- Volume 60 , Number 3
- Page: 335–9
Protective effect of intradermal BCG against leprosy; a case-control study in central Brazil
ABSTRACT
A case-control study was undertaken to evaluate the protective efficacy of intradermal BCG against leprosy in a high-endemic area of leprosy in central Brazil. Sixty-two cases and 186 controls were included in the study. Cases were all newly diagnosed leprosy patients under 16 years of age attending an outpatient health service, and all of them were schoolchildren. Three controls under 16 years old, frequency matched by sex and age group, were selected f rom schools geographically located in the area f rom which the cases came. The presence of BCG was negatively associated with leprosy, indicating a 5.3 risk of leprosy for those nonvaccinatcd and protective efficacy of 81%. Paucibacillary patients were more likely to have a BCG scar than multibacillary patientsRÉSUMÉ
Une étude cas-témoins a été entreprise pour évaluer l'efficacité protectrice du BCG intradermique vis-à-vis de la lèpre dans une région à haute endémicité lépreuse dans le centre du Brésil. Soixante-deux cas et 186 témoins ont été inclus dans l'étude. Les cas étaient tous des patients lépreux nouvellement diagnostiqués, âgés de moins de 16 ans et fréquentant un service sanitaire externe, et tous allaient à l'école. Trois témoins de moins de 16 ans appariés pour le sexe et le groupe d'âge ont été sélectionnés à partir d'écoles localisées géogra-PHIQUEMENT dans la région d'où provenaient les cas. La présence du BCG était associée négativement à la lèpre, indiquant un risque de lèpre pour ceux non vaccinés de 5.3 et une efficacité protectrice de 81%. Les patients paucibacillaires avaient plus de chances d'avoir une cicatrice de BCG que les patients multibacillaires.RESUMEN
Se efectuó un estudio de control de casos para evaluar la eficacia de la aplicación intradérmica de BCG contra la lepra en un área de alta endemia en la región central de Brasil. En el estudio se incluyeron 62 pacientes y 186 controles. Todos los enfermos fueron casos nuevos, escolares de menos de 16 años. Por cada caso, se seleccionaron 3 controles, aparejados por edad y sexo, provinientes de escuelas localizadas en el área de residencia de los pacientes. Se encontró que la presencia de BCG estuvo negativamente asociada con la lepra, indicando un riesgo de lepra de 5.3 para aquellos no vacunados y una eficacia protectora del 81%. Los pacientes paucibacilares fueron más propensos a desarrollar la cicatriz por BCG que los pacientes multibacilares.Brazil is a high-endemic area for leprosy, and it is one of the few countries in Latin America where the leprosy endemic is increasing (7). During the last two decades, an overall prevalence rate of leprosy of 1.8 per 1000 and a detection rate of 17/100,000 with a steady annual increase of 6% was reported by the Brazilian Ministry of Health (10). In 1990, there were more than 270,000 leprosy cases registered in the official control program, representing approximately 85% of the total patients in South America (Ministry of Health, 1990, unpublished data).
Intradermal BCG vaccination against tuberculosis was introduced in the 1970s (9). However, the use of BCG against leprosy as a public health measure has a more recent history, although the possible role of this vaccine in leprosy control was suggested by Fernandez in 1939 (3).
Several well-designed, randomized, controlled trials have been performed to assess the protective efficacy of BCG against leprosy in different regions of the world (1,8,20.21) Although different results were found, there seems to be strong evidence indicating that BCG can offer highly effective protection against leprosy, which is equal to or greater than its protection against tuberculosis (4, 5).
The effect of BCG in a leprosy control program lacks an evaluation. Recent reviews emphasize the necessity of evaluating the effectiveness of BCG vaccination in leprosy control, and propose the case-control method as the appropriate design in these circumstances (17, 18).
Case-control studies to assess the efficacy of BCG against leprosy should be conducted in areas of high prevalence of leprosy and intermediate vaccine coverage, such as the Center-West region of Brazil with 3.2/1000 leprosy prevalence and BCG coverage of 60% to 80%. The efficacy of intradermal BCG in the prevention of leprosy was evaluated in this paper based on a case-control design. Differences in protection between the paucibacillary (PB) and multibacillary (MB) types of the disease were also assessed.
METHODS
Study area
The study was carried out in Goiânia city, Center-West region for the Brazilian official leprosy control program. Data from this region showed prevalence of 3.4/1000 and almost a threefold increase in the detection rates from 1969 (6/100,000) to 1987 (17/100,000), being considered an area with high and increasing prevalence and detection rates of leprosy. Case detection usually occurs by self-reporting in outpatient clinics of the public health sector, and no survey for detection of leprosy has been conducted in this community recently.
