- Volume 60 , Number 3
- Page: 368–75
Recognition of Mycobacterium leprae recombinant 18-KDa proteins in leprosy
ABSTRACT
Three different, purified, Escherichia coli derived, recombinant preparations of the Mycobacterium leprae 18K protein were compared for their immunological recognition in leprosy. The preparations tested were 18K fusion proteins containing 70% (amino acids 38-148) of the full 18K protein fused to either a short leader sequence containing six asparagine residues or to β-galactosidase, and the full length 18K protein. All three recombinant antigens were recognized by IgG antibodies which were restricted mostly to lepromatous leprosy patients. The 18K antigen with the asparagine leader sequence showed better reactivity with IgG antibodies compared with the other two 18K preparations. In lymphocyte proliferation assays, the truncated 18Kand the full-length 18K showed equivalent responses in the same donors with strongest recognition in donors who were also strongly responsive to the M. leprae soluble sonicate. These results indicate that the major human B- and T-cell epitopes are located within the segment 38-148, although some individuals may recognize additional epitopes at the NH2-terminal end.RÉSUMÉ
Trois préparations différentes de recombinants de la protéine 18K de Mycobacterium leprae purifiées obtenues à partir d' Escherichia coli ont été comparées quant à leur pouvoir de reconnaissance immunologique de la lèpre. Les préparations testées étaient des protéines de fusion 18K contenant 70% (acides aminés 38-148) de la protéine 18K complète attachées soit à une courte séquence contenant 6 résidus asparagine ou au β-galactosidase, et la protéine 18K complète. Les trois antigènes recombinants ont été reconnus par des anticorps IgG essentiellement spécifiques à des patients lépromateux. L'antigène 18K avec la séquence asparagine montrait une meilleure réactivité avec les anticorps IgG comparé aux deux autres préparations 18K. Dans les tests de prolifération lymphocytaire, les préparations tronquées et complètes 18K montraient des réponses équivalentes chez les mêmes donneurs avec la reconnaissance la plus forte chez les donneurs qui répondaient également fortement aux sonicats solubles de M. leprae. Ces résultats indiquent que les epitopes principaux des cellules humaines B et T sont localisés dans le segment 38-148, bien que certains individus puissent reconnaître des epitopes additionnels au niveau de l'extrémité NH2.RESUMEN
Tres preparaciones recombinantes de la Mycobacterium leprae de la proteína de 18 kDa purificadas a partir de Escherichia coli, se compararon en cuanto a su reconocimiento inmunológico por los pacientes con lepra. Las preparaciones probadas fueron proteínas de fusión de 18 kDa conteniendo 70% (aminoácidos 38-148) del total de la proteína de 18 kDa completa, fusionadas a una corta secuencia lider conteniendo 6 residuos de asparagina o a la beta-galactosidasa, y la proteína de 18 kDa completa. Los 3 antígenos recombinantes fueron reconocidos por anticuerpos IgG restringidos casi exclusivamente a los pacientes lcpromatosos. El antígeno de 18 kDa con la secuencia líder de asparagina mostró mejor reactividad con los anticuerpos IgG que los otros dos antígenos recombinantes. En los ensayos de proliferación de linfocitos, los antígenos truncados y el antígeno completo de 18 kDa, indujeron respuestas equivalentes en los mismos donadores. Las respuestas más intensas se observaron en aquellos donadores que también dieron respuestas intensas con sonicados solubles del Mycobacterium leprae. Estos resultados indican que los epitopes más importantes reconocidos por los linfocitos T y B, están localizados dentro del segmento 38-148, aunque algunos individuos pueden reconocer epitopes adicionales en el extremo NH2-terminal.An increasing number of Mycobacterium leprae genes have been cloned and sequenced (18). Several of these have been shown to belong to the family of heat-shock proteins, such as the M. leprae 65-kDa and 18-kDa antigens (8,12), and are available through the World Heath Organization IMMLEP bank. Most of the M. leprae proteins exhibit immunologic crossreactivity at the antibody level with proteins from other mycobacteria. However, the determinant of the 18-kDa protein recognized by the monoclonal antibody L7.15 (2), later designated as L5, seemed to be species-specific for M. leprae (6). Human T-cell recognition of the 18-kDa antigen was first reported using crude Escherichia coli lysates containing the antigen as the β-galactosidase fusion protein; these preparations stimulated T-cell clones from M. leprae-vaccinated donors (6). When the 18-kDa β-galactosidase (18K fSgal) fusion protein was purified and used in proliferation assays with human peripheral blood mononuclear cells, there was a substantial antigen-specific response to β-galactosidase which masked T-cell responses to the 18-kDa antigen itself (15). The 18kDa gene was, therefore, recloned and expressed fused to a short leader sequence containing six consecutive asparagine residues (18K Asn) to reduce proteolysis; this protein stimulated T-cell proliferation in a high proportion of patients with the tuberculoid type of leprosy and in healthy contacts of leprosy patients (3). This 18-kDa gene, originally cloned in λgt11 (19) was found to contain only 70% of the whole 18kDa gene as sequenced from a genomic M. leprae cosmid library ('). The full-length 18kDa antigen (18K JDW) has now been cloned, purified and tested for B- and T-cell reactivity in mice (1,4,5,7).
