- Volume 60 , Number 3
- Page: 353–67
Serological response to purified mycobacterial phosphatidylinositol mannoside in healthy controls and in patients with tuberculosis and leprosy
ABSTRACT
The serological response to a monoclonal antibody-defined phosphatidylinositol mannoside (L4-PIM) present in all mycobacteria was examined in patients with various mycobacterial diseases and healthy subjects f rom different populations. IgG but not IgM antibodies were detected in most patients with untreated lepromatous (84%) or borderline lepromatous (65%) leprosy, but in only a minority of those with disease at the tuberculoid end of the leprosy spectrum (< 17% positive). The response to L4-PIM was correlated with the IgM response to disaccharide octyl-bovine serum albumin (dBSA), and decreased with successful treatment. On the other hand, the test proved to be of little value in the diagnosis of untreated tuberculosis (4/15 positive) or atypical mycobacterial infection in patients with AIDS (0/11 positive). IgG antibodies to L4-PIM were also found in a significant proportion of healthy individuals, irrespective of their Mantoux status. These antibodies were shown to be specific for L4-PIM on immunoblotting, and their incidence increased with age in random donors f rom both urban Australia and rural Papua New Guinea. Despite the limited value of thc assay in diagnosis of any particular mycobacterial disease, the présence of antibodics to L4-PIM appears to be a sensitive indi- cator of subclinical infection with environmental mycobacteria in subjeets with an intact immune System.RÉSUMÉ
La réponse sérologique à un phosphatidyle inositol mannoside (L4-PIM) défini par anticorps monoclonaux et présent dans toutes les mycobactéries a été examinée chez, des patients présentant différentes mycobactérioses at des sujets sains de différents populations. Des anticorps IgG mais non pas des IgM ont été détectés chez la majorité des patients présentant une lèpre lépromateuse (84%) ou borderline lépromateuse (65%) non traités, mais seulement chez une minorité de ceux présentant une maladie à l'extrémité tubereuloide du spectre lépreux (< 17% de positifs). La réponse au L4-PIM était corrélée avec la response IgM au disaccharide octyle de la sérum albumine bovine (dBSA), et diminuait avec la réussite du traitement. D'autre part, le test se montrait de faible valeur pour le diagnostic de la tuberculose non traitée (4 positifs sur 15) ou une infection mycobactérienne atypique chez des patients avec la SIDA (0/11 positifs). Des anticorps IgG vis-à-vis du L4-PIM ont également été trouvés chez une proportion significative d'individus en bonne santé, quelle que soit leur réactivité vis-à-vis du test de Mantoux. On a montré par immunoblotting que ces anticorps étaient spécifiques pour le L4-PIM, et leur incidence augmentait avec l'âge parmi des donneurs tirés au sort en provenance de zones urbaines d'Australie et de zones rurales de Papouasie Nouvelle Guinée. En dépit de la valeur limitée du test pour le diagnostic d'une quelconque maladie mycobactérienne spécifique, la présence d'anticorps vis-à-vis du L4-PIM semble être un indicateur sensible d'une infection subcliniquc avec des mycobactéries environnementales chez des individus ayant un système immunitaire intact.RESUMEN
Empleando un fosfatidil inositol manósido (L4-PIM) presente en todas las micobacterias, se examinó la reactividad del suero de pacientes con varias enfermedades micobacterianas y de individuos sanos de diferentes poblaciones. Se detectaron anticuerpos IgG (pero no IgM) en la mayoría de los pacientes con lepra lepromatosa (84%) o lepromatosa subpolar (65%) sin tratamiento, y solo en una minoría de los pacientes con lepra del extremo tubereuloide del espectro (menos del 17%). La respuesta contra el L4-PIM estuvo correlacionada con la respuesta IgM al disacárido octil-albúmina sérica bovina (dBSA), y disminuyó como resultado del tratamiento exitoso. Por otro lado, la prueba resultó de poco valor para el diagnóstico de tuberculosis no tratada (4/15 positivos) o de infecciones por micobacterias atípicas en pacientes con SIDA (0/11 positivos). También se encontraron anticuerpos IgG contra el L4-PIM en una población importante de individuos sanos, independientemente de su reactividad al PPD. Por inmuno-electrotransferencia, estos anticuerpos fueron específicos para el L4-PIM y su incidencia aumentó con la edad. No obstante el valor limitado del ensayo en el diagnóstico de una enfermedad micobateriana en particular, la presencia de anticuerpos contra el L4-PIM podría ser un indicador sensible de infección subclínica por micobacterias ambientales en sujetos con un sistema inmune intacto.The identification, characterization, and purification of protein and nonprotein antigens from Mycobacterium leprae have been facilitated by the development of monoclonal antibody technology and the availability of armadillo-derived M. leprae antigen for biochemical analysis. Three mycobacterial glycolipid antigens have been isolated and fully characterized, including phosphorylated lipoarabinomannan (LAM) (13, l7), the M. leprae-specific phenolic glycolipid-I (PGL-I) (16), and a monoclonal antibodydefined crossreactive dimannosylated phosphatidylinositide (L4-PIM) (12). Immunoassays based on the crossreactive glycolipid LAM have been used with some success in the serodiagnosis of leprosy (30, 40), showing some increase in sensitivity when combined with measurement of IgM antibodies to PGL-I, but with a parallel loss of specificity, being detected in patients with active tuberculosis as well (27). PGL-I and its synthetic analog disaccharide octyl-bovine serum albumin (dBSA) currently form the basis of standard tests for the measurement of M. leprae-specific antibodies (7). While this is the most specific assay available for confirmation of infection in patients with clinical disease, it lacks both the sensitivity for patients with tuberculoid disease and the specificity when used as an epidemiological tool in endemic areas in the sense that it docs not distinguish clearly between exposure to M. leprae and clinical disease (2,11).
The crossreactive glycolipid L4-PIM has been shown to elicit an IgG response in some patients with leprosy which, in preliminary work, appeared to be correlated with the IgM response to dBSA (12). The present study was, therefore, designed to examine the diagnostic value of IgG PIM antibodies in patients with leprosy, tuberculosis, and in patients with M. avium infection in the setting of the acquired immune deficiency syndrome (AIDS). At the same time, the specificity of the assay was evaluated by measuring the response to L4-PIM in sera from healthy subjects of various ages living in urban Sydney, Australia, and from Kalo village, Papua New Guinea, where leprosy has been endemic. The results indicate that, at best, the L4-PIM assay may have a role as an adjunct to PGL-I serology in the diagnosis of clinical mycobacterial infection. The finding, however, of anti-L4-PIM antibodies in some healthy subjects suggests the possible value of this assay for determining the prevalence of subclinical infection with environmental mycobacteria at the population level.
MATERIALS AND METHODS
Monoclonal antibodies
The murine monoclonal antibodies (MAb) L4 (IgG2a) and L22 (IgG1) raised against M. leprae, recognize the mycobacterial crossreactive 4-6-kDa (5) and 65-kDa antigens (6), respectively. L4 and L22 hybridoma lines were grown in tissue culture flasks in RPMI 1640 (Flow Laboratories, McLean, Virignia, U.S.A.) containing 0.85 g/1 NaHCO3; 25 mM HEPES (BDH, Poole, England), 2 mM L-glutamine (Flow), penicillin (50 mg/1; Glaxo), streptomycin (100 mg/1; Glaxo) and 10% fetal calf serum (FCS; Flow). Culture supernatants were harvested twice per week and stored at 4ºC with 0.1% sodium azide.
