- Volume 59 , Number 3
- Page: 482–6
Mycobacterial cell surface proteins revealed by labeling with 125I
To the Editor:
Surface antigens of infecting organisms play an important role in host immunity, since they are the first antigens encountered by the cells of the immune system. In our laboratory, ICRC bacilli- mycobacteria cultured from biopsies of leprosy patients (mainly Mycobacterium avium-intracellu-lare)- are used to prepare an antileprosy vaccine (4). A very high molecular weight, glycolipoprotein fraction of these bacilli, named PP-I, has been found to have good immunogenicity; hence, it is used for the preparation of a subunit vaccine (1). Since this PP-I is purified from the sonicate of ICRC bacilli, its exact location was not known. However, its chemical composition suggested that it may be a cell-wall component. We have now studied this using the technique of iodination of surface proteins in intact cells.
The ICRC bacilli were cultivated as described earlier (3). The organisms were harvested after 12 to 13 days of culture, washed three times with sterile phosphate buffered saline (PBS), and then labeled with 125I using the iodogen method (5). In brief, 109 bacilli suspended in 50 μ1 of PBS were added to a tube coated with 100 μgof iodogen. Carrier-free Na 125I (0.5 mCi; Amersham, U.K.) was then added and the mixture kept on ice for 10 min with intermittent shaking. The reaction was stopped by adding 5 mM potassium iodide (KI) in PBS, and the bacilli were then washed three times with PBS-KI and three times with PBS to remove nonspecifically bound iodine and KI. Radioactivity associated with the cell pellet was recorded; it was found to be in the range 0.5 to 1 x 107 counts per minute (cpm).
The labeled bacilli were sonicated and the soluble extract prepared was analyzed in SDS-PAGE in 10% separating gel and 4.5% stacking gel using the Laemmli buffer system (7). The dried gels were exposed to X-ray plates and autoradiographs developed after 24 to 48 hr of exposure. Two bands of radiolabeled proteins were observed as shown in Figure 1. These proteins did not enter the separating gel; instead, one band was observed at the interphase between stacking and separating gel, and the other at the top of the stacking gel, indicating a very high molecular weight of these proteins. Identical results were obtained under reducing as well as nonreducing conditions (Fig. 1) and even in 5%-20% gradient gels (data not shown).
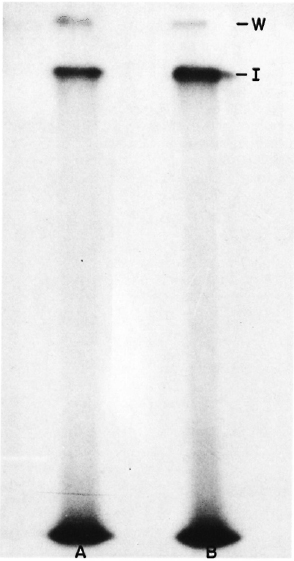
Fig. 1. Autoradiograph of SDS-PAGE gels including A unreduced and B reduced sonicate of 125I-labeled ICRC bacilli. W = position of the wells; I = position of the interphase between the stacking and the separating gel.
Simultaneously, PP-I purified from the sonicate of unlabeled ICRC bacilli as described earlier was also analyzed in SDS-PAGE. Two high molecular weight bands as described above were also observed in the Coomassie blue stained gel (Fig. 2).
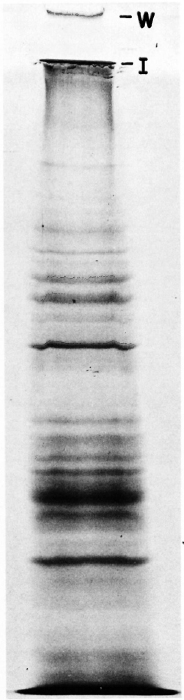
Fig. 2. Coomassie blue-stained gel showing SDS-PAGE analysis for sonicate of unlabeled ICRC bacilli. W = position of the well; I = interphase between stacking and separating gel.
