- Volume 59 , Number 3
- Page: 487–92
Effect of circulating immune complexes of leprosy patients on leukocyte migration inhibition induced by Mycobacterium leprae antigens in healthy volunteers
To the Editor:
Elevated levels of circulating immune complexes (CICs) have been demonstrated in leprosy sera, particularly in patients with borderline lepromatous (BL) and lepromatous (LL)leprosy and in those with erythema nodosum leprosum (ENL) (4,7,16). Recent studies have shown that these CICs have efficient complement activating ability (18,23). Further, it appears that as the disease moves toward the LL pole, the level of CIC increases while the cell-mediated immune (CMI) response against Mycobacterium leprae diminishes (14,22). Whether these elevated CIC levels play any role in suppressing CMI function in leprosy patients is, however, not clear. Leukocyte migration inhibition (LMI) is known to be induced by the production of migration inhibition factor (MIF) by sensitized lymphocytes in the presence of sensitizing antigens (6,14). In the present study, the effect of CIC isolated from leprosy patients was studied on the LMI induced by M. leprae antigens in healthy responders.
CICs were isolated by the polyethylene glycol (2.5% PEG 6000) precipitation method of Creighton, et al. (5) from the sera of 36 leprosy patients consisting of 8 tuberculoid (TT) or borderline tuberculoid (BT) (TT/BT), 8 BT with reaction (BTR), 10 BL or LL (BL/LL) and 10 ENL cases attending the Central JALMA Institute for Leprosy, Agra, India. They were classified according to the Ridley-Jopling scale (17). The control group consisted of 10 healthy laboratory volunteers.
For the LMI test, mononuclear cells (MNC) were taken from the healthy volunteers who had been in constant exposure to leprosy patients for many years and were known to mount a strong CMI response as determined by delayed-type hypersensitivity skin tests, MIF production, and lymphocyte transformation against M. leprae antigens. Prior to the study of the effect of PEG precipitates on the LMI induced by M. leprae antigens, the total protein content of the precipitates was estimated by Lowry's method (10), and the levels of mycobacterial CICs of the IgG and IgM types were determined by an ELISA developed by us (23). The results obtained showed that the PEG precipitates from the BL/LL and ENL groups contained significantly higher levels of protein than those of the normal human sera (NHS) group. The differences in the mean values among the diseased groups, except those of the BTR, were not found to be significant (The Table).
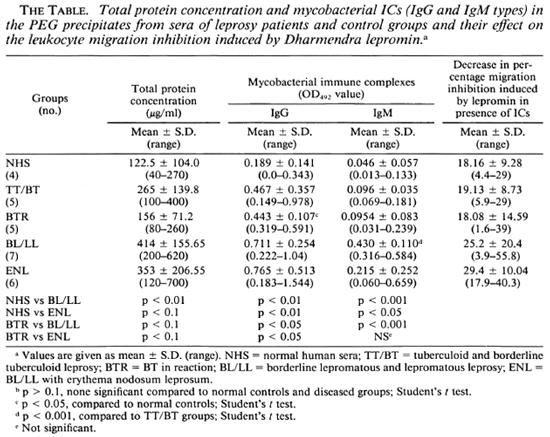
The levels of mycobacterial CICs in the BTR, BL/LL, and ENL groups were found to contain significantly higher levels of the IgG class of mycobacterial CICs than those of the NHS and TT/BT groups. Further PEG precipitates from BL/LL and ENL were also found to have significantly higher levels of IgG ICs than those of the BTR group (The Table). PEG precipitates from BL/LL were also found to have higher levels of the IgM class of ICs than those of the TT/BT and BTR groups (The Table).
PEG precipitates from NHS, TT/BT, and BL/LL groups were tested for their effect on the viability of MNC at various concentrations (0.1 ml, 0.2 ml, 0.3 ml, and 0.4 ml), and were found to have no toxic effect (except the PEG precipitates from BL/LL at 0.4 ml concentration). The viability of the cells was more than 98% (Fig. 2). In a similar experiment, M. leprae antigen (Dhar-mendra lepromin) was tested for its effect on the viability of MNC at various concentrations (106 to 108 bacilli/100 No toxic effect on the MNC except at the concentration of > 108 bacilli could be noted.