Goiânia has a traditional dermatologic outpatient health service, Centro de Saúde Juarez Barbosa, with more than 4000 patients under treatment, representing about 90% of all registered patients in this city. More than 800 new leprosy cases were diagnosed in this health center during 1990 (16).
Intradermal BCG (Morcau strain) has been included in the national immunization program against tuberculosis since 1973. BCG coverage varied from approximately 60% to 80% among children under 1 year of age. Although intradermal BCG has been recommended by the leprosy control program to all household contacts of lepromatous and borderline patients (9), this policy has not been implemented.
Study population
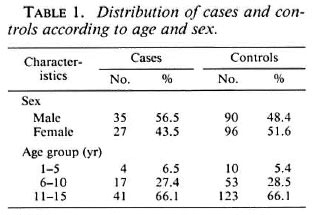
Selection of cases. Cases were selected from the outpatient clinic of Centro de Saúde Juarez Barbosa in the period between August 1989 and August 1990. All newly diagnosed leprosy patients under 16 years of age were included in this study and all of them were schoolchildren. This age group was chosen taking into consideration that intradermal BCG was implemented as a health policy only after 1975. These cases were examined by two dermatologists and were classified according to the operational criteria denned by the Brazilian Ministry of Health (10) as indeterminate (I), tuberculoid (T), borderline (B) and lepromatous (L). Histological and bacilloscopy smear tests were performed in all patients. All indeterminate cases were given lepromin skin tests to assess cellular immunity.
Selection of controls. Controls were selected from among schoolchildren from schools geographically located in the areas from which the cases came. Three controls under 16 years old, frequency matched by sex and age groups (1-5, 6-10 and 11-15 years), were selected for each case. All children were clinically examined at the school by the same dermatologists to exclude any clinical signs of leprosy. The selection of controls in the same geographical area assured a similar socioeconomic background between the cases and the controls. Sixtytwo cases and 186 controls were included in the study. This sample size had a 95% power to detect a protection equal to or greater than 35% associated with BCG vaccination at a 5% level of statistical significance assuming a 50% BCG coverage among controls.
BCG vaccination. Exposure to intradermal BCG for cases and controls was evaluated by looking for the typical scar of BCG over the right deltoid region and, when possible, it was confirmed by a vaccination card.
Statistical analysis. Data were analyzed using the Statistical Package for Social Sciences (SPSS/PC+) 1987. Chi-squared tests were calculated to measure the difference between proportions, and p values (two tailed) < 0.05 were considered statistically significant. The odds ratio (OR) and 95% confidence intervals (CI) were calculated to assess the protection of BCG against leprosy. Vaccine efficacy (VE) was calculated using the formula VE = 1 - Relative Risk (14). The odds ratio was used as the estimation of the relative risk, considering that leprosy is a relatively rare disease.
For analysis, clinical forms of leprosy with similar impaired cell-mediated immunity were considered together. Lepromatous, borderline, and indeterminate leprominnegative patients were pooled and referred to as MB cases. Tuberculoid and indeterminate lepromin-positive patients were also pooled and referred to as PB cases.
RESULTS
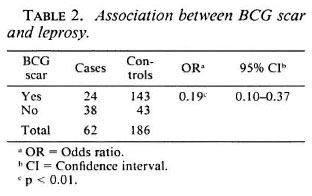
The distribution of leprosy cases and controls according to age group and sex is shown in Table 1; 37 (59.7%) cases were classified as MB and 25 (40.3%) as PB. These two groups had approximately the same sex distribution (56.5% were male). The mean age was 11.4 (S.D. 3.1) for the MB and 10.9 (S.D. 3.4) for the PB groups (t test, p >0.05). The presence of a BCG scar was negatively associated with leprosy, with an oddsratio of 0.19 (95% CI 0.10-0.37), indicating a 5.3 increased risk of leprosy for those nonvaccinated and protective vaccine efficacy of 81% (1-0.19) with 95% CI limits from 63% to 90% (Table 2).
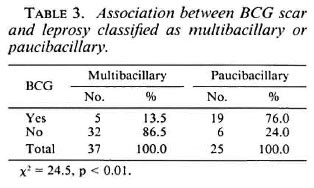
PB patients were more likely to have BCG scars (19/25) than were MB patients (5/37), a statistically significant difference (χ2 24.5, p < 0.01) (Table 3).