The choice of expression systems in the presence or absence of β-galactosidasc (βgal) or a leader sequence and the method of purification may all affect the antigenic epitopes recognized by B and T cells to variable extents. In addition, epitopes recognized by the leprosy patients at both the B- and T-cell level may be different than those recognized by mice. We have, therefore, directly compared these three preparations of the M. leprae 18-kDa protein (18K Asn, 18K βgal and 18KJDW) for their immunological reactivity in patients with leprosy.
MATERIALS AND METHODS
Patients and controls. Untreated leprosy patients presenting at the Marie Adelaide Leprosy Center, Karachi, Pakistan, were recruited for our studies and have been described in detail elsewhere (8-9). Patients are diagnosed clinically as well as histologically on a 4-mm punch biopsy taken from the edge of an active lesion. Ninety-six patients across the leprosy spectrum, two healthy contacts of leprosy patients, and employees working at The Aga Khan University, Karachi, Pakistan, with no known contact with leprosy (endemic controls) were included in the study.
Antigens. The recombinant M. leprae 18kDa preparations used in the study were 18kDa β-galactosidase (18K βgal), and asparagine 18-kDa fusion proteins (18K Asn), both of which contained the carboxy-terminal two-thirds of the full 18-kDa gene(15), and the full length 18-kDa protein (1) (18K JDW). All three recombinant 18K preparations were E. coli-derived; 18K βgal was purified by preparative SDS polyacrylamide gel electrophoresis (SDS-PAGE) and acetone precipitated; 18K Asn was purified by high pressure liquid chromatography (HPLC); 18K JDW was first ammonium sulfate precipitated and further subjected to HPLC; β-galactosidase was prepared in the same way as the 18K βgal antigen after induction of E, coli with isopropyl-β-D-thiogalactopyranoside (IPTG). All proteins were > 95% pure as assessed by SDS-PAGE.
Armadillo-derived, soluble, whole M. leprae sonicate (MLSON) (Batch CD114 and CD 135) was a gift from Dr. R. J. W. Rees, National Institute of Medical Research, London, U.K., through the IMMLEP leprosy antigen bank.
Antisera. Five-ml blood samples collected from both leprosy patients and endemic controls were allowed to separate overnight at 4ºC. The serum was removed and centrifuged at 2000 rpm for 15 min; the clear supernatant was distributed in small aliquots and frozen at -70ºC before use.
Antibody determinations. Plates were coated with 100 µl of antigens at 1 µl/ml in carbonate buffer, pH 9.6, for 4 hr at 37ºC and then overnight at 4ºC. After washing, blocking with 200 µl phosphate buffered saline (PBS) containing 5% bovine serum albumin (BSA) was performed for 2 hr at 37ºC. After washing three times in PBS-Tween 20, 100 fil of sera diluted in PBS-Tween containing 1.0% BSA was added and incubated for a further 2 hr at 37ºC; 100µl alkaline phosphatase-labeled goat anti-human IgG (Fc specific; Jackson ImmunoResearch Laboratories, West Grove, Pennsylvania, U.S.A.) diluted 1/1000 was used. In some assays biotinylated goat anti-human IgG diluted 1/1000 (Fc specific; Jackson) followed by streptavidin-coupled alkaline phosphatase, diluted 1/1000, was used instead of the first conjugate. In the avidin-biotin system, plates were coated with 0.1 µg/ml of antigen due to the high sensitivity of the assay system. In both cases, the reaction was stopped with the addition of 50 µl 3 N NaOH. Sera were initially screened at 1/100 and four sera with high levels of antibodies to 18K were pooled and used as a reference positive control in all subsequent assays. For some of the ELISAs, two additional internal controls representing high and low activity to 18K were also run simultaneously. In addition, at least two control sera with no activity to 18K were repeatedly run to monitor the background binding. Correlation of antibody activity to 18K Asn and 18K βgal was carried out on 65 sera (38 LL/BL and 37 BT/TT) and 18KAsnand 18K JDWon 39 sera (29 LL/BL and 10 BT/TT) from untreated leprosy patients.