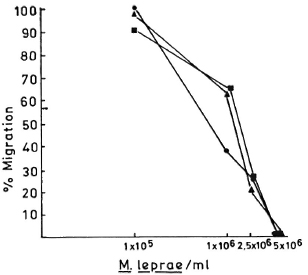
Fig. 1. Dose-dependent effect of M. leprae antigens on the leukocyte migration inhibition in three healthy responding volunteers.
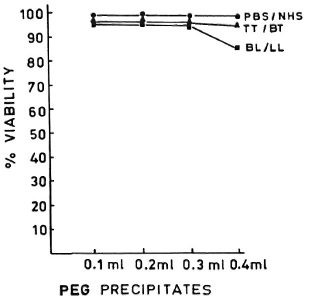
Fig. 2. Dose-dependent effect of PEG precipitates from PBS/NHS ( ), TT/LL (
), TT/LL ( ) and BL/LL (
) and BL/LL (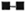 ) on the viability of mononuclear cells. Each dot = mean value of three experiments.
) on the viability of mononuclear cells. Each dot = mean value of three experiments.
In order to determine the optimal dose of PEG precipitates to study their effect on LMI, a dose-dependent experiment to observe the effect of PEG precipitates from NHS and BL/LL groups was carried out using the following concentrations of PEG precipitates: 0.025 ml, 0.05 ml, 0.1 ml, and 0.2 ml. The results showed (Fig. 3) that PEG precipitate from BL/LL at a high concentration (0.2 ml = 72.6 μg of protein) was found to induce moderate migration inhibition (% migration 82 ± 17.17, p < 0.05); at a low concentration (0.025 ml = 9.06 μg protein) no effect was noted on the LMI test. Since 0.025 ml (9.06 μg) protein of PEG precipitate did not show any effect on the leukocyte migration, this concentration of the precipitates obtained from the various groups was used in our subsequent study to find out their effect on the LMI induced by M. leprae antigen. From The Table it can be seen that PEG precipitates from the normal control and the diseased groups had no significant effect on the LMI induced by M. leprae antigen (106 bacilli) [1 x 106 bacilli of Dharmendra lepromin were found to be the optimal dose for the induction of significant migration inhibition (MI)]. In healthy responders, the mean percent MI value induced by 106 M. leprae was found to be 58.53 ± 12.43 (Fig. 1).
Fig. 3. Dose-dependent effect of PEG precipitates from NHS ( ) and BL/LL (
) and BL/LL ( ) on the LMI test in healthy responding volunteers. Each dot = mean % migration value of seven experiments; vertical bars = standard deviations. Difference between the mean value of NHS and BL/LL is statistically significant (p < 0.05).
) on the LMI test in healthy responding volunteers. Each dot = mean % migration value of seven experiments; vertical bars = standard deviations. Difference between the mean value of NHS and BL/LL is statistically significant (p < 0.05).
In a separate experiment in which leukocytes were pretreated with various concentrations (0.1 ml, 0.2 ml, and 0.3 ml) of PEG precipitates obtained from NHS and BL/LL groups, it was found that these precipitates had no significant effect on the LMI induced by M. leprae antigens (Fig. 4).
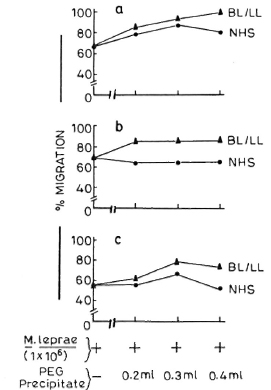
Fig. 4. Effect of pretreatment of MNC (5 x 107 cells/0.5 ml) with various doses of PEG precipitates (0.2 ml, 0.3 ml, and 0.4 ml) from NHS ( ) and BL/ LL (
) and BL/ LL ( ) on the LMI induced by M. leprae antigens. Sections a, b, and c = three different healthy responding individuals.