Antigens
Purified mycobacterial antigens. The phosphatidylinositol mannoside (PIM) recognized by L4 was extracted from freezedricd M. tuberculosis and purified by high pressure liquid chromatography to yield highly purified L4-PIM as previously described (12). Disaccharide conjugated octylbovine serum albumin (dBSA), the synthetic analog of the M. leprae-specific antigen phenolic glycolipid-I, was kindly provided by Dr. H. Engers through the WHO/UNDP/World Bank Immunology of Leprosy program. Purified LAM from M. tuberculosis was the generous gift of Dr. P. Brennan, Colorado State University, Fort Collins, Colorado, U.S.A. Purified soybean phosphatidylinositol (PI) was purchased from Sigma Chemical Company, St. Louis, Missouri, U.S.A. Purified and semipurified mycobacterial antigens were the kind gifts of Drs. M. Riviere, M. Gilleron, A. Venisse and A. Vercellone, the Centre National de la Recherche Scientifique, Toulouse, France. These consisted of the phenolic glycolipids (PGL) PGL B-I and PGL K-I (from M. bovis BCG and M. kansasii, respectively); the glycopeptidolipid antigen of M. xenopi, GPL X-I; a crude mixture of PIM, LAM (lipoarabinomannan) and AM (arabinomannan) derived from M. tuberculosis; a crude cthanol extract from M. bovis BCG; a mixture of BCG-derived arabinogalactan and peptidoglycan complex; purified BCG peptidoglycans and purified lipooligosaccharids (LOS) from M. kansasii.
Bacterial sonicates. Lowenstein-Jensen slope cultures of M. simae, M. fortuitum, M. scrofulaceum, M. phlei and M. avium intracellular e-scrofulaceum (MAIS) were obtained from the Department of Microbiology, Royal Prince Alfred Hospital, Sydney, and the Tuberculosis Laboratory, Queensland Department of Health, Brisbane, Australia. M. bovis (bacillus Calmctte-Guérin, BCG strain) was purchased from the Commonwealth Serum Laboratories, Melbourne, Australia. Armadilloderived lyophilized M. leprae bacilli (batch CD 86) were kindly provided by Dr. R. J. W. Rees through the WHO/UNDP/World Bank Immunology of Leprosy program. Agar cultures of gram-positive organisms {Staphylococcus aureus, beta hemolytic Streptococcus Groups A and B), gram-negative organisms (Proteus mirabilis, Haemophilus influenzae, Pseudomonas aeruginosa and Escherichia coli) and fungi (Aspergillus fumigatis, Candida albicans, Nocardia asteroides and Strcptomyces griseus) and cutaneous Corynebacteria were supplied by the Department of Microbiology, Royal Prince Alfred Hospital, Sydney. Bacilli were suspended in phosphate buffered saline, 0.15 M, pH 7.2 (PBS), and sonicated on ice for 15 min. Sonicates were then sterilized by passage through a 0.22-µm filter (Millipore, Bedford, Massachusetts, U.S.A.), and their protein concentrations determined with Folin's reagent before storage at -70ºC.
Mantoux testing
Mantoux tests were read 48 hr after the intradermal injection of 10 IU of M. tuberculosis-derived PPD (CSL), and interpreted according to the guidelines issued by the National Tuberculosis Advisory Council, Canberra, Australia: Induration < 5 mm, negative; 5-9 mm, weak positive; 10-14 mm, intermediate positive; > 15 mm, strong positive.
Human sera
Sera were obtained from a variety of sources including: a) 87 Mantoux-negative, healthy medical and nursing students of various ethnic backgrounds from the University of Sydney before BCG vaccination, b) 46 members of the same group 8 weeks after BCG vaccination, at which time Mantoux conversion had occurred, c) 24 students from the same peer group with weakto-intermediate Mantoux reactions in the absence of BCG vaccination (range 3-12 mm induration), d) 90 inpatients without mycobacterial disease aged 2 to 16 years from the Royal Alexandra Hospital for Children, Sydney, e) 200 healthy blood donors of unknown Mantoux status who denied known mycobacterial exposure or BCG vaccination (Red Cross Blood Transfusion Service, Sydney). 0 400 healthy subjects from Kalo village, Papua New Guinea, where the majority of the population has been vaccinated with BCG and where leprosy was endemic (2). g) 24 subjects with treated and untreated pulmonary tuberculosis from the Department of Respiratory Medicine, Royal Prince Alfred Hospital, and from Port Moresby General Hospital, Port Moresby, Papua New Guinea, h) 87 patients with untreated leprosy from the Hansen's Disease Clinic at Prince Henry Hospital (Sydney, Australia), from the Boroko Hospital (Port Moresby, Papua New Guinea), and from Anandaban Leprosy Mission Hospital (Kathmandu, Nepal). The leprosy patients from whom the samples were derived had been classified according to the Ridley and Jopling scale by experienced clinicians using clinical and skin-smear evidence, i) 48 close family and school contacts of patients with leprosy who were part of an aboriginal cohort being studied from Thursday Island, Australia, j) 11 patients with documented M. avium bacteremia in the setting of the acquired immunodeficiency syndrome (AIDS) were obtained from the Department of Clinical Immunology, Royal Prince Alfred Hospital, Sydney.
A serum pool from 22 newly diagnosed and untreated Nepalese lepromatous patients, designated LSP5, was used as a positive control for all serological assays. Other leprosy and tuberculosis patients were classified arbitrarily as either treated or untreated on the basis of a duration of treatment of less than or greater than 3 months, respectively.
Enzyme-linked immunosorbent assays
Antibodies to L4-PIM. Flat-bottom, polystyrene, 96-well microtiter plates (Linbro; Flow) were coated with 50 µl of 2.5 µg/ ml of purified L4-PIM in ethanol. The plates were dried under a stream of warm air and then blocked overnight at 4ºC with 3% bovine serum albumin (BSA) in PBS. Subsequently they were washed once with PBS and incubated with 50 µ1 per well of serum diluted 1 in 100 in 10% normal goat serum (NGS) in PBS. After 1 hr at 37ºC, the plates were washed twice with PBS containing 0.05% Tween-20 (PBST) and three times with PBS. Fifty /xl of 1/1000 alkaline phosphatase conjugated goat anti-human immunoglobulin (IgG or IgM; Sigma) in 1% NGS/PBS were added to each well and incubated at 37ºC for 1 hr. Finally, the plates were washed consecutively with PBS (twice), and once each with distilled water and nitrophenyl phosphate (NPP) buffer (NaHCO3 34.8 mM, NaCO3, 15 mM, MgCL2 0.1 mM, pH 9.6) before the addition of p-nitrophenyl phosphate disodium (NPP) 1 mg/ml in NPP buffer. The absorbance was read at 1 hr with a Titertek Multiscan ELISA plate photometer (Flow). The background was calculated from triplicate PIM-containing wells incubated with 10% NGS without human serum and subtracted from the absorbance of triplicate test wells. The response of L4-PIM was expressed as a percentage of the absorbance of the LSP5 positive control on the same plate. The specificity of antibody binding to L4-PIM was examined by comparison of serum binding to phosphatidylinositol (PI), an analogous glycolipid to L4-PIM but lacking the specific terminal mannose residues recognized by the monoclonal antibody L4 (12).