Lymphocyte transformation test. Peripheral blood mononuclear cells (PBMC) were separated from heparinized blood on Ficol-Histopaque 1077 (Sigma, Poole, U.K.). The study group consisted of 4 endemic controls, and 7 untreated leprosy patients (5 BT/TT, 1 indeterminate, 1 BL). Cells were rcsuspended at 2 × 105 PBMC in RPMI 1640 (Sigma) containing 10% autologous plasma. Culture conditions were as described previously (3). The results were expressed as stimulation index [mean counts per minute (cpm) of triplicate cultures with antigen divided by mean cpm of the triplicate cultures without antigen]. A stimulation index of 2.0 or greater was considered significant.
Statistical analysis. Spearman rank correlation was used to determine the association between lymphocyte proliferative responses to the different antigens of M. leprae.
RESULTS
Comparison of ELISAs for 18-kDa recombinant antigens. Figure 1 shows the dose-response curve obtained with the positive serum pool developed with the antihuman IgG antibody conjugated to either alkaline phosphatase (direct) or biotin followed by avidin conjugated to alkaline phosphatase (indirect). Both systems gave acceptable signals when tested with antigen at a concentration of 0.1 µg/ml. Higher signals were observed with the avidin-biotin system than with the direct alkaline phosphatase system. The avidin-biotin system was then used to compare the antibody reactivity of the three 18-kDa antigen preparations.
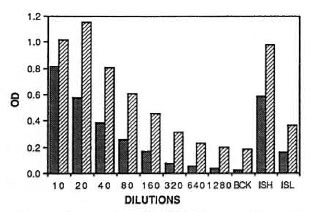
Fig. 1. Comparison of ELISAs for recombinant M. leprae 18K Asn.  = assay using alkaline phosphate conjugate;
= assay using alkaline phosphate conjugate;  = results of the avidin-biotin conjugate system. Double dilution of a high-titered serum pool starting at 1/10 dilution was tested in plates coated with 0.1 µg/ml of antigen. BCK = background responses of blocked wells with no antigen and tested with 1/10 dilution of the positive serum pool; ISH and ISL = individual sera (tested at 1/10 dilution) with high and low antibody activity to 18K Asn.
= results of the avidin-biotin conjugate system. Double dilution of a high-titered serum pool starting at 1/10 dilution was tested in plates coated with 0.1 µg/ml of antigen. BCK = background responses of blocked wells with no antigen and tested with 1/10 dilution of the positive serum pool; ISH and ISL = individual sera (tested at 1/10 dilution) with high and low antibody activity to 18K Asn.
Figure 2 shows the reactivity with the positive pool as well as two individual sera with high and low activity to the 18-kDa antigen. All three antigens were tested under identical conditions in one single assay. The background responses to the βgal control interfered with evaluation of the antibody activity to 18Kβgal. 18K Asn showed higher positivity with the pool than did 18K JDW at all dilutions of the positive pool. However, both 18-kDa preparations (18K Asn and 18K JDW) showed similar reactivity with the individual patient sera. The higher reactivity of 18K Asn with the pool may have been due to a bias in the selection of sera for the pool which were chosen on the basis of their high reactivity with the 18K Asn. To assess the differences in reactivity of the three preparations further, individual sera from leprosy patients were also tested.
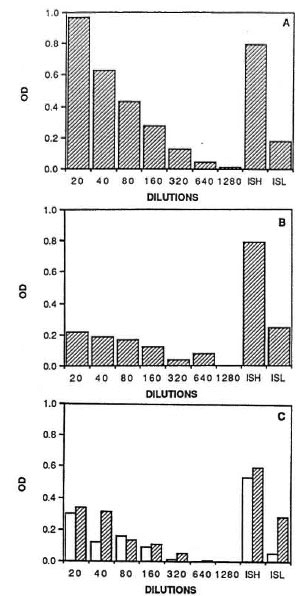
Fig. 2. Comparison of three recombinant M. leprae 18-kDa preparations by ELISA using the avidin-biotin conjugate system. Dilutions of a high-titered serumpool and two individual sera (ISH and ISL) were tested against the 18-kDa antigen as described in the legend for Fig. 1. A = results with 18K Asn; B = 18K JDW; C = 18K-βgal ( ) or βgal (□).
