDT coverage; b) number and training of staff; c) availability of vehicles; d) intensification of case detection before starting MDT, with diagnosis according to a standard MB/PB format; e) screening of fitness for MDT; f) prior health education; g) reliability of records and reports, and quality of base-line data; and h) existence of a pre-prepared plan of operations.120
The MDT coverage of registered leprosy patients has markedly improved in the last 5 years (1986 = 8.7%, 1987 = 14.5%, 1988 = 32.7%, 1989 = 45.3%) to reach a level of 55.7% in 1990.8 This is only the percentage of registered cases (which total 5.3 million), however, and thus is considerably less than the 11-12 million estimated cases.121 Nevertheless, MDT coverage has been shown to be inversely related to leprosy prevalence.8 It is thus important that factors impeding the wider implementation of MDT be identified.
The efficiency of the later intensive and maintenance phases of MDT implementation is indicated by the time taken to bring a maximum case-load under MDT. The shorter the time needed, the more efficient the program.
A more comprehensive approach to the medical care of leprosy patients with integration into the general health services is desirable. Such an approach would make many services, such as physiotherapy, corrective surgery, and rehabilitation, much more accessible and would greatly improve both patient care and patient compliance. Furthermore, it need not mean that all specialized staff and services should disappear, but that leprosy control activities will become the responsibility of the permanent multipurpose health services. Indeed, in some instances the leprosy control infrastructure could be used to strengthen the general health services.
Integration is particularly indicated in light of the disturbing development of AIDS in recent years, particularly in Africa. The prevalence of AIDS is significantly higher in patients with leprosy and, moreover, leprosy may be a presenting feature of AIDS.122, 123
Finally, in the longer term, as MDT takes hold in a community the adverse consequences for leprosy control of a declining number of leprosy patients, particularly for personnel training and interest in the disease generally, should be anticipated.124 Indeed, this problem has already been encountered in Zimbabwe.125
Role of skin-smear examination
Present strategies for MDT rely heavily on the results of the skin-smear examination, which is used to: classify patients into PB and MB types; assess the efficacy of MDT; determine the duration of MDT in MB patients; and diagnose relapse. It is of concern, therefore, that in a recent multi-center study of skin smears the taking of smears was found to be unsatisfactory in 26%, staining unsatisfactory in 22%, and reading unsatisfactory in 36%. 126 Moreover, even in well-established laboratories a need for improved standardization and quality was identified. Other studies have also questioned the handling, reading, and recording of skin smears127 and, in a recent review, it was recommended that one-man peripheral laboratories for skin-smear examinations should be closed and the service confined to central referral laboratories in order to improve the standardization and consistency of the procedure.128
Not only has the procedure been questioned but also the relationship between the BI and cure. The decline in BI (0.6-1 log/ year), for instance, is no faster with MDT than with dapsone monotherapy,129 probably because the removal of dead bacilli by macrophages is the rate-limiting step.130 If the 1982 WHO recommendations were strictly adhered to, therefore, a patient with a BI of 5 would need as many or more years of MDT.
Bacterial negativity is thus a poor and late indicator of cure, and there is currently a trend toward more clinically based management,105 in particular the use of the absence of relapse as a measure of cure. The WHO is not unaware of these developments, however, and in 1988 recognized the need for "non-insistence on slit-skin smears... under certain situations". 5
Finally, the rising specter of AIDS, particularly in Africa122 has been used as a further argument against the routine use of skin smears.
Surveillance after MDT
Despite standard recommendations from the WHO for a post-MDT follow-up of 2 years in PB patients, and a minimum of 5 years in MB patients, 11 the nature of surveillance varies greatly from country to country.131 For PB patients, for instance, follow-up is annually for 4 years in western Nepal; at 3, 6, and 8 months in Ethiopia; and annually for 2 years in India. In MB patients, follow-up is annually for 5 years in India and Ethiopia, annually for 8 years in Nepal, and annually for 10 years in Bhutan.131
Clearly, such interprogram variation is undesirable, and it is essential that prospective studies are conducted to determine the optimal period of follow-up. As already stated, large-scale data on relapse are not yet available, but current findings support a follow-up period of 2-3 years for PB leprosy and on the order of 5 years for MB leprosy. Furthermore, Becx-Bleumink 84 has recommended examination of PB patients at 3-month intervals over the period of follow-up.
The early diagnosis of reaction and relapse depends on the awareness and knowledge of the patient and medical staff, and the motivation of the patient to consult the medical services. Health education is useful in this regard, but it should be borne in mind that, in Africa, programs adopting a policy of health education have still only achieved a 40% attendance of patients one or more times for follow-up examination.132 Other means of keeping in touch with patients should not be neglected, therefore, and in this regard it is encouraging recently to see studies examining the fate of patients "permanently left" or "lost to follow-up."133
Finally, in the longer term as the leprosy problem is brought under control by MDT and the number of patients under chemotherapy declines, the emphasis on post-treatment surveillance is likely to change, with the monitoring of patients for disability becoming increasingly important.
DISCUSSION - THE FUTURE OF MDT
The best drug combination and the duration for which it should be given to obtain maximal cure rates, in the shortest possible time at the lowest risk of drug toxicity, is at present unknown. The future of MDT is therefore still very much in the making, and it seems appropriate to devote a section to the topic.
New drugs and drug formulations
Part of the assessment of MDT should include the efficacy of new drugs and drug formulations. The need for such research is demonstrated by the small number of drugs currently available for use in MDT, particularly new bactericidal compounds to back up rifampin, and overcome the current imbalance in potency between rifampin and the bacteriostatics, dapsone and clofazimine. This would prevent any possibility of the development of M. leprae resistance to MDT.