DISCUSSION
Recently, new vaccines against leprosy employing two microorganisms (BCG plus killed Mycobacterium leprae) are being assessed by large clinical trials in Venezuela, Malawi, and India (2, 6, 13, l5). However, there are practical arguments in favor of using alternative designs to evaluate vaccine efficacy, since BCG has already been part of a routine immunization program against tuberculosis for many decades, and it may be considered unethical to have a placebo group in a vaccine trial. Furthermore, the progressive urbanization and the intensive internal migration in underdeveloped countries, particularly where leprosy is endemic, are also important limitations for a long follow-up study. In Brazil, BCG against leprosy within the routine program needs to be evaluated before considering new vaccines for public health policy.
The most obvious advantages of a case-control approach to evaluate the efficacy of BCG in routine programs is that case-control studies are relatively quick and cheap to conduct and, more important, they can address the question ofefficacy in the actual vaccination program in a specific region and population, and not in a planned experimental situation, as in clinical trial.
Our findings suggested that BCG offered at least a 63% protection against leprosy. This result is similar to the case-control study in Malawi that estimated 50% BCG protection against leprosy, although in that study cases and controls were not selected in a field situation but were from the Lepra Evaluation Project data set based on a total population survey (5).
We are aware of the difficulties in making a highly specific diagnosis of leprosy. The eventual inclusion of nondiseased subjects as cases could have the effect of underestimating the BCG efficacy.
BCG protection against leprosy was assessed comparing the different clinical forms of leprosy and considering the degrees of clinical severity and cellular immunity between the two groups of patients. We have found that a BCG scar was seen in 76% of the PB cases and in only 13.5% of the MB cases. No cases with a positive smear presented a BCG scar. These results suggest that the protective effect of BCG vaccination varies according to the different clinical forms of leprosy, and that a higher BCG coverage in the population might decrease the proportion of MB forms. In fact, when different forms of leprosy were considered as in a recent study conducted in India, BCG vaccination was found to be a "risk factor" for indeterminate forms and to have a protective effect for the tuberculoid and borderline types of leprosy (12).
It seems worth mentioning that in casecontrol studies finding a proper control group for comparison may present some difficulties. Socioeconomic conditions are a potentially important confounder related to both exposure (vaccine coverage) and outcome (incidence of leprosy) (19). Considering that all leprosy cases were schoolchildren, the selection of controls from schools geographically related to each case led to the same degree of socioeconomic matching. Nevertheless, this strategy may not be sufficient to balance the difference between cases and controls, particularly when the main purpose of the study is to measure vaccine efficacy.
In Brazil more than 50% of the incident cases are classified as LL or B types, almost 10% of those leprosy cases are under 15 years of age, and low coverage of BCG vaccinations are still observed in the country as a whole. This seems to be a good scenario for epidemiological Research to test a possible shift of types of leprosy by increasing BCG vaccine coverage.
REFERENCES
1. BAGSHAWE, A., SCOTT, G. C, RUSSELL, D. A., WIG-LEY, S. C, MERIANOS. A. and BERRY, G. BCG vaccination in leprosy: final results of the trial in Karimui, Papua New Guinea, 1963-79. Bull. WHO 67(1989)389-399.
2. CONVIT, J., ARANZAZU, N., ULRICH, M., ZUNIGA, M., ARAGON, M. E., ALVARADO, J. and REYES, O. Investigations related to the development of a leprosy vaccine. Int. J. Lepr. 51(1983)531-539.
3. FERNANDEZ, J. M. M. Comparative study of the Mitsuda reaction with tuberculin reaction. Rev. Argent. Dcrmatosifil. 23(1939)425-453.
4. FINE, P. E. M. BCG vaccination against tuberculosis and leprosy. Br. Med. Bull. 44( 1988)691,703(51 réf.).
5. FINE, P. E. M., PONNIGHAUS, J. M., MAINE, N., CLARKSON, J. A. and BLISS, L. Protective efficacy of BCG against leprosy in Northern Malawi. Lancet 2(1986)499-502.
6. GANAPATI, R., REVANKAR, C. R., LOCKWOOD, D. N. J., WILSON, R. C, PRICE, J. E., ASHTON, P., ASHTON, L. A., HOLMES, R. M., BENNETT, C, STANFORD, J. L. and REES, R. J. W. A pilot study of three potential vaccines for leprosy in Bombay. Int. J. Lepr. 57(1989)33-37.
