- Volume 60 , Number 3
- Page: 376–81
Comparison of Bis-di-octadecylamide of trehalose dicarboxylic acid (BDA.TDA) with glycolipid SL-IV as ELISA antigens for the serodiagnosis of leprosy
ABSTRACT
Two glycolipids-one synthetic and nonnatural (BDA.TDA), the other natural and Mycobacterium tuberculosis species-specific (SL-IV)-were tested to determine their serological activity in sera obtained f rom leprosy patients, and to determine their discriminating ability in the detection of disease. The ELISA results obtained in the IgG antibody class show that both were useful substances capable of detecting multibacillary and paucibacillary disease in about 2 out of 3 leprosy patients. When these antigens were tested in parallel, the sensitivity of the ELISA test was increased by 10% without a decrease in specificity.RÉSUMÉ
Deux glycolipides, l'un synthétique et non naturel (BDA.TDA). l'autre naturel et spécifique pour le Mycobacterium tuberculosis (SL-IV). ont été testés pour déterminer leur activité sérologiquc sur des sérums obtenus chez des malades de la lèpre, et pour déterminer leur capacité discriminante dans la détection de la maladie. Les résultats des tests ELISA obtenus pour les anticorps de la classe des IgG montrent que tous deux étaient des substances utiles capables de détecter la lèpre multibacillairc et paucibacillairc chez environ deux patients sur trois. Quand ces antigènes furent testés en parallèle, la sensibilité du test ELISA fut augmentée de 10% sans diminution de la spécificité.RESUMEN
Se probaron 2 glicolipidos -uno sintético (BDA.TDA) y otro natural y especifico del Mycobacterium tuberculosis (SL-IV) -para determinar su reactividad con los sueros obtenidos de pacientes con lepra y para establecer su capacidad discrimiante en la detección de la enfermedad. Los resultados de un ELISA para anticuerpos IgG, mostraron que ambas substancias fueron útiles para detectar la enfermedad multibacilar y paucibacilar en aproximadamente 2 de cada 3 pacientes. Cuando estos antigenos se probaron en paralelo, la sensibilidad del ensayo de ELISA se incrementó en un 10%, sin disminuir su especificidad.With about 10 million cases world-wide (1) leprosy is, after tuberculosis, the second most frequent mycobacterial disease affecting mankind. Leprosy is caused by Mycobacterium leprae, a microorganism which has not yet been successfully cultivated in vitro. Accordingly, bacteriological diagnosis of this disease is limited to the demonstration of the presence of acid-fast bacilli (AFB) in clinical specimens.
Leprosy exhibits a spectrum of specific immune states ranging from the polar tuberculoid (TT), in which cell-mediated immunity (CMI) predominates and few if any AFB can be found, to the polar lepromatous (LL), where humoral immunity overshadows CMI and large numbers of AFB ban be demonstrated in clinical specimens. Because of this, it is difficult to confirm the disease bacteriologically in patients at the tuberculoid end of the spectrum. It would, therefore, be of great interest to develop an alternate diagnostic procedure for leprosy such as an enzyme-linked immunosorbent assay (ELISA). A few such tests have been described and many different antigens have been tried; among them the most species specific was phenolic glycolipid-I (PGL-I)(2,3). PGL-I, although effective in the serodiagnosis of leprosy cases at the LL end of the spectrum, is less useful for the diagnosis of paucibacillary forms because of the low sensitivity observed (7,16 ).
Antibodies to sulfolipids have been demonstrated in sera of tuberculosis (5,12) and leprosy patients (4,11) . An ELISA based on the use of purified sulfoglycolipid-IV (SL-IV) has shown that this antigen could contribute significantly to the diagnosis and follow up of leprosy because, although the serological patterns were found to be similar to PGL-I patterns, the IgG responses toward SL-IV predominated over the IgM responses.