Antibodies to other mycobacterial antigens. All mycobacterial antigens (except for LAM and dBSA) were dissolved initially in hot ethanol at 45ºC, then diluted further in ethanol before coating of polystyrene microtiter plates as described above. On the basis of preliminary assays, antigens were applied at optimal concentrations of either 10 µg/ml (PGL B-I, BCG peptidoglycans, crude LAM/AM/PIM mixture, ethanol extract of BCG) or 2.5 µg/ml (the remaining antigens). In each assay the serological responses to purified L4-PIM and PI (negative control) were retested simultaneously on the same plate.
Antibodies to LAM and L4-PIM were also measured on the same plate. Following application of L4-PIM and PI in ethanol and drying in warm air, either 50 µl per well of 2 µg/ml LAM in carbonate buffer (pH 9.6) or carbonate buffer alone was applied to separate wells and incubated for 16 hr at 4ºC. The plates were then washed three times in PBS and blocked for 2 hr with 3% filtered BSA in PBS at 37ºC, followed by the application of serum diluted 1/100 in 10% NGS to wells coated with L4-PIM or LAM, and to negative control wells coated with PI or bicarbonate buffer alone. The assay was completed as described above. Antibodies to the synthetic disaccharide conjugate of bovine serum albumin of PGL-I from M. leprae (dBSA) were assayed as previously described (40).
Detection of L4-PIM in bacterial sonicates. Triplicate wells of polystyrene, 96well, flat-bottom microtiter plates (Linbro, Flow) were coated overnight at 4ºC with 50 µl of mycobacterial, bacterial, or fungal sonicates at a protein concentration of 50 µg/ ml. The next morning the plates were washed once with PBS, then blocked with 3% BSA in PBS for 2 hr at 37ºC. After a further wash with PBS, test wells were coated with 50 id of neat L4 culture supernatant for 1 hr at 37ºC, washed three times with PBS, and the binding of L4 was detected by incubating with alkaline phosphatase conjugated sheep anti-mouse IgG (Sigma) for 1 hr at 37ºC. The monoclonal antibody L22, which recognizes the ubiquitous mycobacterial 65kDa protein antigen (6) was used as a positive control for binding of mycobacterial sonicates to ELISA plates.
Because comparable monoclonal antibodies were not available for the nonmycobacterial sonicates, binding was demonstrated using the biotinylated lectins concanavalin A (ConA) and wheat germ agglutinin (WGA; E-Y Laboratories, San Mateo, California, U.S.A.). ELISA plates were coated with 50 id of bacterial sonicates at a protein concentration of 50 µg/ml, blocked as described above, and then incubated for 1 hr at 37ºC with 50 id per well of either ConA 1µg/ml in Tris (hydroxymethyl amino methane; Sigma) buffered saline (TBS, 0.005 M, with 1 mM CaCl2, 15 mM NaCl, pH 7), or 2 µg/ml of WGA in PBS plus 1 mM CaCl2. Plates were washed three times with PBS, incubated for a further hour at 37ºC with alkaline phosphatase conjugated Streptavidin (Sigma) in 0.1% BSA in PBS, and the color reaction developed in the standard way.
SDS-PAGE fractionation and immunoblotting with human sera
Mycobacterial sonicates were separated by discontinuous gel electrophoresis (25) using separating and stacking gels of 12% and 4%, respectively, of 0.75-mm thickness. Twenty µg of protein from mycobacterial sonicates of M. bovis BCG, M. leprae, M. scrofulaceuni and M. phlei were loaded per gel track and electrophoresis was carried out as previously described (4).
Mycobacterial antigens were transferred from the SDS-PAGE gel to nitrocellulose (Bio-Rad) by electroblotting in 10% methanol, 12 mM Tris, 86 mM glycine, pH 8.3 (46). Nitrocellulose strips containing antigen were blocked in 0.22 µm fdtered 3% BSA/ PBS overnight at 4ºC. After washing three times in Tris-buffered saline with Twecn (TBST) the strips were incubated at 4ºC with pools of 0.22 iim fdtered human serum diluted 1/100 in 2.5% NGS in TBST for a further 24 hr. The strips were then washed three times in TBST and incubated for 2 hr with biotinylated goat anti-human antibody (Amersham, England) diluted 1/400 in TBST. After a further three washes in TBST, the strips were incubated for 2 hr with horseradish peroxidase conjugated Streptavidin (Amersham) 1/400 in TBST, washed three times in Tris-buffered saline and the color reaction developed with a freshly made solution of 4-chloro-napthol (4CN; Sigma).
Statistical analysis
Statistical analysis of serological data was performed with "Statview" for the Macintosh computer using the Mann-Whitney U test for comparisons between clinical groups of nonparametric data with normal approximation for large values of "U," and regression analysis for comparison of paired data. Data were interpreted as having statistical significance with p < 0.05.
RESULTS
Serological assay for anti-L4-PIM antibodies. Preliminary assays of sera from healthy controls and patient groups failed to demonstrate the presence of an IgM response to L4-PIM. Thus the binding of IgM to L4-PIM in serum samples from 13 patients with untreated tuberculosis, 20 patients with untreated lepromatous leprosy and 20 patients with treated lepromatous and tuberculoid leprosy was no greater than that observed in the control subjects (12 Mantoux-ncgativc healthy medical students) and not significantly different from binding to the negative control antigen, phosphatidylinositol (PI; data not shown).
By contrast, a significant IgG response to L4-PIM was observed in some patient groups. Optimum differentiation between sera from patients and healthy Mantouxnegative controls was obtained by diluting the samples 1/100 in NGS without the addition of Tween. When sera from 87 Mantoux-negative healthy students were studied, the distribution of antibodies to L4-PIM detected by this method was skewed to the left with an arithmetic mean, median, and mode of 8.6%, 3.9% and 0%, respectively, of the LSP5 lepromatous serum pool. That this was not due to nonspecific binding of serum to L4-PIM was confirmed by the absence of binding to either PI (negative control antigen) or ethanol alone (data not shown). Furthermore, the background IgG response to L4-PIM was unaffected by vaccination with M. bovis BCG, as shown by the fact that the values for serum samples taken before and 8 weeks after vaccination from 46 medical students were correlated (r = 0.9, p = 0.0001) and remained unchanged, being 13.7 ± 13.7% and 11.4 ± 12.2% of LSP5, respectively. An IgM response to BCG vaccination was not observed.