) or βgal (□).
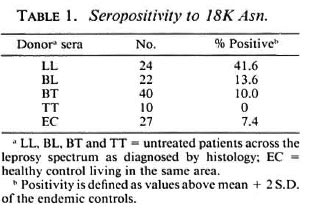
IgG antibody to the 18-kDa antigens in leprosy sera. Sera were tested in both the direct alkaline phosphatase and the avidinbiotin system. In general, more consistent results were obtained with the direct alkaline phosphatase system. The avidin-biotin system gave not only variable backgrounds with endemic-control sera but, in addition, the values obtained in this assay system were found to be less consistent with the sera of leprosy patients. Therefore, results are only shown for the direct alkaline phosphatase system. Figure 3 shows correlation between the antibody activity to 18K Asn and the other two preparations. Antibody reactivity with 18K Asn was consistently higher than with 18K JDW or 18K βgal. The cut off for normal endemic sera (mean + 2 S.D.) is shown as vertical or horizontal lines which were used to determine the seropositivity. Only 9.2% (N = 6) were positive to both 18K Asn and 18K βgal while 25% (N = 16) were positive to 18K Asn alone in the same set (Fig. 3A). A slightly higher percentage was positive to both 18KAsnand 18KJDW (18%) and to 18K Asn alone (26%) (Fig. 3B). The major discrepancy was in sera with lower activity. Sera highly positive to 18K Asn (> 0.8 OD) generally show positivity to theother two preparations. The exceptions were two sera with the 18K ital preparationwhere the high reactivity was due to antibodies to βga1 (data not shown). Because of the higher immunological activity of 18K Asn, this preparation was further used to determine the seropositivity across the leprosy disease spectrum (Table 1). Patients with lepromatous leprosy showed the highest antibody responses to 18K Asn. It was interesting to note that seropositivity to 18K Asn was restricted even within the lepromatous leprosy group (41.6%) which usually showed close to 100% positivity to M. leprae sonicate in both the IgM and IgG isotypes.
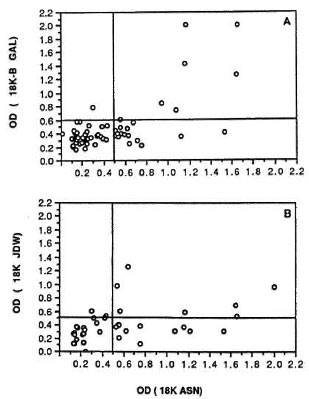
Fig. 3. Comparison of 18K Asn antibody reactivityin leprosy sera to M. leprae 18K βgal and I8K JDW. A: N = 65; B: N = 39; horizontal and vertical lines indicate a cut-off value (endemic control mean ± 2S.D.) for seropositivity.
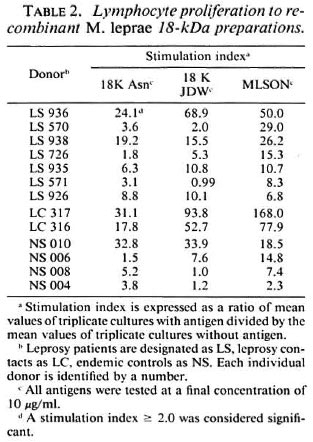
Lymphocyte proliferation to the 18-kDa antigens. These antigens were next compared for their ability to induce lymphocyte proliferation. Lymphocytes from peripheral blood obtained from leprosy patients, healthy contacts of leprosy patients, or healthy endemic controls with no known contact with leprosy patients were tested against M. leprae soluble sonicate (MLSON), 18K Asn and 18K JDW. 18K βgal was not included in these experiments because of the previously reported high T-cell reactivity to βgal itself (15). T-cell reactivity to these three antigens in individual donors is shown in Table 2. Donors with positive lymphocyte proliferation to MLSON are shown. Significant correlation was observed with MLSON for both 18K Asn (p < 0.048) and 18K JDW (p < 0.005). The strongest responses to the 18-kDa antigens were observed in the donors showing the highest responses to M. leprae. Four out of seven leprosy patients showed similar lymphocyte proliferative responses. One leprosy patient and the two contacts of leprosy patients responded more strongly to the full-length molecule, while some donors failed to respond to one or the other 18-kDa antigen.