It has recently been shown that synthetic, pseudo-cord-factor-like glycolipids were recognized by sera from TB patients and that these compounds were highly efficient in the ELISA serodiagnosis of tuberculosis(13). We therefore thought that it would be of interest to examine one of these compounds, bis-dioctadecylamide of trehalose dicarboxylic acid (BDA.TDA) to see if it was also recognized by the sera of leprosy patients, and whether it could be of use in the serodiagnosis of this disease. We also wanted to determine if dual testing with SL-IV would significantly enhance the discriminating powers of single antigen testing of an ELISA-based serodiagnosis of leprosy.
MATERIALS AND METHODS
Sera. One-hundred-thirty-nine (139) human sera were tested in this study, of which 81 were taken from leprosy patients. According to the Ridley-Jopling classification (14), 10 sera belonged to the polar tuberculoid (TT) form, 16 to the polar lepromatous (LL) form, 8 to the borderline tuberculoid (BT) form, 7 to the indeterminate (IND) form, 8 to the mid-borderline (BB) form, and 7 to the borderline lepromatous (BL) form. Twenty-five (25) other human leprosy sera were obtained from the Institute Fame Pereo in Port au Prince, Haiti; 9 were classified as multibacillary and 16 paucibacillary. All leprosy sera were obtained during chemotherapy treatment. The other 58 sera were obtained from the Canadian Red Cross and from a local hospital's outpatient clinic. The latter sera belonged either to healthy individuals or to patients suffering from a variety of diseases other than tuberculosis or leprosy.
Antigens. Trehalose dicarboxylic acid bis (N,N-dioctadccylamide) (BDA.TDA) a "mirror amide" pseudo-cord-factor was supplied by one of us (MBG) (9) while SL-IV (6) was obtained from the Pasteur Institute of Paris. The chemical structure of SL-IV is currently being revised but until its definitive structure is established, the SL-IV designation is still used in this paper.
ELISA. This procedure has been described elsewhere (13). In short, 25 µl hexane containing 100 ng of glycolipid antigen was coated onto Dynatech Immulon-3 polystyrene microtiter plate wells using a single pipet and dried at 37ºC. Wells similarly treated but without antigen were used to check for nonspecific scrum absorption. After overnight saturation at 4ºC with phosphate buffered saline (PBS) containing 5% bovine scrum albumin (BSA), the plates were washed with PBS without Tween in a Titertek Microplate Washer (ICN Biomedicals, Inc., Huntsville, Alabama, U.S.A.). Test sera diluted 1/250 in PBS were added in 100-µl volumes into each well. After 90 min of incubation followed by further washing, goat anti-human IgG and IgM (H + L) β-galactosidase conjugates (Biosys, Compiegne, France) were added to the wells which were then incubated for 120 min. After an additional washing, o-nitrophenyl-β-D-glactoside (ONPG) (Sigma Chemical Co., St. Louis, Missouri, U.S.A.) substrate was added, and the plates were incubated at 37ºC for 60 min. Plates were read at 414 nm by a Titertek Multiskan MCC/340 reader (ICN Biomedicals); ∆414 OD values were determined by subtracting blank absorbance values from test absorbance values and by using a correction factor obtained by making the absorbance in wells containing the conjugate plus the substrate (v/v, 100µl) equal to the 100% response; results were confirmed once.
Data analysis. Sensitivity, specificity and predictive values were calculated by Bayesian methods (15). To determine the mean, standard deviation, coefficient of variation as well as the sensitivity and specificity values at any chosen cut-off point, data were entered into a "LOTUS 123" especially designed program. In order to compare the antigens we strove to choose cut-off points yielding maximum specificity values. Graphs were prepared using the "PRINT-GRAPH" program of "LOTUS 123." Dual modes of testing (8) were compared and analyzed with a special designed LOTUS program in which antigens are compared one on one and the Bayesian characteristics calculated accordingly.