New drugs would also allow more treatment options, as in the case of patients who object to the skin discoloration produced by clofazimine and yet cannot take ethionamide or rifampin because of liver disease, in whom dapsone monotherapy is still the only choice. The ideal characteristics of a new antileprosy drug would be:84 -134 a) has bactericidal rather than bacteriostatic action; b) is able to destroy "persister" organisms; c) is effective when taken orally; d) acts by a different mechanism from currently available drugs; e) requires little or no supervision; f) patient compliance is not critical; g) is specific with few side effects; h) is acceptable to patients; and i) is cheap.
Among new compounds being tested against M. leprae, only the fluoroquinolones135 and the mycobacterial ribonucleotide reductase inhibitors136 currently appear promising. In addition, an ability of the an-tituberculous drug pyrazinamide to kill M. leprae persistors has recently been demonstrated.137 Examples of new drug formulations have included a long-acting preparation of dapsone which can be administered monthly, apparently without risk of toxicity, and a combined preparation of dapsone and clofazimine, but evaluation of many of these is still lacking.
Finally, one factor which has severely limited research into new drugs and drug formulations for leprosy has been the lack of financial incentive for drug companies. Indeed, most of the current antileprosy drugs resulted from research into more "profitable" diseases, as with rifampin and tuberculosis. However, research into atypical mycobacterial infections occurring in AIDS patients may prove to be productive.
Alternative MDT regimens
There is a need to find viable alternative regimens for MDT, both to provide an alternative in cases of intolerance to MDT and, in a wider sense, to further improve the efficacy of treatment. Two proposed regimens for MB leprosy, for instance, comprising sulfamethoxazole-trimethoprim and prothionamide or sulfamethoxazole-trimethoprim and isoniazid together with rifampin138 have been claimed to enable therapy of just 6 months. They have not been evaluated fully yet, but even if their efficacy was no greater than the current regimen, they would have the advantage of being effective against tuberculosis, which has been known to occur in association with leprosy.
Bactericidal/bacteriostatic combination
In a remarkable editorial in the Indian Journal of Leprosy in 1988 a fundamental attack was made on the combination of bactericidal and bacteriostatic drugs in MDT, with the claim that the two modes of activity cancel out one another.139 The author even proposed replacing MDT with a staggered form of therapy comprising an initial 6-8 weeks of daily rifampin followed by 10-12 months of daily dapsone and clofazimine. The only piece of evidence presented in favor of this view was one study in mice140 which has since been refuted by a number of similar studies.141 On this basis, the author extrapolated the well-known antagonism between the bactericidal drug penicillin and the bacteriostatic chloramphenicol to all drugs, without any further justification. Perhaps the best piece of evidence against the author's proposals, however, was his inability to account for the many demonstrations of the efficacy of MDT in the literature.
PB leprosy
Three-drug therapy. The MDT regimen for PB leprosy recommended by the WHO comprises rifampin given together with dapsone in order to minimize the risk of rifampin resistance in patients "wrongly diagnosed as paucibacillary." Given this acceptance of the fallibility of leprosy classification, however, and the dangers of primary resistance in both PB and MB patients already alluded to, the value of this regimen could be questioned, in particular with regard to the absence of a third drug.
Further evidence which casts doubt on this regimen are findings of bacillary loads in these patients far exceeding previous estimates of 1 million,142 which has implications for the dangers of secondary drug resistance, and of poorer cell-mediated immunity than previously supposed.94
The addition of clofazimine to the WHO regimen has therefore been proposed, to effectively extend three-drug MDT to all leprosy patients. Alternatively, the tailoring of therapy by such factors as lepromin status and the number of skin patches has been proposed,88 but studies have observed no relationship between these factors and disease activity at the end of 6 months of therapy94 and, in practice, this approach to therapy has proved overly cumbersome.143
Duration of therapy. Some debate exists over the optimal duration of MDT for PB leprosy, in particular, whether therapy should be extended from 6 months based on findings of continued improvement as regards clinical inactivity. On balance, the evidence probably favors extending the duration of therapy but with proposals for treatment to inactivity,20 and even for maintenance with dapsone monotherapy,87 it is clear that further trials are needed to enable a firm consensus to be reached.
Initial intensive rifampin therapy. Initial intensive rifampin therapy has been used as part of the treatment of PB leprosy in some countries,144 but to date no advantage over MDT has been demonstrated.12
MB leprosy
Nature of the "third drug." Despite wide acceptance of the basic principles and objectives of MDT in MB leprosy, some uncertainty surrounds the nature of the third drug to accompany rifampin and dapsonc, namely, clofazimine versus ethionamide/ prothionamide. Both thioamides have been tried in place of the WHO-recommcnded clofazimine,145-147 but evaluation of the relative efficacy and toxicity of these regimens has so far been inconclusive. One of the studies proposed a regimen of 375 mg ethionamide daily in place of clofazimine, to be used in selected hospitalized patients in whom close supervision for side effects was possible. It was hoped that a relatively quick, cosmetically acceptable "cure" would be produced by this regimen, but after 2 years of therapy the clinical inactivity rate (65%) was identical to the WHO regimen.
The thioamides are widely accepted as being more toxic than clofazimine and, indeed, this was the basis for the WHO recommendation of clofazimine. There is some concern over the dangers of liver toxicity from these drugs, particularly when used together with rifampin. In one study, for instance, hepatotoxicity occurred in 20% of the patients.148 Finally, implications of the higher cost of the thioamides need assessment.