) on the LMI induced by M. leprae antigens. Sections a, b, and c = three different healthy responding individuals.
The specific CMI responses against M. leprae antigens in BL/LL and ENL patients have been found to be reduced to a great extent, and in some it is almost completely absent (8,11,12). However, the effect of CIC on the CMI response in leprosy patients is not known. There are reports in which ICs have been shown to act as immunomodulators in the CMI response (13,21). Furthermore, soluble ICs (2,3), aggregated IgG (9), and IgG-coated sheep red blood cells (1) have been reported to stimulate production of lymphokine (IL-1 or IL-1 -like factors) in murine or human monocytes and macrophages (1). Our observation showed a moderate induction of migration inhibition of leukocytes by PEG precipitates obtained from the BL/LL groups at a high concentration only. This might be due to the presence of a significant quantity of mycobacterial antigens in the CIC (4,16) which have induced the production of lymphokine.
PEG precipitates at a lower concentration (0.025 ml) obtained from various leprosy types were found to exert no effect on the LMI induced by M. leprae antigens. At this juncture, no explanation could be offered for this observation because at this low concentration of PEG precipitates the mycobacterial antigens and antimycobactcrial antibodies are at such a low concentration that it is probably unlikely that they would exert an effect on the LMI.
PEG precipitates obtained from the diseased groups were found to exert no significant suppressive effect on the LMI induced by M, leprae antigen. The LMI of 18.16% ± 9.28 induced by NHS PEG precipitates is not significant (The Table). This insignificant suppression may be due to the background level of antimycobacterial antibodies present in the IC of these individuals.
It has been shown that the Fc fragment of IC can induce lymphocyte blast transformation and polyclonal antibody synthesis in murine B lymphocytes (2,3). The immunochemical analysis of CIC isolated from the PEG precipitates of serum samples of patients has been shown to contain IgG, IgM, and IgA (4,16) and, hence, is very rich in Fc contents. It is possible that CIC isolated from leprosy sera may have been in a solubilizcd form; hence the Fc portions were incapable of binding to the Fc receptors of the cells.
Takahashi, et al. (20) have shown that while solubilized ICs retain a little capacity to activate the complement system, they lose their ability to bind to the Fc receptors of various blood cells such as leukocytes, monocytes, macrophages, and certain cells of the B-lymphocyte series (15,19,20).
From these observations it may be concluded that CICs in leprosy patients probably have no impairing effect on the production of MIF induced by M. leprae antigens. Further studies are, however, needed to determine the role of CICs on the other functional parameters of CMI.
- Padmawati Tyagi, M.Sc.
Bhawneshwar K. Girdhar, M.D.
Kiran Katoch, M.D.
Utpal Sengupta, Ph.D.
Central JALMA Institute for Leprosy
Taj Ganj, Agra 282001, India
- Vadakkuppattu D. Ramanathan, Ph.D.
Tuberculosis Research Centre
Chetput, Madras, India
REFERENCES
1. Arend, W. P., Joslin, F. G. and Massoni, J. Effect of immune complexes on production by human monocytes of interlcukin 1 or an interleukin 1 inhibitor. J. Immunol. 134(1985)3868-3875.
2. Berman, M. A., Spiegelberg, H. L. and Weigle, W. O. Lymphocyte stimulation with Fc fragments. I. Class, subclass, and domain of active fragments. J. Immunol. 122(1979)89-96.
3. Berman, M. A. and Weigle, W. O. B-lymphocyte activation by the Fc region of IgG. J. Exp. Med. 146(1977)241-256.
4. Chakrabarty, A. K., Maire, M, Saha, K. and Lambert, P. H. Identification of components of IC purified from human sera. II. Demonstration of mycobacterial antigens in immune complexes isolated from sera of lepromatous patients. Clin. Exp. Immunol. 51(1983)225-231.
5. Creighton, W. D., Lambert, P. H. and Miescher P. A. Detection of antibodies and soluble antigen complexes by precipitation with polyethylene glycol. J. Immunol. 111(1973)1219-1227.