IgG anti-L4-PIM responses in patient groups and healthy controls. Since IgG antibodies to L4-PIM were not normally distributed in the Mantoux-negative controls (no history of mycobacterial disease), the 95% level of the empirical distribution of optical density readings from these subjects (39.3% of the LSP5 pool) was used to define a positive serological response. The distribution of antibodies to L4-PIM in all patient groups was statistically different from the healthy control population (Mann Whitney U test, p < 0.05). For example, the majority of patients with borderline lepromatous (BL, 21/32; 65.6%) and lepromatous leprosy (LL, 37/44; 84.1%) were positive for antibodies to L4-PIM. The assay, however, lacked both sensitivity and specificity with positive responses obtained in less than a quarter of the patients with untreated tuberculoid (TT, 3/18 positive; 16.7%) or borderline tuberculoid leprosy (BT, 5/31 positive; 16.1%), while 26.7% (4/ 15 positive) of the patients with untreated tuberculosis had a positive antibody titer (Fig. 1).
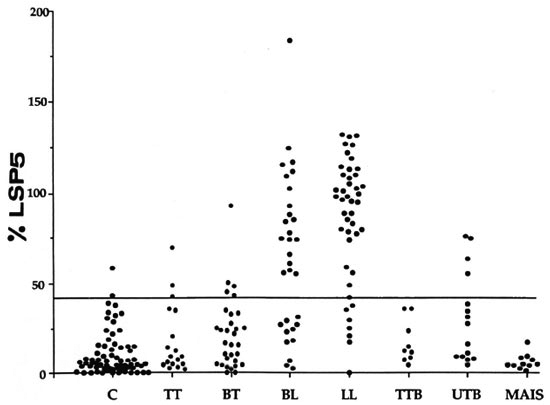
Fig. 1. Serological response to L4-PIM. IgG antibodies to L4-PIM were measured by ELISA and expressed as a percentage of the absorbance of a lepromatous serum pool, LSP5. Assays were performed in 87 Mantoux-negative controls (C) and in subjects with untreated tuberculoid (TT; 18), borderline tuberculoid (BT; 31),borderline lepromatous (BL; 32), and lepromatous leprosy (LL; 44) as well as in patients with treated tuberculosis(TTB; 9), untreated tuberculosis (UTB; 15), and AIDS patients with ig bacteremia (MAIS; 11). Thehorizontal line represents an upper limit of normal of 39.3% of the LSP5 scrum pool calculated from the 95%level of the empirical distribution of Mantoux-negative control subjects.
In view of the lack of specificity of L4-PIM antibodies for M. leprae infection and the presence of L4-PIM in MAIS (Fig. 2), the value of the assay for detecting the latter infection in patients with AIDS was examined. No patient (out of a total of 11) with proven M. avium bacteremia had a detectable response, presumably on the basis of the well-documented inability of this group of patients to mount antigen-specific B-cell responses (3).
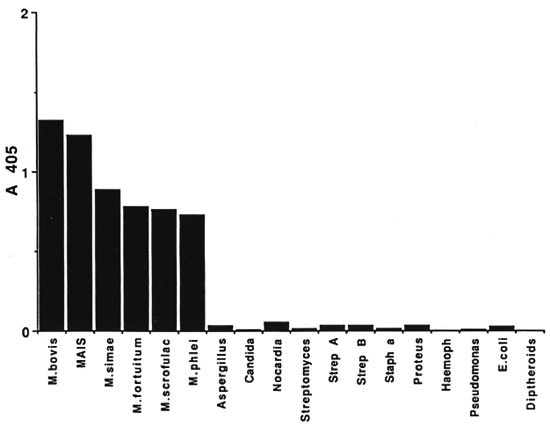
Fig. 2. Detection of L4-PIM in bacterial sonicates. The presence of L4-PIM in various bacterial species wasdetermined by the finding of L4 monoclonal antibody to wells coated with 50 pg/ml of bacterial sonicates in adirect ELISA.
The IgG anti-L4-PIM and the IgM antidBSA responses were correlated in untreated leprosy patients. Thus, both assays identified a similar proportion of untreated cases with sensitivities for detection of TT, BT, BL and LL leprosy of 17%, 16%, 66% and 84%, respectively, in the case of L4-PIM and 9%, 14%, 94% and 85%, respectively, in the case of dBSA. Secondly, when parallel assays were performed on sera from 18 untreated leprosy patients (12 LL/BL and 6 TT/BT) and 46 treated patients (21 LL/BL and 25 TT/BT), IgM antibodies to dBSA and IgG antibodies to L4-PIM were found to be correlated (r = 0.74 and 0.88, respectively; p = 0.0001 in both cases; data not shown). Thirdly, IgG antibody levels to L4-PIM decreased with treatment in some leprosy patients in parallel with IgM levels to dBSA (data not shown), suggesting that either or both assays might be useful in monitoring patients undergoing treatment for leprosy. On the other hand, there was no correlation between the two assays in household or school contacts of leprosy patients (r = 0.20; p > 0.16) despite the occurrence of high IgM dBSA antibodies in some of them.
Specificity of IgG anti-L4-PIM antibodies. As mentioned previously, most healthy control subjects did not make a significant antibody response to L4-PIM when the cut off was set at 39.5% of the LSP5 positive control serum pool. Indeed, only 2.3% (2/ 87) were positive by this criterion. The cutoff level for a positive response to L4-PIM, however, was arbitrary, and it was unclear whether L4-PIM antibody levels above the upper limit of normal in controls represented a continuous spectrum of nonspecific binding to the antigen (unlikely in view of the absence of any significant binding of sera to PI) or a bimodal distribution with clinical significance. If the latter were correct, the presence of high levels of antibodies to L4-PIM might indicate prior exposure to L4-PIM contained in environmental mycobacteria, or to an identical or crossreactive antigen in other common microorganisms.
To examine this possibility, four experiments were performed. In the first, L4 M Ab was used to detect the presence of L4-PIM in various gram-positive, gram-negative, and fungal organisms as well as several species of mycobacteria. As shown in Figure 2, L4 reacted with all species of mycobacteria but to none of the other microorganisms tested, even though the latter had clearly bound to the plate as shown by the capacity of biotinylated lectins to react with ELISA plates coated with these organisms (data not shown).
Secondly, pools of four individual sera were prepared from healthy students with -high- (mean: 33.0% of LSP5) compared to -low- (13.0% of LSP5) levels of anti-L4-PIM antibodies and from two similar groups of patients with tuberculosis (75.6% versus 6.6% of LSP5). When these serum pools were immunoblotted with sonicates of M. leprae, M. bovis BCG, M. scrofulaceum and M. phlei, pools with "high" but not "low" levels of antibodies to L4-PIM (by ELISA) were shown to react with all four species of mycobacteria tested (Fig. 3), particularly in the low molecular weight region (known to contain immunoreactivc PIMs recognized by the MAb L4 (4, 12). This result suggested that the presence of such antibodies in otherwise healthy subjects was likely to reflect exposure to environmental mycobacteria.