Initial intensive rifampin therapy. At the time of the WHO recommendations for MDT in 1982, the Indian Association of Leprologists (IAL) recommended an alternative regimen for MB leprosy which differed by including additional initial daily rifampin.54 As with PB leprosy, however, there is as yet no evidence that it offers any advantage over the WHO regimen.76, 96
Duration of therapy. The WHO has recommended MDT in MB patients for a minimum of 2 years, and up to skin-smear negativity where possible. 11 This policy of continuation to smear negativity has been questioned and, indeed, is a practice based more on tradition from the dapsone era than on scientific grounds. The many limitations of the skin-smear examination were discussed earlier, but of particular concern is the slow decline in the BI, such that even after 5 years of MDT in one study 15% of MB patients were found to remain skin-smear positive.149 The fact that smear pos-itivity is not incompatible with "cure" is well-demonstrated by a study in Malta, which observed that of 20.7% of treated MB patients who remained smear positive for 5-12 years, not one relapsed.130 Moreover, many patients continue to show bacteriological improvement after the cessation of MDT.96
There is some evidence that these findings are leading to changes in treatment practice, and many programs now routinely stop therapy after 2 years of MDT in MB patients regardless of bacteriological status. Moreover, relapse rates in these programs have been encouraging. Apart from being unnecessary, the disadvantages of MDT exceeding 2 years, namely, reduced patient compliance, increased risk of drug toxicity, and increased expense,84 should be remembered. In a more general sense, but just as serious, is the possibility that prolonged MDT would lead to the belief among both health workers and patients that leprosy is still "incurable."
Fixed duration trials of MDT are now needed to determine the optimal duration of MDT in MB leprosy, ideally incorporating long-term follow-up, to confirm cure by the absence of relapses. Indeed, one such study has been started by the All Africa Leprosy Rehabilitation and Training Centre (ALERT) in Ethiopia.
Administration of clofazimine. More minor proposed modifications to the WHO regimen for MB leprosy involve the administration of clofazimine on alternate days only.94 This follows reports of a lower percentage of oral absorption of clofazimine with higher doses, and the inability of a loading dose to ensure greater absorption of the drug.150 Monthly administration of clofazimine could thus be argued to be wasteful, both financially and therapeutically. Assessment of the alternate day clofazimine regimen has not been favorable. In one study none of 56 patients achieved bacteriological negativity by 2 years of treatment, although some clinical improvement was observed, with reduction of the average clinical score as calculated by Iyer's method, 151 16 to 2. 94 The study was further limited by not having a control group receiving the WHO regimen.
Other topics for future research
Other interesting topics for future research include the role of combined im-munochemotherapy and the relationship between the presence of pcrsistors and the risk of relapse following cessation of MDT. There is also great scope for further operational studies of MDT, into such areas as the reasons for noncompliance. "Patient" factors are often considered but "service" factors are not, particularly methods to improve service shortcomings.
CONCLUDING REMARKS
In this essay, the impact of MDT on various aspects of leprosy treatment and control has been discussed with emphasis on the improvements in efficacy and acceptability of treatment. One unforeseen effect of MDT was to provide an impetus to leprosy control program managers to "set their house in order," which led to radical operational and technical changes within programs. The dependence of many aspects of MDT efficacy on the nature and extent of these changes has been demonstrated.
In conclusion, the way forward in the treatment and control of leprosy depends to a large degree on the meticulous implementation of MDT as recommended by the WHO. In addition, however, it is essential that research into such areas as new drugs and formulations and alternative MDT regimens should be vigorously pursued. Ongoing critical analysis of MDT is important in identifying potential improvements in leprosy chemotherapy.
- John S. Gilbody, B.Sc.(Hons.)
Acknowledgment. My thanks go to the Library staff of the Wills Library at Guy's Hospital Medical School, London, for their help with literature searching, and to Miss Patricia Charnet for translation assistance.
1. Hansen, G. A. and Looft, C. Leprosy: In Its Clinical and Pathological Aspects. Bristol: John Wright & Co., 1895.
2. Adams, A. R. D. Clinical Tropical Diseases. 8th ed. London: Blackwell Scientific Publications, 1984.
3 Lyons, A. S. Medicine: An Illustrated History. New York: Harry N. Abrams, 1978.
4. Sansarricq, H. Leprosy in the world today. Lepr. Rev. 52 Suppl. 1 (1981) 15-31.
5. World Health Organization. Report of third coordinating meeting on implementation of multidrug therapy (MDT) in leprosy control programmes. Geneva: World Health Organization, 1988. WHO/CDS/ LEP/88.4.
6. WHO Expert Committee on Leprosy. Sixth report. Geneva: World Health Organization, 1988. Tech. Rep. Ser. 768.
7. World Health Organization Study Group. Leprosy, second coordinating meeting on the implementation of multidrug therapy in leprosy control. Wkly. Epidem. Rep. 62 (1987) 101-102.
8. Noordeen, S. K. A look at world leprosy from the Chief of the WHO Leprosy Unit (Clayton Memorial Lecture). London School of Hygiene and Tropical Medicine, London, 1 October 1990.
9. Ridley, D. S. and Jopling, W. J. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34 (1966) 255-273.
10. World Health Organization. Standard protocol for chemotherapy trials in non-lcpromatous leprosy. Geneva: World Health Organization, 1982. TDR/THE-LEP/PROTOCOL/82.1.