6. Federlin, K., Maini, R. N., Russel, A. S. and Dumonde, D. C. A micro-method for peripheral leucocyte migration in tuberculin sensitivity. J. Clin. Pathol. 24(1971)533-541.
7. Furukawa, F., Yoshida, H., Sekita, K., Ozaki, M., Imamura, S. and Hamashima, Y. Different mode of circulating immune complexes and anti-ssDNA antibodies in sera of lepromatous leprosy and systemic lupus erythematosus. Lepr. Rev. 55(1984)291-299.
8. Godal, T., Myklestad, B., Samuel, D. R. and Myrvang, B. Characterization of the cellular immune defect in lepromatous leprosy: a specific lack of circulating Mycobacterium leprae reactive lymphocytes. Clin. Exp. Immunol. 9(1971)821-831.
9. Haakenstad, A. O. and Manik, M. The biology of immune complexes. In: Autoimmunity; Genetic, Immunologic, Virologic and Clinical Aspects. Talal, N., ed. New York: Academic Press, 1977, pp. 277-298.
10. Lowry, O. H., Rosebrough, N. J., Farr. L. A. and Randall, R. j. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193(1951)265-275.
11. Mehra, V., Mason, L. H., Fields, J. P. and Bloom, B. R. Lepromin-induced suppressor cells in patients with leprosy. J. Immunol. 123(1979)1813-1817.
12. Mendes, E., Raphael, A., Mota, N. G. S. and Mendes, N. F. Cell-mediated immunity in leprosy and transfer of delayed hypersensitivity reactions. J. Allergy Clin. Immunol. 53(1974)223-229.
13. Morgan. E. L. and Weigle, W. O. Biological activities residing in the Fc region of immunoglobulin. Adv. Immunol. 40(1987)61-134(457 ref.).
14. Myrvang, B., Godal, T., Ridley, D. S., Fro-land, S. S. and Song, Y. K. Immune responsiveness to Mycobacterium leprae and other mycobacterial antigens throughout the clinical and histopathological spectrum of leprosy. Clin. Exp. Immunol. 14(1973)541-553.
15. Nussenzweig. V. Receptors for immune complexes on lymphocytes. Adv. Immunol. 19(1974)217-258.
16. Ramanathan, V. D., Parkash, O., Ramu, G., Parker, D., Curtis, J., Sengupta, U. and Turk, J. L. Isolation and analysis of circulating immune complexes in leprosy, Clin. Immunol. Immuno-pathol. 32(1984)261-268.
17. Ridley, D. S. and Jopling, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
18. Saha, K., Sharma, V., Chakrabarty, A. K.. and Sehgal, V. N. Breakdown of factor B as an index of complement activation in lepromatous leprosy and its relation with bacillary load. Scand. J. Immunol. 17(1983)37-43.
19. Scharfstein, J., Correa, E. B., Gallo, G. R. and Nussenzweig, V. Human C4-binding protein. Association with immune complexes in vitro and in vivo. J. Clin. Invest. 63(1979)437-442.
20. Takahashi, M., Takahashi, S. and Hirose, S. Solubilization of antigen-antibody complexes: a new function of complement as a regulator of immune reactions. Prog. Allergy 27(1980)134-166(47 ref.).
21. Theofilopoulos, A. N. and Dixon, F. J. The biology and detection of immune complexes. Adv. Immunol. 28(1979)89-220(978 ref.).
22. Touw Langenduk, E. J. M., Warndorf Van Diepen, T., Harboe, M. and Belehu, A. Relation between anti-Mycobaclerium leprae antibody activity and clinical features in borderline tuberculoid leprosy. Int. J. Lepr. 51(1983)305-311.
23. Tyagi, P., Ramanatiian, V. D., Girdhar, B. K., Katoch, K., Bhatia, A. S. and Sengupta, U. Activation of complement by circulating immune complexes isolated from leprosy patients. Int. J. Lepr. 58(1990)31-38.