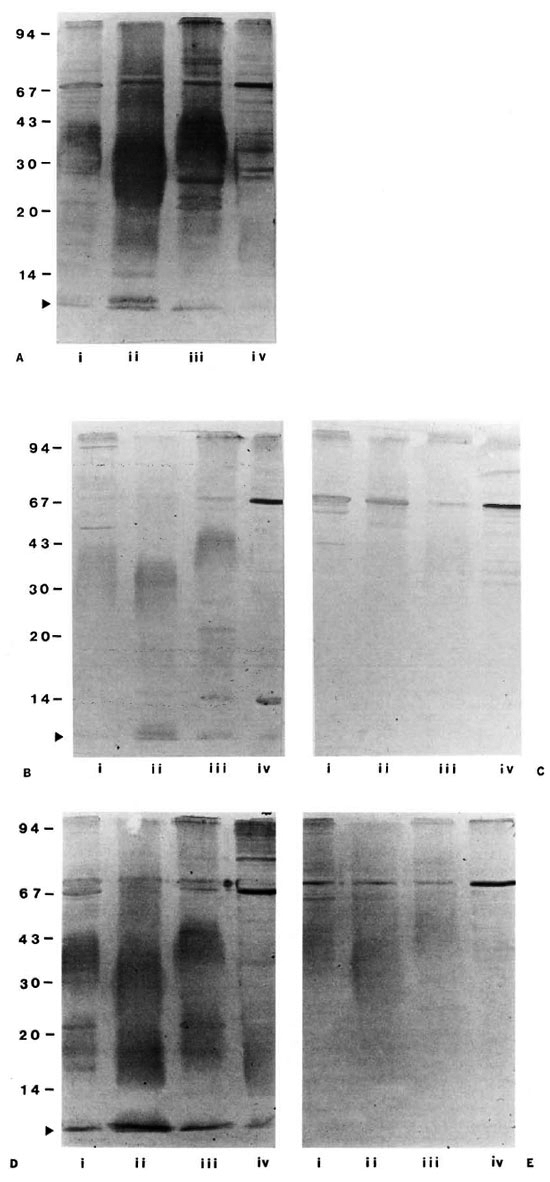
Fig. 3. Immunoblots of mycobacterial sonicates. Mycobacterial sonicates of (i) M. bovis BCG, (ii) .M. leprae, (iii) M. scrofulaceum, and (iv) M. phlei were immunoblotted with serum pools diluted 1/100 from: (A) lepromatous leprosy patients (LSP5), Mantoux-negative students with either relatively high (B; 33% of LSP5) or low (C; 13% of LSP5) levels of antibodies to L4-PIM, and tuberculosis patients with either relatively high (D; 75.6%of LSP5) or low (E; 6.6% of LSP5) antibody levels to L4-PIM; ► = position of the low molecular band containing L4-PIM.
Thirdly, the serological response to L4-PIM was compared with that to other purified and semipurified mycobacterial antigens to determine whether antibodies to these antigens were present in subjects with high anti-L4-PIM antibodies. While there was a strong correlation between the IgG response to L4-PIM and that to a crude PIMcontaining extract from M. tuberculosis (r = 0.82), no such relationship was found with the other mycobacterial antigens tested such as LAM or the species-specific phenolic glycolipids (r < 0.25 in all cases).
Finally, IgG anti-L4-PIM antibodies were measured in a subset of 26 nonBCG-vaccinated healthy medical and nursing students without known mycobacterial exposure, but with weak-to-intermediate Mantoux reactions ranging from 3 mm to 12 mm in diameter. The rationale for this experiment was the observation that such weak reactions may reflect exposure to environmental mycobacteria (33, 41). While no correlation was found between the diameter or area of the Mantoux reaction and the magnitude of the serological response to L4-PIM (r = 0.11), higher antibody levels to L4-PIM were detected in the Mantoux-positive subgroup compared to their Mantouxnegative peers (arithmetic mean and median of 11.4% and 10.0% of LSP5 versus 8.6% and 3.9% of LSP5, respectively; p = 0.04; Mann Whitney U test). In other words, a relationship may exist between natural mycobacterial exposure as assessed by delayed hypersensitivity testing with PPD and the serological response to L4-PIM, even though no increase in antibody level was noted after BCG vaccination (see above).
Epidemiology of antibody responses to L4-PIM. The presence of antibodies to L4-PIM in healthy subjects and the apparent restriction of this antigen to mycobacteria implied that such responses probably reflected asymptomatic infection with environmental mycobacteria. Since such exposure was likely to increase with age, IgG anti-L4-PIM antibodies were measured in the sera collected from subjects of various ages in Sydney, Australia, and the results compared with those of sera collected from Kalo village, Papua New Guinea, where exposure to environmental mycobacteria might be expected to be greater.
Interestingly, an age-related increase in the mean, median, and scatter of the serological response to L4-PIM was observed in subjects from Sydney as well as from Kalo (Fig. 4). This finding reflected a true increase in specific antibodies to L4-PIM and was not due to an increase in nonspecific binding to L4-PIM with age when binding to L4-PIM and PI were compared (data not shown). These findings once again created the problem of determining a negative -control- population from which to discriminate negative from positive responses. Since estimates of antibodies to L4-PIM in cord sera would reflect only maternal IgG, and levels in sera from very young subjects could be potentially confounded by the immaturity of their immune system (32), an arbitrary cut off for a positive result was calculated based on the 95% level of the empirical distribution of antibodies to L4-PIM in subjects from Sydney aged 11 to 20 years (Fig. 4A). Using this cut-off level (19.5% of the LSP5 serum pool), the percentage of subjects with positive serological responses to L4-PIM in Sydney rose from zero (aged 0 to 10 years) to a maximum of 26% (aged 41 to 50 years).
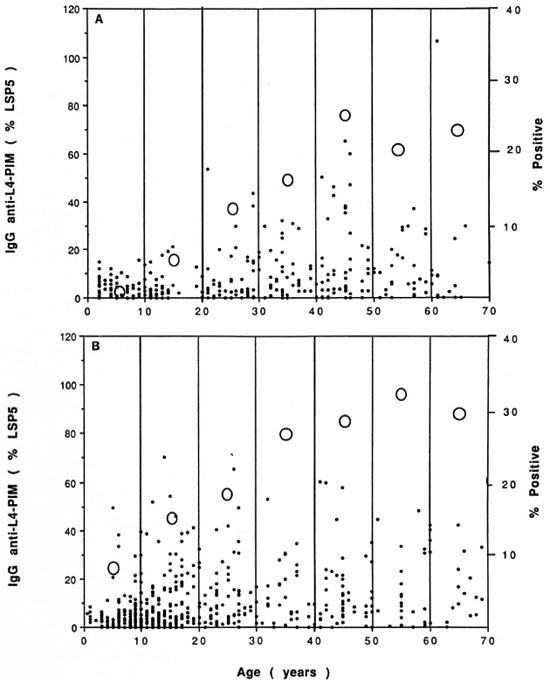
Fig. 4. Antibodies to L4-PIM in subjects of various ages. IgG antibodies to L4-PIM were measured byELISA and expressed as a percentage of the absorbance of LSP5 (left axis,●). Serum samples were collectedfrom random donors in Sydney, Australia (A) and from Kalo village, Papua New Guinea (B), and expressed asa function of age. Percentage of subjects positive for antibodies to L4-PIM in each age group is also shown (rightaxis, O).