11. WHO Study Group. Chemotherapy of leprosy for control programmes. Geneva: World Health Organization, 1982. Tech. Rep. Ser. 657.
12. Katoch, K.., Ramu, G., Ramanathan, U. and De-sikan, K. V. Comparison of three regimens containing rifampin for treatment of paucibacillary leprosy patients. Int. J. Lepr. 55 (1987) 1-8.
13. Ramanan, C, Manglani, P. R. and Chandrakar, K. A. Retrospective study of leprosy in an industrial hospital. J. Commun. Dis. 16 (1984) 169-175.
14. Grugni, A., Nadkarni, N. J. and Kini, M. S. Multidrug therapy in paucibacillary' leprosy - a five-year experience. Indian J. Lepr. 60 (1988) 589-592.
15. Waters, M. F. R., Ridley, D. S. and Ridley, M. J. Clinical problems in the initiation and assessment of multidrug therapy. Lepr. Rev. 57 Suppl. 3 (1986) 92100.
16. Guidelines for multidrug treatment in endemic districts. National Leprosy Eradication Programme. New Delhi: Directorate General of Health Services. Ministry' of Health and Family Welfare, 1989.
17. Ridley, D. S. Therapeutic trials in leprosy using serial biopsies. Lepr. Rev. 29 (1958) 45-52.
18. Pfaltzgraff, R. E. The management of reaction in leprosy. Int. J. Lepr. 57 (1989) 103-109.
19. Wolcott, R. R. and Ross, Sister H. Exacerbation of leprosy during present day treatment. Int. J. Lepr. 21 (1953) 437-440.
20. Ramu, G. Multi-drug therapy of leprosy. (Editorial) Indian J. Lepr. 57 (1985) 465-485.
21. Pettit, J. H. S. and Rees, R. J. W. Sulphone resistance in leprosy; an experimental and clinical study. Lancet 2 (1964) 673-674.
22. Neelan, P. N., Noordccn, S. K., Balakrishnan, S. and Rajasckara Pandian, P. Prevalence of secondary dapsone resistance in leprosy in Kanchecpuram and Tiruvannamalai control units of Tamil Nadu. Lepr. India 55 (1983) 222-230.
23. Baquillon, G., Ferracci, C, Van Loo, G. and Pat-tyn, S. R. Further results on dapsone-rcsistant leprosy in Bamako (Mali). Lepr. Rev. 54 (1983) 19-21.
24. Pearson, J. M. H., Hailc, G. S., Barnetson, R. St. C. and Rees, R. J. W. Dapsone-resistant leprosy in Ethiopia. Lepr. Rev. 50 (1979) 183-199.
25. Meade, T. W., Pearson, J. M. H., Rees, R. J. W. and North, W. R. S. The epidemiology of sulfone resistant leprosy. (Abstract) Int. J. Lepr. 41 (1973) 684.
26. Pearson, J. M. H., Rees, R. J. W. and Waters, M. F. R. Sulphone resistance in leprosy; a review of one hundred proven clinical cases. Lancet 2 (1975) 69-72.
27. Pettit, J. H. S., Ridley, D. S. and Rees. R. J. W. Studies on sulfone resistance in leprosy. Int. J. Lepr. 34 (1966) 375-390.
28. Guelpa-Lauras, C.-C, Grosset, J.-H., Constant-Desportes, M. and Brucher, C. Nine cases of rifampin resistant leprosy. (Letter) Int. J. Lepr. 52 (1984) 101 - 102.
29. Hastings, R. C. and Jacobson, R. R. Rifampicin resistant leprosy. Health Co-operation Papers 1 (1981) 47-54.
30. Warndorff. Van Diepcn. T. Clofaziminc-resistant leprosy-a case-report. Int. J. Lepr. 50 (1982) 139- 142.
31. Pattyn, S. R., Rollier, M.-T., Rollier, R., Verdoo-laegne-Van Loo, G. Sensibilité envers la dapsone, la sulfamcthoxypyridazine et l'éthionamidc, de Mycobacterium leprae provenant de malades traités par ces substances. Int. J. Lepr. 43 (1975) 356-363. (English Abstract 362-363).
32. Pattyn. S. R. and Colston, M. J. Cross-resistance amongst thiambutosine, thiaectazonc, ethionamide and prothionamide with Mycobacterium leprae. (Letter) Lepr. Rev. 49 (1978) 275-281.
33. Pearson, J. M. H., Haile, G. S. and Rees, R. J. W. Primary dapsone resistant leprosy. Lepr. Rev. 48 (1977) 129-132.
34. Londono, F. Primary sulphone resistance. (Letter) Lepr. Rev. 48 (1977) 51.
35. Russell, D. A., Worth, R. M., Scott, G. C, Vincin, D. R., Jano, B., Fasal, P. and Shepard, C. C. Experience with accdapsone (DADDS) in the therapeutic trial in New Guinea and the chemoprophylactic trial in Micronesia. Int. J. Lepr. 44 (1976) 170-176.
36. Guinto, R. S., Ccllona, R. V., Fajardo, T. T. and delà Cru/, E. G. Primary dapsone resistant leprosy in Cebu, Philippines. Int. J. Lepr. 49 (1981) 427-430.
37. UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases. 6lh Programme report. Geneva: World Health Organization, 1983, p. 261. TDR/PR-6/83.
38. Sreevatsa, Girdhar, B. K., Ramu, G. and Desikan, K. V. Parallel dapsone and rifampicin resistance: a prospective study. Jpn. J. Lepr. 53 (1984) 28-31.