RESULTS
Table 1 shows the mean values, standard deviations, and coefficients of variation for sera from the leprosy patient group and for sera belonging to presumably healthy individuals and patients suffering from diseases other than tuberculosis. The mean values of nonleprosy sera are higher for the synthetic antigen BDA.TDA than for the natural antigen SL-IV in both the IgG and IgM antibody classes. However, the coefficient of variation was almost twice as high for SL-IV than for BDA.TDA. The table also shows that sera from leprosy patients recognize the synthetic antigen more strongly than the natural antigen (OD 0.524 vs OD 0.307) but only in the IgG class, and that the coefficient of variation for the synthetic product is lower than that of SL-IV (53% vs 76%).
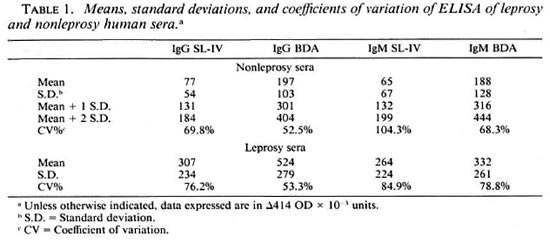
The discriminating power of ELISA testing of these two substances in the serodiagnosis of leprosy in the IgG and IgM classes is depicted in The Figure which shows the variation of the sensitivity and specificity parameters as a function of the increasing value of the cut-off point.
The figure. Variation of sensitivity and specificity parameters as a function of increasing OD values of the cut-off point: A = IgG SL-IV; B = IgM SL-IV; C = IgG BDA.TDA; D = IgM BDA.TDA.
High cut-off points, i.e., mean plus two standard deviations, can be chosen to maximize the specificity of the test and to facilitate the comparison of the performance of the antigens (Table 2). The two antigens have similar capabilities in the IgG class, i.e., BDA.TDA shows a sensitivity of about 63% at a specificity of about 97% with the cut-off value at OD 0.350 versus about 63% at a specificity of about 98%, respectively, for SL-IV with the cut-off value at OD 0.150. The data obtained in the IgM class using the corresponding cut-off values yielded lower sensitivity levels, especially in the case of BDA.TDA.
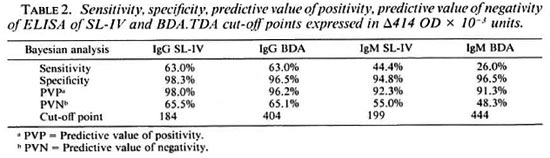
Of the 81 leprosy sera included in this study, 41 can be considered as paucibacillary (TT, BT, IND) and 40 as multibacillary (BB, BL, LL). Table 3 shows the means, standard deviations and coefficients of variation obtained in sera from multibacillary (MB) and paucibacillary (PB) leprosy patients with both SL-IV and BDA.TDA. It is evident from this table that the level of antigen recognition is higher in MB patients than in PB patients for SL-IV in both the IgG and IgM antibody classes. This is not the case for BDA.TDA, since the mean values vary only as a function of antibody class, i.e., IgG means are higher than IgM means, but there are no differences in the level of recognition between PB and MB disease.
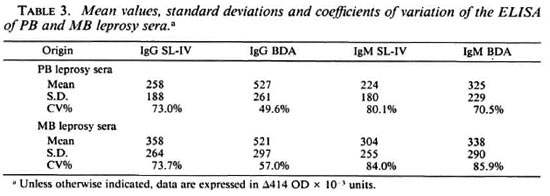
At the cut-off points which were chosen above and for data obtained in the IgG class, SL-IV detected about 61% (25/41) of the PB cases and about 65% (26/40) of the MB cases; BDA.TDA detected about 59% (24/ 41) and about 63% (25/40), respectively.
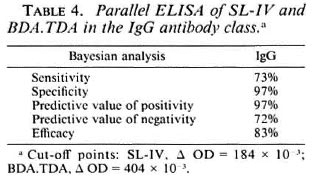
Table 4 shows the results obtained by parallel testing of both antigens in the IgG class. Dual parallel testing of SL-IV and BDA.TDA increases the sensitivity of the test by about 10% to 73%, the specificity remaining unchanged.