The high molecular weight of the surface labeled proteins was confirmed by gel filtration. The sonicate of labeled bacilli was fractionated on a Sephacryl S-300 gel permeation column. The major radioactive peak obtained was in the void volume of the column (Fig. 3) and contained the high molecular weight proteins mentioned above. A similar pattern was obtained with PP-I (data not shown). Exclusion from Sephacryl S-300 indicates a molecular weight greater than 1.5 x 106 daltons. These results indicate that proteins from the PP-I preparation are located at the surface of the bacilli.
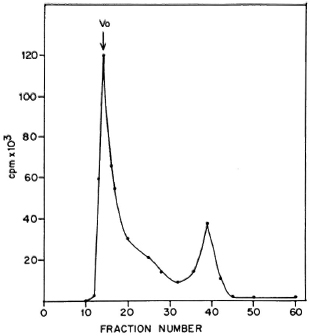
Fig. 3. Fractionation of sonicate of 125I-labelcd ICRC bacilli on Scphacryl S-300. Vo = void volume.
Another objective of the work undertaken was to radiolabel M. leprae for studies on the processing and presentation of its antigens. Since M. leprae has not yet been cultivated, internal or metabolic labeling of its proteins cannot be carried out. The technique of iodinating whole bacilli, if successful, would be appropriate for such studies since it offers two advantages. It restricts the studies only to the surface proteins, which have been shown to be the major immunodominant antigens of M. leprae (10).
In addition, studies using these whole, labeled bacilli simulate a natural infection in which proteins are encountered by cells, not in soluble form but in intact organisms.
M. leprae obtained from biopsies of leprosy patients as well as from a lepromin preparation could be successfully labeled with 125I using the method described above. SDS-PAGE analysis of the sonicate of these bacilli also indicated high molecular weight bands similar to those in the ICRC bacilli (Fig. 4 a and b). Our finding of high molecular weight proteins located at the surface of M. leprae agrees with the results reported from Dr. Brennan's laboratory. Similar proteins have been demonstrated by them in a peptidoglycan-protein component prepared from cell walls of M. leprae (6,9).
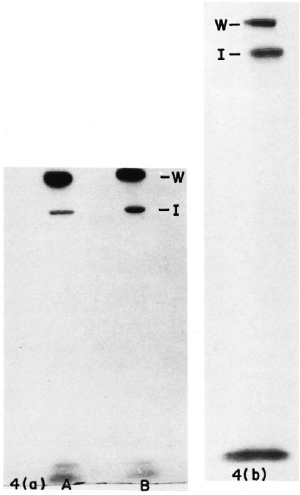
Fig. 4. Autoradiograph of SDS-PAGE gels including sonicates of 125I-labeled (a) unreduced M. leprae (A) and reduced M. leprae (B); (b) reduced lepromin. W = position of wells; I = interphase between stacking and separating gel.
Since the M, leprae used in these studies were obtained from human tissue and the ICRC bacilli were cultured in a medium containing human serum, we wanted to rule out the possibility that the high molecular weight radioiodinated proteins were components of the human serum or tissue. Therefore, sonicates prepared from radiolabeled ICRC bacilli and M. leprae were immunoprecipitated as described by Richard, et al. (11), with a few modifications. The sonicates were allowed to react with rabbit antisera against ICRC bacilli and M. leprae at 37ºC for 2 hr. The antigen antibody complexes formed were allowed to react with Protein A coupled to Sepharose CL-4B beads (Sigma Chemical Co., St. Louis, Missouri, U.S.A.) at 4º for 1 hr, and the beads were then washed three times with PBS. The immunoprecipitated proteins were then eluted by boiling in sample buffer and subjected to SDS-PAGE followed by autoradiography. Both the bands observed for the sonicate in earlier studies were found to be precipitated (data not shown). This indicated that the proteins in the sonicates are derived from the bacilli and ruled out that they were due to contamination with human serum or tissue components.
The technique of surface iodination of whole cells has been widely used for eu-karyotic cells and has also been applied to microorganisms, such as Pseudomonas (8) and Trypanosoma (13) species. There is one report of surface iodination of M. smeg-matis (2) but, to the best of our knowledge, it has not been applied to M. leprae and hence is reported here. This would be important in experiments aimed at studying the intracellular processing of surface proteins of M. leprae and other mycobacteria.