39. Bigger, J. W. Treatment of staphylococcal infections with penicillin by intermittent sterilisation. Lancet 2 (1944) 497-500.
40. Toman, K. Bacterial persistence in leprosy. Int. J. Lepr. 49 (1981) 205-217.
41. Dharmendra and Ramu, G. Prognosis in lepro-matous leprosy. In: Leprosy, Volume I. Dharmcndra, cd. Bombay: Kothari Medical Publishing House, 1978.
42. Ramu, G. and Desikan, K. V. A study of scrotal biopsy in subsided cases of lepromatous leprosy. Lepr. India 51 (1979) 341-347.
43. Vellut, C. Ten years follow-up of lepromalous patients on DDS treatment. Lepr. India 40 (1968) 111 - I 14.
44. Waters, M. F. R., Rees, R. J. W., McDougall, A. C. and Weddcll, A. G. M. Ten years of dapsone in lepromatous leprosy: clinical, bacteriological and histological assessment and the findings of viable leprosy bacilli. Lepr. Rev. 45 (1974) 288-298.
45. Sreevatsa, Girdhar, B. K. and Desikan, K. V. Persister M. leprae after introductory rifampicin followed by dapsone therapy. Lepr. India 53 (1981) 350-353.
46. Waters, M. F. R., Rees, R. J. W., Pearson, J. M. H., Laing, A. B. G., Hclmy, H. S. and Gelber, R. H. Rifampicin for lepromatous leprosy: nine years' experience. Br. Med. J. 1 (1978) 133-136.
47. Ellard, G. A. Drug compliance in the treatment of leprosy. Lepr. Rev. 52 (1981) 419-427.
48. Gicl, R. and van Luijk, J. N. Leprosy in Ethiopian society. Int. J. Lepr. 38 (1970) 187-193.
49. Langhorne, P., DufTus, P., Berkeley, J. S. and Jc-sudasan, K. Factors influencing attendance during the multidrug therapy of leprosy. Lepr. Rev. 57 (1986) 1730.
50. Hagan, K. J., Smith, S. E., ct al. The reliability of self-administration of dapsone by leprosy patients in Burma. Lepr. Rev. 50 (1979) 201-211.
51. Low, S. J. M. and Pearson, J. M. H. Do leprosy-patients take dapsone regularly? Lepr. Rev. 45 (1974) 218-223.
52. Hertroijs, A. R. A study of some factors affecting the attendance of patients in a leprosy control scheme. Int. J. Lepr. 42 (1974) 419-427.
53. WHO Expert Committee on Leprosy. Fifth report. Geneva: World Health Organization, 1977. Tech. Rep. Ser. 607.
54. Indian Association of Leprologists (IAL). Summary of the seminar on "Consensus on treatment regimens in leprosy and problems of drug delivery." Indian J. Lepr. 56 (1984) 158-159.
55. Warndorff, J., Bourland, J. and Pattyn, S. R. Follow-up on short course 2 months' rifampicin treatment of paucibacillary leprosy. Lepr. Rev. 53 (1982) 9-17.
56. Chemotherapy of leprosy. (Editorial) Lancet 2 (1982) 77-78.
57. Subcommittee on Clinical Trials of the Chemotherapy of Leprosy (THELEP). Scientific Working Group of the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases. Persisting Mycobacterium leprae among THELEP trial patients in Bamako and Chingleput. Lepr. Rev. 58 (1987) 325-337.
58. Van Brakel, W., Kist, P., Noble, S. and O'Toole, L. Relapses after multidrug therapy for leprosy: a preliminary report of 22 cases in West Nepal. Lepr. Rev. 60 (1989) 45-50.
59. Jopling, W. H., Ridley, M. J., Bonnici. E. and Depasquale, G. A follow-up investigation of the Malta-Project. Lepr. Rev. 55 (1984) 247-253.
60. Becx-Bleumink, M. Implementation of multidrug therapy in the ALERT Leprosy Programme in the Shoa Region of Ethiopia. First results with paucibacillary patients. Lepr. Rev. 57 (1986) 111-119.
61. Keeler, R. F. Multidrug therapy in leprosy in Trinidad and Tobago; a preliminary report. Lepr. Rev. 55 (1984) 391-396.
62. Revankar, C. R., Ganapati, R. and Naik, D. D. Multidrug therapy for paucibacillary leprosy; experience in Bombay. Indian J. Lepr. 57 (1985) 773-779.
63. Rose, P. Short course multidrug therapy of paucibacillary leprosy in Guyana: preliminary communication. Lepr. Rev. 55 (1984) 143-147.
64. Balakrishnan, S., Kumar, A., Rao, B. R. and Patro, T. P. Implementation of tests for monitoring drug compliance of leprosy out-patients under multi-drug therapy. Indian J. Lepr. 58 (1986) 555-559.
65. Almeida, J. G. and Chacko, C. J. G. Computerized mathematical model of M. leprae population dynamics during multiple drug therapy. Indian J. Lepr. 57 (1985) 780-789.
66. World Health Organization. Report of an informal working group in educational material for patients convened by the WHO Action Programme on Essential Drugs hosted by the WHO South-East Asia Regional Office, New Delhi, 1985 (DAP/85.10).
67. McDougall, A. C. and Georgiev, G. D. Educational material for the patient with leprosy. Lepr. Rev. 60 (1989) 221-228.
68. Valencia, L. B. Leprosy-as seen by the patients. World Health Forum 9 (1988) 59-62.
69. Revankar, C. R. and Sorenscn, B. H. Blister calendar packs for the treatment of patients in leprosy control programmes with multiple drug therapy (MDT). (Letter) Lepr. Rev. 59 (1988) 84.