DISCUSSION
The results of this comparison study have shown that an entirely synthetic "mirroramide"-pseudo-cord-factor (BDA.TDA) is recognized more strongly than a natural species-specific glycolipid of M. tuberculosis (SL-IV) by IgG class antibodies by sera belonging to leprosy patients. A similar result has recently been reported for tuberculosis(13). The fact that both tuberculosis and leprosy patients' sera can recognize an artificial molecule, unlikely to be found in nature, suggests that these patients might be reacting to either a BDA.TDA related, natural, yet still undiscovered antigen, or to a known natural substance such as cord factor substantially modified by the host to show structural resemblance to BDA.TDA. The response to BDA.TDA of leprosy sera measured in the IgM class is almost half as strong as in the IgG class. This is what one could expect if the latter hypothesis were true, since IgM is the predominant early antibody frequently seen in the immune response to antigenically complex infectious organisms.
Results obtained in the IgG ELISA also show that both BDA.TDA and SL-IV are useful substances capable of detecting disease in two out of three patients suffering from leprosy, both MB and PB disease. These results differ from those obtained with other antigens, such as the M. leprae-specific PGL-I which was found to more efficiently detect the MB forms of the disease. In the case of SL-IV, one would also expect less recognition of PB disease since ∆414 OD levels are lower than in MB disease. However, both substances tested in this study detect an equivalent percentage of both PB and MB disease. This, according to our calculations (The Figure), is likely due to the choice of cut-off points, i.e., both OD 0.300 and 0.400 cut-off points guarantee 100% specificity for SL-IV but decreased sensitivity to 38% and 21%, respectively, in this study. High cut-off values have a tendency, in the case of SL-IV, to influence detection levels of PB forms more than those of MB forms since the mean values of the former are lower than those of the latter (Table 2).
We are assuming, since BDA.TDA sensitivity and specificity values in this study are similar to those of SL-IV, that this synthetic immunoreactant might be as useful as the natural antigen in detecting disease. This assumption deserves to be tested independently in a large-scale evaluation testing of a wider selection of leprosy sera comprising more cases belonging to the different types of the disease. This evaluation might still reveal a more selective discrimination at least in the case of SL-IV. The synthetic molecule BDA.TDA is, however, expected to behave equally well in detecting PB and MB disease since we could not show any significant difference in mean ∆414 levels (Table 2).
Finally, this study shows that when these two substances are tested in parallel, the sensitivity of dual testing is 10% higher than that of single testing without a significant and concomitant decrease in specificity.
Acknowledgment. The authors wish to express their gratitude to Dr. Claude Péan, Laboratory Director, Institute Fame Pereo, Port-au-Prince, Haiti, for the provision of some of the leprosy sera used in this study as well as to Dr. Peter Gill, Director of the Canadian Red Cross Reference Laboratory, and Dr. Eva Kemenyi for the nonleprosy sera. They would also like to thank Mrs. I. Laszlo for the elaboration of the "LOTUS 123" programs necessary for the Bayesian analysis of the data.
REFERENCES
1. ANONYMOUS. Leprosy. Health Technology Directions 9 No. 3. Seattle: PATH International, 1989.
2. BRETT, S. J., DRAPER, P., PAYNE, S. N. and REES, R. J. W. Serological activity of a characteristic phenolic glycolipid from Mycobacterium leprae in sera from patients with leprosy and tuberculosis. Clin. Exp. Immunol. 52(1983)271-279.
3. CHO, S.-N., YANAGIHARA, D. L,, HUNTER, S. W., GELBER, R. H. and BRENNAN, P. J. Serological specificity of phenolic glycolipid I from Mycobacterium leprae and use in serodiagnosis of leprosy. Infect. Immun. 41(1983)1077-1083.