A similar increase in the IgG response to L4-PIM with advancing age was observed in subjects from Kalo, a coastal village in Papua New Guinea with an 8.8% prevalence of clinical leprosy (2). In this group, however, antibody levels were generally higher for any given age group than those from their Sydney counterparts, particularly in villagers aged 11 to 20 years (Fig. 4B), although the differences were not statistically significant. The latter result is consistent with previous findings of a peak incidence of positive IgM responses to dBSA in clinically healthy subjects from the same age group, which was concluded to be due to subclinical infection with M. leprae (Baumgart, personal communication). If the same cut-off level of 19.5% LSP5 serum pool was used, positive responses rose from 7.2% in subjects younger than 10 years to a maximum of 33.3% in those aged 51 to 60 years. Serological responses to L4-PIM, however, were not simply indicative of transmission of M. leprae. Not only were IgG antibodies to L4-PIM and IgM antibodies to dBSA not correlated in these villagers (r = 0.06), but the same age-related change in L4-PIM antibodies persisted, even when subjects with positive responses to dBSA were removed from analysis (data not shown). Thus, the responses to L4-PIM in this village (as in Sydney) most likely reflected exposure to environmental mycobacteria other than M. leprae.
DISCUSSION
The PIM family of glycolipids constitutes a heterogeneous group of molecules composed mainly of mono- and dimannosides thought to play a structural role in the cell membrane (31). Although primarily restricted to mycobacteria, they have also been identified in a range of actinomycetes and corynebacteria (19, 26). There is a substantial body of literature indicating that crude PIM extracts can elicit an antibody response in both experimental animals (22, 45) and man. Thus, antibodies have been detected in patients with newly diagnosed tuberculosis, lepromatous leprosy, and atypical mycobacterial infection with frequencies of up to 80%, 92% and 39%, respectively, as well as in up to 13% of healthy individuals (10, 21, 34-36, 44).
In the current study, antibodies to highly purified L4-PIM were measured in sera from Mantoux-positive and -negative healthy subjects resident in Australia and Papua New Guinea, and from patients with a variety of mycobacterial infections. The large overlap in antibody levels between patients and controls necessitated the creation of an arbitrary cut-off level for the assay. When this was set at 39.5% of LSP5, the majority of patients with untreated lepromatous and borderline lepromatous leprosy as well as some patients with untreated tuberculosis had a positive response (Fig. 1). Patients with untreated leprosy had high levels of IgG (but not IgM) antibodies to L4-PIM, which were correlated with IgM antibodies to dBSA and decreased with duration of successful treatment. While this predominance of IgG antibodies to mycobacterial sonicates is well described (1, 14), the absence of a detectable IgM response to L4-PIM in untreated leprosy and tuberculosis is surprising when one consideres that the other two major mycobacterial glycolipid antigens-LAM and PGL-I -elicit antibodies of both IgM and IgG class (24, 50). The possibility of a transient IgM response following infection as opposed to clinical disease, however, cannot be excluded (14), although we were unable to demonstrate such an effect following BCG vaccination.
The assay clearly lacked specificity for a particular mycobacterial infection and, in addition, proved to be relatively insensitive, in that positive responses were obtained in only a minority of patients with paucibacillary leprosy or untreated tuberculosis. It was thus of no greater value than the dBSA assay for diagnosing patients with tuberculoid (BT, TT) disease (8, 27, 29). On the other hand, significant levels of IgG anti-L4-PIM antibodies were detected in a proportion of healthy contacts of leprosy cases, including some who were negative for the dBSA antibody assay (data not shown). One possible conclusion from these results is that a combination of the two assays may be of more value than the dBSA assay alone for detecting subclinical infection in populations exposed to M. leprae. The situation, however, is complicated by the wide-spread distribution of L4-PIM in environmental mycobacteria other than pathogenic species, as well as the presence of antibodies to L4-PIM in a significant proportion of the population at large which increases with age, even in areas where leprosy is not endemic (Fig. 4a).
The presence of anti-L4-PIM antibodies in a small but significant proportion of healthy subjects is intriguing. Nonspecific binding of serum to L4-PIM can be excluded on two counts: first, the sera did not react with PI, the equivalent of L4-PIM without the mannose residues carrying the B-cell epitope for L4 (12). Secondly, the specific reactivity of positive sera was confirmed on immunoblotting (Fig. 3) as well as by ELISA. On the other hand, the increase in anti-L4-PIM reactivity with age (Fig. 4) raises the possibility that these antibodies might be directed to crossreactive epitopes or to L4-PIM itself present in other environmental bacteria to which the healthy subjects had been exposed. The demonstration of binding to a range of mycobacteria but not to other common microorganisms (Fig. 2) makes this phenomenon likely to be due to infection with environmental mycobacteria expressing L4-PIM per sc. Indeed, precedents for such a conclusion already exist in the literature.
For example, a small percentage of healthy subjects have been reported to produce antibodies to other crossreactive antigens such as LAM (27). Furthermore, environmental mycobacteria have been frequently isolated in both rural and urban settings (41), including M. xenopi and M. kansasii from tap water (28) and M. avium-intracellulare-scrofulaceum complex from house dust and soil (9, 37, 38). Not only has isolation of these or ot on nasganisms been associated with outbreaks of infection (20, 24), but they may be the agents responsible for the "low PPD reactivity" observed on skin testing of healthy populations (33, 42, 43). This conclusion is supported by: a) work demonstrating a correlation between microorganisms identifiable in the local environment and delayed hy persensitivity skin testing to the identicalmicroorganism in the local population (41); b) recent studies in South Australia indicating similar geographical variations in human PPD reactivity of healthy populations,from a low of 1.1% (urban) or 3.2% (rural) of 14-year-old school-aged children risingto 6.7% and 12.5% positive, respectively, iftested with M. avium preparations (Robinson, personal communication); c) thefinding reported here of higher anti-L4-PIMantibody levels in subjects with low/intermediate Mantoux reactions than in subjectswith negative tests.
On the other hand, three observations argue against the conclusion that the presenceof anti-L4-PIM antibodies is indicative ofexposure to -environmental- mycobacteria. The first is the absence of a detectableantibody response to L4-PIM in patientswith advanced HIV infection infected with M. avium. This observation, however, can be readily explained by the well-known inability of patients with AIDS to mount antigen-specific B-cell responses despite elevation in overall immunoglobulin levels 3-4 8 4,). Secondly, there was no correlation between the levels of antibodies against L4-PIM and those to other mycobacterial antigens such as LAM. While more difficult to explain, this finding may simply reflect the greater immunogenicity of L4-PIM. Thirdly, BCG vaccination failed to increase the levels of anti-L4-PIM antibodies despite 1S 23 previous reports to the contrary (15, 18, 23, 47). In practice, antigen presented intracutaneously is well known to be less efficient than natural exposure by the mucosal routes in eliciting an antibody rather than a T-cell response. On balance, therefore, it is reasonable to conclude that the measurement of antibodies to a ubiquitous antigen like L4-PIM, although of little additional value to the dBSA assay in the diagnosis of leprosy, may provide a useful epidemiological tool for studying the prevalence of subclinical infection with environmental mycobacteria or related organisms.