70. Jesudasan, K., Vijayakumaran, P., Pannikar, V. K. and Christian, M. Impact of MDT on leprosy as measured by selective indicators. Lepr. Rev. 59 (1988) 215-223.
71. World Health Organization. Report of the second coordinating meeting on "Implementation of multidrug therapy in leprosy control programmes." Geneva: World Health Organization, 1987. WHO/CDS/LEP/ 87.2.
72. Chum, H. J. The impact of MDT implementation in the Tanzania National TB-Lcprosy Programme. Lepr. Rev. 57 Suppl. 3 (1986) 63-66.
73. Ekambaram, V. and Rao, M. K. Changing picture of leprosy in North Arcot district, Tamil Nadu, after MDT. Indian J. Lepr. 61 (1989) 31-43.
74. Noordeen, S. K. Global review of MDT implementation. (Paper presented to WHO/SEARO inter-country seminar on "Implementation and evaluation of multidrug therapy in leprosy control programmes through primary' health care.") New Delhi: SEARO, 7-11 December 1987.
75. Rose, P. Changes in epidemiological indices following the introduction of WHO MDT into the Guyana leprosy control programme. Lepr. Rev. 60 (1989) 151-156.
76. Chopra, N. K., Agarwal, J. S. and Pandya, P. G. Impact of multidrug therapy on leprosy in Baroda district (Gujarat). Indian J. Lepr. 61 (1989) 179-189.
77. Boerrigtcr, G. and Ponnighaus, J. M. Preliminary evaluation of the effect of WHO-MDT on disabilities in leprosy patients in Malawi (Central Africa). Lepr. Rev. 57 Suppl. 3 (1986) 101-105.
78. Leprosy, NLEP in India. Report of the second independent evaluation of NLEP. New Delhi: Leprosy Division, 1987.
79. Berne, D., Haimanot, R. T., Tedla, T. and Tad-desse, T. Epidemiological pattern of leprosy in Ethiopia: a review of the control programmes. Lepr. Rev. 61 (1990) 258-266.
80. Fine, P. E. Leprosy-the epidemiology of a slow bacterium. Epidemiol. Rev. 4 (1982) 161-188.
81. Kar, P. K. and Sohi, A. S. Study of multidrug therapy in paucibacillary leprosy. J. Indian Med. Assoc. 87 (1989) 34-36.
82. Girdhar, B. K., Girdhar, A., Ramu. G. and De-sikan, K. V. Short course treatment of paucibacillary (TT/BT) leprosy cases indian J. Lepr. 57 (1985) 491-498.
83. Vellut, C. Some facts about 25 years of dapsonc monotherapy at Polambakkum. Indian J. Lepr. 56 Suppl. (1984) 458-459.
84. Becx-Blcumink, M. Operational aspects of multidrug therapy. Int. J. Lepr. 57 (1989) 540-551.
85. Puavilai, S. and Timpatanapong, P. Short-course multi-drug therapy for leprosy patients. J. Med. Assoc. Thai. 72 (1989) 33-36.
86. Katoch, K., Ramu, G. and Ramanathan, U. Che-motherapeutic trials with different regimens containing rifampicin in paucibacillary type of leprosy cases-a preliminary report. Indian J. Lepr. 57 (1985) 499-506.
87. Dhir, R., Guha, P. K. and Singh, G. Short-term chemotherapy of paucibacillary leprosy. Indian J. Lepr. 58 (1986) 549-554.
88. Revankar, C. R., Karjivkar, V. G., Gurav, V. J. and Ganapati, R. Clinical assessment of paucibacillary' leprosy under multidrug therapy-three years followup study. Indian J. Lepr. 61 (1989) 355-359.
89. Tiwari, V. D., Tutakne, M. A., Singh, G. and Dut-ta, R. K. Multidrug therapy in hospitalised leprosy cases. Indian J. Lepr. 60 (1988) 71-76.
90. Pattyn, S. R., Husser, J. A., Baquillon, G., Maiga, M. and Jamet, P. Evaluation of five treatment regimens, using either dapsonc monotherapy or several doses of rifampicin in the treatment of paucibacillary leprosy. Lepr. Rev. 61 (1990) 151-156.
91. Palande, D. D. and Ramu, G., convenors. Multidrug therapy (in India): report of an informal get together of leprologists involved in MDT in India. Int. J. Lepr. 56 (1987) 120-121.
92. Samuel, N. M., Samuel, S., Nakami, N. and Mur-mu, R. Multidrug treatment of leprosy -practical application in Nepal. Lepr. Rev. 55 (1984) 265-272.
93. Almeida, J. G. and Chacko, C. J. G. Evaluation of dapsone monotherapy for lepromatous leprosy in Gudiyatham Taluk. Lepr. India 56 Suppl. 2 (1984) 454-459.
94. Katoch. K., Ramu, G., Ramanathan, U., Scngup-ta, U. and Srcevatsa. Follow up of BL/LL patients on a slightly modified WHO regimen of multidrug therapy. Ind'ian J. Lepr. 59 (1987) 36-43.
95. Kaur, S., Sharma, V. K., Kumar. E., Singh, M. and Kaur, I. Multidrug therapy in bacilliferous leprosy-two years experience. Indian J. Lepr. 57 (1985) 483-490.
96. Ganapati. R., Rcvankar, C. R. and Rashmi, R. P. Three years assessment of multidrug therapy in mul-tibacillary leprosy cases. Indian J. Lepr. 59 (1987) 4449.