7. LOMBARDI, C. Evaluation of leprosy epidemiology in 12 countries of the Americas, 1980-1983. Bull. PAHO 23(1989)284-294.
8. LWIN, K.., SUNDARESAN, T., GYI, M. M., BECHELLI, L. M., TAMONDONG, C., GARUAJOSA, P. G., SANSARRICQ, H. and NOORDEEN, S. K. BCG vaccination of children against leprosy: fourteen year findings of the trial in Burma. Bull. WHO 63(1985)1069-1078.
9. MINISTÉRIO DA SAÚDE, SECRETARIA NACIONAL DE PROGRAMAS ESPECIAIS DE SAÚDE. DIVISÃO DE PNEUMOLOGIA SANITARIA. Controle da tuberculose: uma proposta de integração ensino-serviço. Rio de Janeiro: DNPS/NUTES, 1987.
10. MINISTÉRIO DA SAÚDE, SECRETARIA NACIONAL DE PROGRAMAS ESPECIAIS DE SAÚDE. DIVISÃO DE DERMATOLOGIA SANITARIA. Portaria no. 1. Diário Oficial, 3-1-1990.
11. MOTA, C. P. and ZUNIGA, M. G. Time trends of Hansen's disease in Brazil. Int. J. Lepr. 58(1990)453-461.
12. MULIYIL, J., NELSON, K. E. and DIAMOND, E. L. Effect of BCG on the risk of leprosy in an endemic area. A case control study. Int. J. Lepr. 59(1991)233-248.
13. NOORDEEN, S. K. The present status of leprosy vaccine development. Southeast Asian J. Trop. Med. Pub. Health 19(1988)525-534.
14. ORENSTEIN, W. A., BERNIER, R. H. and HINMAN, A. R. Assessing vaccine efficacy in the field; further observations. Epidemiol. Rev. 10(1988)212-241.
15. PONNIGHAUS, J. M. and FINE, P. E. M. Sensitization studies with potential leprosy vaccine preparations in northern Malawi. Int. J. Lepr. 54(1986 )25-37.
16. QUARESMA, M. E. P., RODARTE, A. R., FERREIRA, M. G. and SILVA, M. T. P. Estudo epidemiológico da hanscniasc, com base na casuística da Rede Pública cm Goiánia-Goiás. Rev. Patolog. Trop. 18(1989)81-97.
17. SMITH, P. Evaluating interventions against tropical diseases. Int. J. Epidemiol. 16(1987)159-166.
18. SMITH, P. G. Epidemiological methods to evaluate vaccine efficacy. Br. Med. Bull. 44(1988)679 -690.
19. SMITH, P. G., RODRIGUES, L. C. and FINE, P. E. M. Assessment of the protective efficacy of vaccines against common diseases, using case-control and cohort studies. Int. J. Epidemiol. 13(1984)87-93.
20. STANLEY, S. J., HOWLAND, C, STONE, M. M. and SUTHERLAND, I. BCG vaccination of children against leprosy in Uganda: final results. J. Hyg. Camb. 87(1981)233-248.
21. TRIPATHY, S. P. BCG trial in leprosy in India. Indian J. Lepr. 56(1984)686-687.
1. M.Sc., Dr.Med., Public HealthDepartment; Institute de Patologia Tropical e Saude Publica, Universidade Federal de Goias, P.O. Box 131, 74000 Goiânia, Goias, Brazil.
2. Dr.Med., Research Fellow CNPq; Institute de Patologia Tropical e Saude Publica, Universidade Federal de Goias, P.O. Box 131, 74000 Goiânia, Goias, Brazil.
3. Ph.D.; Institute de Patologia Tropical e Saude Publica, Universidade Federal de Goias, P.O. Box 131, 74000 Goiânia, Goias, Brazil.
4. M.Sc.; Institute de Patologia Tropical e Saude Publica, Universidade Federal de Goias, P.O. Box 131, 74000 Goiânia, Goias, Brazil.
5. M.Sc., Institute de Patologia Tropical e Saude Publica, Universidade Federal de Goias, P.O. Box 131, 74000 Goiânia, Goias, Brazil.
6. Ph.D., Organizacion Pan Americana de la Salud, Maracay, Venezuela.
Reprint requests to Dr. Ana Lúcia S. S. Andrade, Rua 1136 No. 630, Setor Marista, 74000 Goiânia-Goias, Brazil.
Received for publication on 2 January 1992.
Accepted for publication on 19 Mareh 1992.