DISCUSSION
The gene for the M. leprae 18-kDa protein was one of the first leprosy genes cloned in Xgt11 (19). Several laboratories have been using 18-kDa preparations obtained by different purification protocols which may result in differential preservation of antigenic epitopes. We have, therefore, compared IgG antibody responses to three 18-kDa preparations obtained using different protocols (two 18-kDa antigens containing amino acids 38-148 and the full-length protein) in leprosy. Our observation that the IgG responses to the asparagine 18-kDa antigen was higher than to the other two 18-kDa antigens implies that the major human B-cell epitopes are located within the carboxy-terminal two-thirds of the molecule. It is perhaps surprising that the 18K Asn gave higher antibody responses than the full-length molecule; the asparagine leader sequence which protects the recombinant antigen from degradation during its expression in E. coli (14) may provide the protein with increased solubility as well as additional charges giving better binding to the plastic surfaces during ELISAs. It was difficult to assess antibody binding to the purified asparagine leader sequence since small peptides tend to wash off the plastic more easily, resulting in inconsistent binding. Despite this technical problem, extensive testing with the leader peptide failed to demonstrate significant antibody recognition to this peptide (data not shown), indicating that the antibody responses were directed to the 18-kDa portion of the molecule. This is further supported by the observation that comparable antibody responses were obtained with other 18-kDa preparations lacking the asparagine leader sequence.
The purified, full-length 18-kDa protein and the synthetic peptides covering its sequence have been used previously to define B-cell epitopes in mice (BALB/c) immunized with the synthetic peptides. An IgG response to six peptides was observed, five of which were also recognized by B10.BR mice (4). Helper T-cell responses were inferred by the responses in the IgG isotypc to four peptides (1-20, 16-35, 31-50 and 76-95) in B10.BR mice. Interestingly, mouse T-cell proliferative responses mapped to 116-120 (7) which is adjacent to the L5 epitope located in the region of 109-115 (5), indicating that T-helper cells providing help for IgG isotype switching may be distinct from those inducing proliferative responses. Such a dichotomy of T-cell proliferation and IgG antibody responses has been shown for murine immune responses to human basement collagen (16) which was shown to be under Ir gene control. Our results with lepromatous leprosy patients who are high responders for IgG antibody to M. leprae 18K Asn but show little or no proliferative responses (3) would indicate a similar dichotomy of T-cell help for antibody and proliferative responses. Furthermore, the restricted antibody response to the 18-kDa protein, even within the lepromatous leprosy patients, may indicate some genetic restriction which also has been suggested by studies in mice; high, intermediate and low responder strains have been identified in terms of their IgG responses to the 18-kDa proteins which show linkage to genes in the H2 Complex (4).
Epitopes recognized by human T cells during proliferative responses may be distinct from those recognized by mouse T cells primed using immunization protocols. Human T-cell responses to the 18-kDa protein were first reported using a crude lysate containing the 18-kDa antigen as a βgal fusion protein with T-cell clones derived from volunteers immunized with killed M. leprae vaccine (11). Five out of 10 human T-cell clones, selected to respond to M. leprae but not to PPD or M. bovis BCG, recognized the 18-kDa antigen, indicating that this protein contained a leprosy-specific, dominant, T-cell epitope. However, these clones did respond to M. scrofulaceum (11) and, in one case, to Mycobacterium w (10). The T-cell epitope recognized by human T-cell clones was shown to be located in the region of 38- 50, and was recognized in a DR4 Dw4-restricted fashion by the four T-cell clones tested (13).
We have been looking at T-cell recognition in leprosy patients and their contacts who may respond differently during an active or subclinical infection than donors immunized with M. leprae vaccine. We have previously shown that the 18K Asn (38- 148) with a short asparagine leader sequence, but without the β-galactosidase, was strongly recognized by long-term healthy leprosy contacts (3). Recombinant antigens derived from E. coli may also be contaminated with different amounts of endotoxin and E. coli-derived proteins which may interfere with the evaluation of the immune response to the antigen of interest, particularly at the T-cell level. We believe that our immune responses are directed to the 18-kDa proteins since T-cell proliferation to the 18-kDa proteins correlated well with proliferation to the M. leprae soluble sonicate. In addition, all three proteins showed comparable T-cell responses in the majority of the donors, and some have shown higher T-cell reactivity to 18K JDW which contains little or no endotoxin (H. M. Dockrell and S. K. Young, unpublished observations).