97. Li, W., Ye, G., Yang, Z., Tao, M., Luo, J., Wang, C. and Ji, F. Effect of three-year multidrug therapy in multibacillary leprosy patients. Proc. CAMS PUMC 5 (1990) 37-40.
98. Reddy, P. K. and Mohinuddin, S. K. Pattern of relapses in paucibacillary leprosy patients treated with MDT (WHO 1982). Indian J. Lepr. 60 (1988) 581-588.
99. Reddy, P. K. Occurrence of reversal reactions in BT patients during WHO paucibacillary' leprosy MDT (1982). Indian J. Lepr. 60 (1988) 453-456.
100. Bhate, R. D., Gupta, C. M., Chattopadhay, S. P. and Singh, I, P. Experience with multidrug therapy in paucibacillary' leprosy. Indian J. Lepr. 58 (1986) 244250.
101. Pandian, T. D., Sithambaram, M., Bharathi, R., and Ramu, G. A study of relapse in nonlcpromatous and intermediate groups of leprosy. Indian J. Lepr. 57 (1985) 149-158.
102. Pearson, J. M. H. and Ross, W. F. Nerve involvement in leprosy -pathology, differential diagnosis and principles of management. Lepr. Rev. 46 (1975) 199212.
103. Waters, M. F. R. and Ridley, D. S. Tuberculoid relapse in lepromatous leprosy. (Abstract) Int. J. Lepr. 47 (1979) 350.
104. Pattyn, S. R. Incubation times of relapses after treatment of paucibacillary leprosy. Lepr. Rev. 55 (1984) 115-120.
105. Georgiev, G. D. and McDougall, A. C. A re-appraisal of clinical and bacteriological criteria in the implementation of multiple drug therapy for leprosy control programmes and proposals for their better use. Lepr. Rev. 64 (1990) 64-72.
106. Lowe, J. The late results of sulphone treatment of leprosy in East Nigeria. Lepr. Rev. 25 (1954) 113124.
107. Touw-Langendijk, E. M. j. and Naafs, B. Relapses in leprosy after release from control. Lepr. Rev. 50 (1979) 123-127.
108. Ekambaram, V. Duration of treatment for disease arrest of non-lepromatous cases and relapse rate in these patients. Lepr. Rev. 50 (1979) 297-302.
109. Jesudasan, K., Christian, M. and Bradley, D. Relapse rates among nonlepromatous patients released from control. Int. J. Lepr. 52 (1984) 304-310.
110. Ramu, G. and Ramanujam, K. Relapse in borderline leprosy. Lepr. India 46 (1974) 19-25.
111. Davey, T. F. "Present trends and future prospects in the realm of therapy." Discussion of the 7th International Congress on Leprology, Tokyo, November 12th, 1958. Tokyo: CIBA, 1959, p. 12.
112. Annual Report. Agra: Central JALMA Institute for Leprosy, 1983.
113. Chopra, N. K., Agarwal, J. S. and Pandya, P. G. A study of relapse in paucibacillary leprosy in a multidrug therapy project, Baroda district, India. Lepr. Rev. 61 (1990) 157-162.
114. A Nollet, E., Janssens, L., Groenen, G., Bourland, J., Pattyn, S. R. and the Collaborative Study Group on the Treatment of Leprosy. Incubation time for relapse in multibacillary leprosy. (Abstract) Int. J. Lepr. 52 (1984) 686.
115. Pattyn, S. R. Efficacy of different regimens in multibacillary leprosy. Lepr. Rev. 57 Suppl. 3 (1986) 265271.
116. Smith, W. C. S. Hypersensitivity reaction to dapsone. (Letter) Lepr. Rev. 57 (1986) 179-180.
117. Richardus, J. H. and Smith, T. C. Increased incidence in leprosy of hypersensitivity reactions to dap-sone after introduction of multidrug therapy. Lepr. Rev. 60 (1989) 267-273.
118. Dharmshaktu, N. S. Progress of multi drug therapy under National Lepropsy Eradication Programme. Indian J. Lepr. 62 (1990) 19-21.
119. Birch, M. C. Leprosy treatment in Nepal with multidrug regimens. Lepr. Rev. 55 (1984) 255-264.
120. Manual for Multiple Drug Therapy (MDT) in Ethiopia. National Leprosy Control Programme, 1983.
121. World Health Organization. Report of a meeting on action plans for leprosy control, New Delhi, August 23-25, 1982. Geneva: World Health Organization, 1983. WHO/LEP/83.1/CORR.1.
122. Meeran, K. Prevalence of HIV infection among patients with leprosy and tuberculosis in rural Zambia. Br. Med. J. 298 (1989) 364-365.
123. Nunn, P. P. and McAdam, K. P. Mycobacterial infections and AIDS in tuberculosis and leprosy. Br. Med. Bull. 44 (1988) 801-813.
124. Lechat, M. F. Leprosy: the long hard road. World Health For. 9 (1988) 69-71.
125. Warndorff, D. K. and Warndorif, J. A. Leprosy control in Zimbabwe: from a vertical to a horizontal programme. Lepr. Rev. 61 (1990) 183-187.
126. Vettom, L. and Pritze, S. Reliability of skin smear results: experience with quality control of skin smears in different routine services in leprosy control programmes. Lepr. Rev. 60 (1989) 187-197.
127. De Rijk, A. J., Nilsson, T. and Chonde, M. Quality control of skin smear services in leprosy programmes: preliminary experience with inter-observer comparison in routine services. Lepr. Rev. 56 (1985) 177-191.