Acknowledgment. This work was supported by thc Leprosy Eradication and Education Program of Lions International; the Centre National de la Recherches Scientifique. France (JJF); and the National Health and Medical Research Council of Australia (RJM, BM, KM, AB). We wish to thank Mr. P. Roche and Drs. W. Britton. P. Gatenby and R. Garsia for the generous gift of patient sera. The assistance of the Department of Community Medicine of the University of Papua New Guinea in the collection of sera from Kalo village is gratefully acknowledged. Strains of environmental mycobacteria used in this work were supplied by Mr. D. J. Dawson and Mrs. Z. Blacklock. Tuberculosis Laboratory. Queensland Department of Health, Australia. We are grateful to Professor Y. Cossart and Dr. C. McClcod for facilities in which to grow these mycobacteria. Useful discussions were held with Dr. P. Robinson. Department of Thoracic Medicine, Royal Adelaide Hospital, and we are grateful for permission to quote his unpublished work. We wish to acknowledge the assistance of Dr. D. Handelsman for assistance with the statistical analysis. Finally, this work would not have been possible without the help and cooperation of medical and nursing students at thc University of Sydney, the staffand volunteer blood donors from the Red Cross Blood Transfusion Service, Sydney, Australia, and the villagers of Kalo, Papua New Guinea.
REFERENCES
1. BARDANA, E. J.. MCCLATCHY. J. K., FARR, R. S. and MINDEN, P. Universal occurrence of antibodies to tubercle bacilli in sera from non-tuberculous and tuberculous individuals. Clin. Exp. Immunol. 13(1973)65-77.
2. BAUMGART, K., BRITTON, W., BASTEN, A. and BAGSHAWE, A. Use of phenolic glycolipid I for scrodiagnosis in a high prevalence village in Papua New Guinea. Trans. R. Soc. Trop. Med. Hyg. 81(1987)1030-1032.
3. BOWEN, D. L., LANE, H. C. and FAUCI, A. S. Immunological abnormalities in the acquired immunodeficiency syndrome. Prog. Allergy 37(1986)207-223.
4. BRITTON, W. J., HELLQVIST. and BASTEN, A. Separate antigenic determinants on cell wall associated carbohydrate antigens of Mycobacterium leprae defined with monoclonal antibodies. Int. J. Lepr. 54(1986)545-555.
5. BRITTON, W. J.. HELLQVIST, L.. HASTEN. A., and RAISON, R. L. Mycobacterium leprae antigens involved in human immune responses. I. Identification of four antigens by monoclonal antibodies. J. Immunol. 135(1985)4171-4177.
6. BRITTON, W. J., HELLQVIST, L., GARSIA. R. J. and BASTEN, A. Dominant cell wall proteins of Mycobacterium leprae recognized by monoclonal antibodies. Clin. Exp. Immunol. 67(1987)31-42.
7. BUROESS, P. J., FINE. P. E. M., PONNIGHAUS, J. M. and DRAPER, C. Serological tests in leprosy, the sensitivity, specificity and predictive value of ELISA tests based on phenolic glycolipid antigens, and the implications for their use in epidemiological studies. Epidemiol. Infect. 101(1988)159-171.
8. CHO, S.-N., YANAGIIIARA, D. L.. HUNTER S. W., GELUER. R. H. and BRENNAN. P. J. Serological specificity of phenolic glycolipid I from Mycobacterium leprae and use in serodiagnosis of leprosy. Infect. Immun. 41(1983)1077-1083.
9. DAWSON, D.J. Potential pathogens among strains of mycobacteria isolated from house-dusts. Med. J. Aust. 1(1971)679-680.
10. FAVEZ. G., JEQUIER. S. and VULLIEMOZ. P. Demonstration and discrimination of distinct circulating antibodies during active tuberculosis in man. Am. Rev. Respir. Dis. 94(1966)905-913.
11. FINE. P. E., PONNIGHAUS, J. M., BURGESS, P., CLARKSON, J. A. and DRAPER, C. C. Serocpidemiological studies of leprosy in northern Malawi based on an enzyme-linked immunosorbent assay using synthetic glycoconjugatc antigen. Int. J. Lepr. 56(1988)243-254.
12. FOURNIE, J.-J., MULLINS, R. J. and BASTEN. A. Isolation and structural chareteristics of a monoclonal antibody-defined cross-reactive phospholipid antigen from Mycobacterium tuberculosis and Mycobacterium leprae. J. Biol. Chem. 266(1991)1211-1219.
13. GAYLORD, H., BRENNAN, P. J.. YOUNG, D. B. and BUCHANAN, T. M. Most Mycobacterium leprae carbohydrate-reactive monoclonal antibodies are directed to lipoarabinomannan. Infect. Immun. 55(1987)2860-2863.
14. GRANGE, J. M. The humoral immune response in tuberculosis: its nature, biological role and diagnostic usefulness. Adv. Tuberc. Res. 21(1984)1-78.
15. HOEPPNER, V. H., JACKETT, P. S., BECK, J. S., KARDJITO, T., GRANGE, J. M. and IVANYI. J. Appraisal of the monoclonal antibody-based competition test for the serology of tuberculosis in Indonesia. Scrodiagn. Immunother. 1(1987)59-77.
16. HUNTER. S. W.. FUJIWARA. T. and BRENNAN. P. J. Structure and antigenicity of the major specific glycolipid antigen of Mycobacterium leprae. J. Biol. Chem. 257(1982)15072-15078.
17. HUNTER, S. W., GAYLORD, H. and BRENNAN, P. J. Structure and antigenicity of the phosphorylated lipopolysaccharide antigens from the leprosy and tubercle bacilli. J. Biol. Chem. 261(1986)12345- 12351.
18. KARDJITO, T., HANDOYO I. and GRANGE, J. M. Diagnosis of tuberculin reactivity by immunological methods: 1: the effect of tuberculin reactivity and previous BCG vaccination on the antibody levels determined by ELISA. Tubercle 63(1982)269-274.
19. KATAOKA, T. and NOJIMA, S. The phospholipid compositions of some actinomyectes. Biochem. Biophys. Acta 144(1967)681-683.
20. KAUSTOVA, J., OLSOVSKY. Z., KUIIIN, M.. ZATLOUKAL. O. PELIKAN, M. and HRADIL, V. Endemic occurrence of Mycobacterium kansasii in water-supply systems. J. Hyg. Epidem. Immunol. 25(1981)24-30.
21. KHULLER. G. K. and SUDRAHMANYAM. D. Antibodies to mannophosphoinositides in leprosy patients. Int. J. Lepr. 38(1970)365-367.
22. KHULLER, C. K. and SUDRAHMANYAM, D. Antigenicity of phosphatidyl inositomannosides of Mycobacterium tuberculosis. Immunochemistry 8(1971)251-256.
23. KRAMHOVITIS, E. Detection of antibodies to Mycobacterium tuberculosis plasma membrane antigen by enzyme-linked immunosorbent assay. J. Med. Microbiol. 21(1986)257-264.
24. KUHIN, M., SVANDOVA, E., MEDEK, B., CHOBOT, S. and OLSOVSKY, Z. Mycobacterium kansasii infection in an endemic area of Czechoslovakia. Tubercle 61(1980)207-212.
25. LAEMMILI, U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 227(1970)680-685.
26. LECIIEVALIER, M. P., DE BIERVE, P. and LECHE-VALIER. H. Chemotaxonomy of aerobic actinomyectes: phospholipid composition. Biochem. System. Ecol. 5(1977)249-260.