Most donors who gave lymphocyte proliferation to the 18K Asn antigen also responded to the full-length 18-kDa protein. It was interesting to note that 5 out of 13 donors responded more strongly to the fulllength molecule, indicating that the region between 1-38 amino acids may contain an additional T-cell epitope. The only human T-cell epitope reported so far is located within the region of 38-50. Our results further indicate that the 18-kDa antigen may also contain an epitope crossreactive with M. bovis BCG, as shown by the responses in BCG-vaccinated donors in the U.K. (3) and as shown in this study by the responses to the 18-kDa proteins in healthy endemic controls who are likely to have been vaccinated with BCG. Further epitope mapping should identify if one of the T-cell epitopes is species-specific for M. leprae.
Acknowledgment. This investigation received financial support from the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases. HMD and NGS received additional support from the Medical Research Council, London, U.K.
The authors would like to thank Drs. Qadeer Ehsan, Khekashan Hussain, and Mustafa Ali Khan for their help in obtaining patient material at the Marie Adelaide Leprosy Center, Karachi, Pakistan. Histopathological diagnosis was performed by Dr. Sebastian Lucas, University College and Middlesex School of Medicine, London, U.K. Excellent technical assistance was provided by Miss Sarwat Jamil, Mrs. Maqboola Dojki and Neville Lyall. The antigens were provided with the help of Drs. Roger Booth and Ross Prcstidge, University of Auckland, New Zealand; Kathie Grant, London School of Hygiene and Tropical Medicine; and Dr. R. J. W. Rees, National Institute for Medical Research, London, U.K. The authors are grateful to Dr. A. W. Sturm for reviewing the manuscript. Secretarial help was provided by Miss Regina Paul.
REFERENCES
1. BOOTH, R. J., HARRIS, D. P., LOVE, J. M. and WATSON, J. D. Antigenic proteins of Mycobacterium leprae: complete sequence of the gene for the 18-kDa protein. J. Immunol. 140(1988)597-601.
2. BRITTON, W. J., HELLQVIST, L., BASTEN, A. and RAISON, L. Mycobacterium leprae antigens involved in human immune responses. I. Identification of four antigens by monoclonal antibodies. J. Immunol. 135(1985)4171-4176.
3. DOCKRELL, H. M., STOKER, N. G., LEE, S. P., JACKSON, M., GRANT, K. A., JOUY, N. F., LUCAS,S. B., HASAN, R., HUSSAIN, R. and MCADAM, K. P. W. J. T-cell recognition of the 18-kilodalton antigen of Mycobacterium leprae. Infect. Immun. 57(1989)1979-1983.
4. DOHERTY, T. M., BACKSTROM, B. T., PRESTIDGE, R. L., LOVE, S. G., HARDING, D. R. K. and WATSON, J. D. Immune responses to the 18-kDa protein of Mycobacterium leprae; similar B cell epitopes but different T cell epitopes seen by inbred strains of mice. J. Immunol. 146(1991)1934-1940.
5. DOHERTY, T. M., BOOTH, R. K., LOVE, S. G., GIBSON, J. J., HARDING, D. R. K. and WATSON, J. D. Characterisation of an antibody-binding epitope from the 18-kDa protein on Mycobacterium leprae. J. Immunol. 142(1989)1691-1695.
6. ENGERS. H. D.. ABE, M., BLOOM, B. R.. MEHRA, V., BRITTON, W., BUCHANAN, T. M., KHANOLKAR, S. K.. YOUNG, D. B., CLOSS, O., GILLIS, T., HARBOE, M., IVANYI, J., KOLK, A. H. J. and SHEPARD, C. C. Results of a World Health Organisation-sponsored workshop on monoclonal antibodies to Mycobacterium leprae. Infect. Immun. 48(1985)603-605.
7. HARRIS, D. P., BACKSTROM, B. T., BOOTH, R. J., LOVE, S. G., HARDING, D. H. and WATSON, J. D. The mapping of epitopes of the 18-kDa protein of Mycobacterium leprae recognized by murine T cells in a proliferation assay. J. Immunol. 143(1989)2006-2012.