128. Georgicv, G. D. and McDougall, A. C. Skin smears and the bacterial index (BI) in multiple drug therapy leprosy control programs: an unsatisfactory and potentially hazardous state of affairs. (Letter) Int. J. Lepr. 56 (1988) 101-104.
129. Grosset, J. H. Recent developments in the field of multidrug therapy and future research in chemotherapy of leprosy. Lepr. Rev. 57 Suppl. 3 (1986) 223224.
130. Jopling, W. H. A report on two follow-up investigations of the Malta-Project, 1983 and 1986. Lepr. Rev. 57 Suppl. 3 (1986) 47-53.
131. Neville, J. After multidrug therapy (MDT): who is responsible for continuing care? (Editorial) Lepr. Rev. 59 (1988) 1-3.
132. Leprosy Control Department. All Africa Leprosy and Rehabilitation Center (ALERT). Annual Report for 1987. Addis Ababa: ALERT, 1987, pp. 70-134.
133. Muliyil, J. and Thangavcl, N. Status of multi-bacillary patients "lost to follow-up." Indian J. Lepr. 61 (1989) 229-232.
134. Hooper, M. The search for new drugs for the treatment of leprosy. Lepr. Rev. 56 (1985) 57-60.
135. Grosset, J.-H., Jarlier, V., TrufTot-Pernot, C, Guelpa-Lauras, C.-C. and Lecoeur, H. Activité des nouvelles quinolones sur les mycobactérics. In: Les Nouvelles Quinolones. Paris: Arnette Edit., 1985, pp. 181-189.
136. Van Saanc, P. and Timmerman, H. Pharmaco-chemical aspects of leprosy. Recent developments and prospects for new drugs. Pharm. Weekbl. 11 (1989) 3-8.
137. Katoch, K., Srcevatsa, Ramanathan, U. and Ramu, G. Pyrazinamide as part of combination therapy for BL and LL patients-a preliminary report. Int. J. Lepr. 56 (1988) 1-9.
138. Freerksen, E., Rosenfeld, M. and Spannuth, G. New forms of multidrug therapy for the treatment of leprosy. First report for the practice on rifampicin + sulfamethoxazole-trimethoprim + protionamide and rifampicin + sulfamethoxazole-trimethoprim + isoniazid. Chemotherapy 35 (1989) 133-139.
139. Chatterjee, B. R. On multi-drug combinations in the treatment of leprosy. (Editorial) Indian J. Lepr. 60 (1988) 493-497.
140. Gelber, R. H. The neonatally thymectomized rat as a model of the lepromatous patient. Int. J. Lepr. 55 Suppl. (1987) 879-881.
141. Grosset, J. H., Ji, B.-H. and Noordecn, S. K. Comments on an editorial by B. R. Chatterjee "On multi-drug combinations in the treatment of leprosy." Indian J. Lepr. 60 (1988) 493-497. (Editorial) Indian J. Lepr. 61 (1989) 315-327.
142. Raval, S. N., Scngupta, U., Ramu, G., Prabhunc, P. V. and Desikan, K. V. A study of continuous bacil-laemia in borderline and lepromatous type of leprosy. Lepr. India 54 (1982) 623-633.
143. Jesudasan, K., Bradley, D., Smith, P. G. and Christian, M. Management of patients with non-lep-romatous leprosy -the Karigiri experience. (Abstract) Int. J. Lepr. 52 Suppl. (1984) 709.
144. Rao, C. K. Drugs against leprosy. World Health Forum 9 (1988) 63-67.
145. Ji, B.-H., Chen, J., Wang, C. and Xia, G. Hepatotoxicity of combined therapy with rifampicin and daily prothionamide for leprosy. Lepr. Rev. 55 (1984) 282-289.
146. Depasquale, G. The Malta experience; Isopro-dian-rifampicin combination treatment for leprosy. Lepr. Rev. 57 Suppl. 3 (1986) 29-37.
147. Freerksen, E. and Rosenfeld, M. Leprosy eradication project of Malta. Chemotherapy 23 (1977) 356386.
148. Pattyn, S. R., Jansscns, L., Bourland, J., Saylan, T., Davies, E. M., Grillone, S., Feraci, C. and the Collaborative Study Group for the Treatment of Leprosy. Hepatotoxicity of the combination of rifampin-ethionamide in the treatment of multibacillary leprosy. Int. J. Lepr. 52 (1984) 1-6.
149. Becx-Bleumink, M. MDT in the leprosy programme of ALERT-a review. Paper prepared for the Pre-Congress Workshop on Leprosy Control. Evaluation and Integration, XIII International Leprosy Congress, 1988.
150. Mathur, A., Vcnkatcsan, K., Bharadwaj, V. P. and Ramu, G. Evaluation of the effectiveness of clofazimine therapy. I. Monitoring the absorption of clofazimine from the gastrointestinal tract. Indian J. Lepr. 57 (1975) 146-148.
151. Iyer, C. G. S., Balakrishnan, S. and Ramu, G. A comparison of low and conventional dosage of dapsonc in treatment of lepromatous leprosy. Lepr. India 49 (1977) 372-386.
* This review, written in 1990 by John S. Gilbody, a second-year medical student at Guy's Hospital Medical School, was a prize-winning essay in the annual competition sponsored by the British Leprosy Relief Association (LEPRA) for essays on various aspects of leprosy. We take pleasure in publishing this review. The author's present address is: J. S. Gilbody, Crombie, 78 Stopples Lane, Hordle Near Lymington, Hampshire S041 OGL, England.