4. CRUAUD, P., POTAR, M. C., DAVID, H. L., PAPA, F., TORGAL-GareIA, J., MAROJA, F. and ORSI-SOUSA, A. T. IgG and IgM antibodies im munoreacting with a 2,3-diacyl trehalose-2'-sulphate in sera from leprosy patients. Zbl. Bakt. 273(1990)209-215.
5. CRUAUD, P. H.. BERLIE, C, TORGAL-GareIA, J., PAPA. F. and DAVID, H. L. Human IgG antibodies immunoreacting with specific sulfolipids from Mycobacterium tuberculosis. Zbl. Bakt. 271(1989)481-485.
6. DAFFÉ, M. PAPA. F., LASZLO, A. and DAVID, H. L. Glycolipids of recent clinical isolates of Mycobacterium tuberculosis: chemical characterization and immunoreactivity. J. Gen. Microbiol. 135(1989)2759-2766.
7. DAVID, H. L., MAROJA, M. F. and CRUAUD, P. H. Quantitative Relationship between Anti-PGL-I-specific antibody levels and the lepromin reaction. Int. J. Lepr. 59(1991)332-334.
8. GALEN, R. S. Use of predictive value theory in clinical immunology. In: Manual of Clinical Laboratory Immunology. 3rd edn. Washington, D.C.: American Society for Microbiology, 1986, pp. 966-970.
9. GOREN, M. B. and JIANG, K. S. Pseudo-cord-factors: derivatives of α-D-glucopyranuronosyl (1-1)α-D-glucopyranuronoside. Chcm. Phys. Lipids 25(1979)209-224.
10. HUNTER. S. W. and BRENNAN, P. J. A novel phe nolic glycolipid from Mycobacterium leprae possibly involved in immunogenicity and pathogenicity. J. Bacteriol. 147(1981)728-735.
11. KHULLER, G. K., MALIK, M. and KUMAR, B. An tibodies to sulfolipids in human leprosy sera. Int. J. Lepr. 50(1982)319-321.
12. KHULLER, G. K., MALIK, U. and NALINI, P. An tibodies to mycobacterial sulfolipids in human tuberculous sera. ICRS Med. Sci. (1981) 492.
13. LASZLO, A., BAER, H. H., GOREN, M. B.. HANDZEL. V., BARRERA, L. and DE KANTOR, I. N. Evaluation of synthetic pseudo-cord-factor-like glycolipids for the serodiagnosis of tuberculosis. Res. Microbiol. 143(1992)217-223.
14. RIDLEY, D. S. and JOPLING, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
15. TOMAN. K. Sensitivity, specificity and predictive values of diagnostic tests. Bull. Int. Union Tuberc. 56(1981)18-28.
16. TORGAL-GareIA, J., PAPA, F. and DAVID, H. L. Immunological response to homologous and heterologous phenolic glycolipid antigens in tuberculosis and leprosy. In: Proceedings of the 4th International Meeting on Mycobacteria. Institut Pasteur. I989 Rastogi N. and David. H. L., eds. Acta Leprol. 7 Suppl.(1989)102-106.
1. Ph.D., Laboratory Center for Disease Control, Department of Health and Welfare, Ottawa, Canada Kl A 0L2.
2. M.Sc. (Pharm.);Unite de la Tuberculose et des Mycobactcries. Institut Pasteur, 75724 Paris 15, France.
3. Ph.D., National Jewish Center for Immunology and Respiratory Medicine. Denver, Colorado 80206, U.S.A.
4. A.R.T., Mycobacteriology. Laboratory Center for Disease Control, Department of Health and Welfare, Ottawa, Canada K1A 0L2.
5. M.Sc. (Pharm.);Unite de la Tuberculose et des Mycobactcries. Institut Pasteur, 75724 Paris 15, France.
6. M.D., Ph.D., Unite de la Tuberculose et des Mycobactcries. Institut Pasteur, 75724 Paris 15, France.
Received for publication on 11 February- 1992.
Accepted for publication in revised form on 21 May 1992.