27. LEVIS, W., MEEKER, H. C, SCHULLER-LEVIS, G., SERSEN, E., BRENNAN, P. J. and FRIED, P. Mycobacterial carbohydrate antigens for serological testing of patients. J. Infect. Dis. 156(1987)763-769.
28. MCSWIGGEN, D. A. and COLLINS, C. H . The isolation of M. kansasii and M. xenopi from water systems. Tubercle 55(1974)291-297.
29. MILLER, R. A.. GORDER. D. and HARNISCH, J. P. Antibodies to phenolic glycolipid-I during longterm therapy: serial measurements in individual patients. Int. J. Lepr. 55(1987)633-636.
30. MILLER. R. A., HARNISCH, J. and BUCHANAN, T. M. Antibodies to mycobacterial arabinomannan in leprosy: correlation with rcactional states and variation during drug treatment. Int. J. Lepr. 52(1984)133-139.
31. MINNIKIN, D. E. Lipids: complex lipids, theirchemistry, biosynthesis and roles. In: The Biology of the Mycobacteria. Volume I. Ratledge, C. and Stanford, J., eds. London: Academic Press, 1983, pp. 95-184.
32. OXELIUS, V. A. Quantitative and qualitative investigations of serum IgG subclasses in immunodeficiency diseases. Clin. Exp. Immunol. 36(1979)112-116.
33. PALMER, C. E. Tuberculin sensitivity and contact with tuberculosis; further evidence of non-specific sensitivity. Am. Rev. Respir. Dis. 68(1953)678-694.
34. REGGIARDO, R. Z. and MIDDLEBROOK, G. Serologically active glycolipid families from Mycobacterium bovis BCG. II. Serological studies on human sera. Am. J. Epidemiol. 100(1975)477-486.
35. REGGIARDO, Z. and VAZQUEZ. E. Comparison of enzyme-linked immunosorbent assay and hemagglutination test using mycobacterial glycolipids. J. Clin. Microbiol. (1981)1007-1009.
36. REGGIARDO, Z.. VAZQUEZ. E. and SCHNAPER, L. ELISA tests for antibodies against mycobacterial glycolipids. J. Immunol. Methods 34(1980)55-60.
37. REZNIKOV. M. and DAWSON. D. J. Mycobacteria of the intracellulare-scrofulaceum group in soils in the Adelaide area. Pathology 12(1980)525-528.
38. REZNIKOV, M. and LEGGO. J. H. Examination of soil in the Brisbane area for organisms of the Mycobacterium avium-intracellulare-scrofulaceum complex. Pathology 6(1974)269-273.
39. RIDLEY, D. S. and JOPLING, W. H. Classification according to immunity: a live-group system. Int. J. Lepr. 34(1966)255-273.
40. ROCHE, P. W., BRITTON. W. J.. FAILBUS, S. S.. LUDWIG, H., THEUVENET, W. J. and ADIGA, R. B. Heterogeneity of serological responses in paucibacillary leprosy-differential responses to protein and carbohydrate antigens and correlation with clinical parameters. Int. J. Lepr. 58(1990)319-327.
41. SHIELD, M.J. The importance of immunologically effective contact with environmental mycobacteria. In: The Biology of the Mycobacteria. Volume 2. Ratledge, C. and Stanford. J., cds. London: Academic Press, 1983, pp. 343-415.
42. SINGER, E. and RODDA, G. M. J. Non-specific sensitization to old tuberculin: antigenic studies. Tubercle 44(1963)268-280.
43. SINGER, E. and RODDA. G. M. J. Non-specific sensitization to old tuberculin: mycobacteria in water. Tubercle 46(1965)209-213.
44. TAKAHASHI, Y. Specific serum agglutination of kaolin particles sensitized with tubercle phosphatide and its clinical evaluation as a sérodiagnostic test for tuberculosis. Am. Rev. Respir. Dis. 85(1962)708-719.
45. TAKAIIASHl,Y..ONODERA,TlindYAMAMOToK.-I.The behaviour of three différent kinds of antibodies in tuberculosis: antiprotein. antipolysaccharide and antiphosphalide: I. Experimental tuberculosis. J. Exp. Med. 14(1961)555-579.
46. Towbin, H., Staehelin, T. and GORDON, J. Electrophoretic transfer of proteins from Polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sei. U.S.A. 76(1979)4350-4354.
47. TURNEER, M., VAN VOOREN. J. P.. NYABENDA. J.. LEGORS, F., LECOMTE. F., THIRIAUX, J., SERRUYS. E. and YERNAULT, J. C. The humoral immune response after BC G vaccination in humans: consequences for the serodiagnosis of tuberculosis. Eur. Respir. J. 1(1988)589-593.
48. VAN VOOREN. J. P., FARBER, C.-M.. MOTTE, S., DE BRUYN, J., LEGROS, F. and YERNAULT, J. C. Assay of specific antibody response to mycobacterial antigen for the diagnosis of a pleural effusion in a patient with AIDS. Tubercle 69(1988)303-305.
49. WINTER, S. M., BERNARD, E. M., GOLD. J. W. M. and ARMSTRONG, D. Humoral response to disseminated infection by Mycobacterium avium-Mycobacterium intracellular in acquired immunodeficiency syndrome and hairy cell leukemia. J. Infect. Dis. 151(1985)523-527.
50. YOUNG, D. B., DISSANAYAKE, S., MILLER, R. A.. KHANOLKAR, S. R. and BUCHANAN. T. M. Humans respond predominantly with IgM immunoglobulin to the species-specific glycolipid of Mycobacterium leprae. J. Infect. Dis. 149(1984)870-873.
1. M.B.B.S, F.R.A.C.P., F.R.C.P.A.; D.Phil (Oxon), F.T.S., Director, Centenary Institute of Cancer Medicine and Cell Biology. University of Sydney, New South Wales 2006, Australia.
2. B.Sc; D.Phil (Oxon), F.T.S., Director, Centenary Institute of Cancer Medicine and Cell Biology. University of Sydney, New South Wales 2006, Australia.
3. M.B.B.S., F.R.A.C.P., F.R.C.P.A.; D.Phil (Oxon), F.T.S., Director, Centenary Institute of Cancer Medicine and Cell Biology. University of Sydney, New South Wales 2006, Australia.
4. M.B.B.S., F.R.A.C.P., F.R.C.P.A.. D.Phil (Oxon), F.T.S., Director, Centenary Institute of Cancer Medicine and Cell Biology. University of Sydney, New South Wales 2006, Australia.
5. Ph.D., Centre National de la Recherche Scientifique, 118 Route de Narbonne, 31062 Toulouse, France.
6. M.B.B.S., F.R.A.C.P., F.R.C.P.A., Department of Infectious Disease, Prince Henry Hospital, Little Bay, Sydney, New South Wales 2036, Australia.
7. M.B.B.S.. Student Health Service, University of Sydney, Sydney. New South Wales 2006, Australia.
Reprint requests to Prof. Basten.
Received for publication on 13 December 1991.
Accepted for publication on 31 Mareh 1992.