Leprosy presents a spectrum of cell-mediated immune responses against M. leprae, ranging from hypersensitivity in the tuberculoid form to unresponsiveness in lepro-matous leprosy. Several possibilities have been suggested to explain these responses (12), e.g., processing of M. leprae antigens by macrophages may differ in different individuals leading to inappropriate antigen presentation. Surface labeled M. leprae offers a very good tool for exploring these possibilities using macrophages and Schwann cells.
- Neelima J. Deuskar, Ph.D.
Research Officer
- Madhav G. Deo, M.D., Ph.D., F.A.M.S., F.N.A.
Research Director
Cancer Research Institute
Tata Memorial Centre
Bombay 400012, India
REFERENCES
1. Bhatki, W. S., Chullawala, R. G., Chaturvedi, R. M, Dixit, G. M. and Deo, M. G. Lepromin conversion induced by a subunit vaccine from ICRC bacilli. Indian J. Med. Res. 87 (1988) 545554.
2. Caldwell, H. D. and Buchanan, T, M. Immunochemical and structural integrity of surface protein antigens of mycobacteria during separation from armadillo liver tissue. Int. J. Lepr. 47 (1979) 469-476.
3. Chirmule, N. B., Mulhekkak, R. and Deo, M. G. Antigenic profile of ICRC bacilli with special reference to isolation of immunogenic subunit. Int. Arch. Allergy Appl. Immunol. 86 (1988) 19-27.
4. Deo, M. G., Bapat, C. V., Bhalerao, V., Chaturvedi, R. M., Bhatki, W. S. and Chullawala, R. G. Antileprosy potentials of the ICRC vaccine. A study in patients and healthy volunteers. Int. J. Lepr. 51 (1983) 540-549.
5. Fraker, P. J. and Speck, J. C, Jr. Protein and cell membrane iodinations with a sparingly soluble chloroamide, l,3,4,6-tetrachloro-3a,6a-di-phenylglycoluril. Biochem. Biophys. Res. Commun. 80 (1978) 849-857.
6. Hunter, S. W., McNeil, M., Modlin, R. L., Meh-ra, V., Bloom, B. R. and Brennan, P. J. Isolation and characterization of the highly immunogenic cell wall-associated proteins of Mycobacterium leprae. J. Immunol. 142 (1989) 2864-2872.
7. Laemli, U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227 (1970) 680-685.
8. Lambert, P. A. and Booth, B. R. Exposure of outer membrane proteins on the surface of f'seu-domonas aeruginosa PA01 revealed by labelling with (125I) lactoperoxidase. FEBS Lett. 14 (1982) 43-45.
9. Melancon-Kaplan, J., Hunter, S. W., McNeil, M., Stewart, C, Modlin, R. L., Rea, T. H., Convit, J., Salgame, P., Mehra, V., Bloom, B. R. and Brennan, P. J. Immunological significance of Mycobacterium leprae cell walls. Proc. Natl. Acad. Sci. U.S.A. 85 (1988) 1917-1921.
10. Mutis, T., van Schooten, W. C. A. and de Vries, R. R. P. A peptidoglycan protein complex puri-
fied from M. leprae cell walls contains most or all immunodominant M. leprae T-cell antigens. Int. J. Lepr. 57 (1989) 788-793.
11. Richard, J., Pink, L. and Ziegler, A. Radiola-beling and immunoprecipitation of cell surface macromolccules. In: Immunological Methods. Lefkovits, I. and Pernis, 15., eds. New York: Academic Press, 1979, pp. 169-179.
12. Watson, J. D. Prospects for new generation vaccines for leprosy: progress, barriers and future strategics. Int. J. Lepr. 57 (1989) 834-842.
13. Zingales, B., Andrews, N. W., Kuwajima, V. Y. and Colli, W. Cell surface antigens of Trypanosoma cruzi: possible correlation with the intcrior-ization process in mammalian cells. Mol. Bio-chem. Parasitol. 6 (1982) 111 - 124.