8. HASAN, R., DOCKRELL, H. M., CHIANG, T. and HUSSAIN, R. Quantitative antibody ELISA for leprosy. Int. J. Lepr. 57(1989)766-776.
9. HUSSAIN, R.. JAMIL, S., KIFAYET, A., FIRDAUSI, F., DOCKRELL, H. M., LUCAS, S. and HASAN, R. Quantitation of IgM antibodies to the M. leprae synthetic disaccharide can predict early bacterial multiplication in leprosy. Int. J. Lepr. 58(1990)491-502.
10. MUSTAFA, A. S. Identification of T cell-activating recombinant antigens shared among three candidate antilcprosy vaccine, killed M. leprae, M. bovis BCG, and Mycobacterium to. Int. J. Lepr. 56(1988)265-273.
11. MUSTAFA, A. S., GILL, H. K., NERLAND, A.. BRITTON, W. J., MEHRA, V., BLOOM, B. R., YOUNG, R. A. and GODAL, T. Human T-cell clones recognise a major M. leprae protein antigen expressed in E. coli. Nature 319(1986)63-66.
12. NERLAND, A. H., MUSTAFA, A. S., YOUNG, R. A., SWEETSER, D. and GODAL, T. A protein antigen of Mycobacterium leprae is related to a family of small heat shock proteins. J. Bacteriol. 170(1988)5919-5921.
13. OFTUNG, F., SHINNICK, T. M., MUSTAFA, A. S., LUNDIN, K. E. A., GODAL, T. and NERLAND, A. H. Heterogeneity among human T cell clones recognizing an HLA-DR4, Dw4-restricted epitope from the 18-kDa antigen of Mycobacterium leprae, defined by the synthetic peptides. J. Immunol. 114(1990)1478-1483.
14. SUNG, W. L., YAO, F.-L., ZAHAB, D. M. and NARANG, S. A. Short synthetic oligodeoxy ribonucleotide leader sequences enhance accumulation of human proinsulin synthesized in Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 83(1986)561-565.
15. STOKER, N. G., GRANT, K. A., DOCKRELL, H. M., HOWARD, C. R., JOUY, N. F. and MCADAM, K. P. W. J. High level expression of genes cloned in phage λgt11. Gene 78(1989)93-99.
16. TITE, J. P., FOELLMER, H. G., MADRI, J. A. and JANEWAY, C. A.. JR. Inverse Irgene control of the antibody and T cell proliferative responses to human basement membrane collagen. J. Immunol. 139(1987)2892-2898.
17. YOUNG, D., LATHIGRA. R., HENBRIX. R., SWEET-SER, D. and YOUNG, R. A. Stress proteins are immune targets in leprosy and tuberculosis. Proc. Natl. Acad. Sci. U.S.A. 85(1988)4267-4270.
18. YOUNG, D. B., KAUFMANN, S. H. E., HERMANS, P. W. M. and THOLE, J. E. R. Mycobacterial protein antigens: a compilation. Mol. Microbiol. 6(1992)133-145.
19. YOUNG, R. A., MEHRA, V., SWEETSER, D., BUCHANAN, T., CLARK-CURTISS, J., DAVIS, R. W. and BLOOM, B. R. Genes for the major protein antigens of the leprosy parasite Mycobacterium leprae. Nature 316(1985)450-452.
1. Ph.D.. M.R.C. (Path.), Department of Microbiology, The Aga Khan University, P.O. Box 3500, Karachi, Pakistan.
2. Ph.D., Department of Clinical Sciences, London School of Hygiene and Tropical Medicine, London, U.K.
3. M.Sc, Department of Microbiology, The Aga Khan University, P.O. Box 3500, Karachi, Pakistan.
4. M.B.B.S., Department of Microbiology, The Aga Khan University, P.O. Box 3500, Karachi, Pakistan.
5. Ph.D., Department of Molecular Medicine, School of Medicine. University of Auckland. Auckland, New Zealand.
6. M.B.B.S.. M.Sc, Marie Adelaide Leprosy Center, Karachi, Pakistan.
7. Department of Clinical Sciences, London School of Hygiene and Tropical Medicine, London, U.K. Department of Molecular Medicine, School of Medicine. University of Auckland. Auckland, New Zealand.
Received for publication on 25 Mareh 1992.
Accepted for publication in revised form on 1 June 1992.