- Volume 60 , Number 3
- Page: 382–9
Enzyme linked immunosorbent assay of a 12-kDa protein of Mycobacterium leprae with sera f rom leprosy patients
ABSTRACT
A low molecular weight protein was obtained f rom a sonicate of armadillo-derived Mycobacterium leprae cells and f rom a λgt11 phage lysate of Escherichia coli (specifying the M. leprae 12-kDa protein) by a single step of ultrafiltration. Both proteins had an approximate molecular weight of about 12,000 (by SDS-PAGE) and were recognized by the M. leprae 12-kDa-specific monoclonal antibody ML06 by immunoblotting. Sera f rom 79 leprosy patients across the clinical spectrum, 17 contacts, and 12 normal healthy individuals were screened in an enzyme-linked immunosorbent assay (ELISA) using the 12-kDa proteins as the antigens. Antibodies to the 12-kDa protein (f rom lysate as well as sonicate) were detected in patients' sera across the clinical spectrum (44%-100% positivity), while no detectable reactivity was observed with control or contact sera. Sera f rom patients who had undergone a year or more of chemotherapy exhibited no reactivity compared to those f rom patients with only 3-6 months of chemotherapy. The 12-kDa proteins were also recognized by rabbit hyperimmune M. leprae antiserum.
RÉSUMÉ
Une protéine de bas poids moléculaire a été obtenue d'un sonicat de cellule de Mycobacterium leprae en provenance de tatous et de lysats de phages gt11 d'Escherichia coli (spécifiant la protéine de 12-kDa du M. leprae) par ultrafiltration en une étape. Les deux protéines avaient un poids moléculaire approximatif d'environ 12.000 (par SDS-PAGE), et étaient reconnues par immunoblotting par l'anticorps monoclonal ML06 spécifique de 12-kDa de M. leprae. Des sérums de 79 patients lépreux de tout le spectre clinque, 17 contacts et 12 individus en bonne santé ont été testés par un test en/ymatique (ELISA) utilisant les protéines de 12-kDa comme antigène. Des anticorps vis-à-vis de la protéine de 12-kDa (à partir du lysat aussi bien que du sonicat) ont été détectés dans les sérums de patients de l'ensemble du spectre clinique (44 à 100% de positivite) tandis qu'aucune réactivité détectable n'a été observée dans le sérum des contrôles ou des contacts. Les sérums des patients qui avaient suivi une année de chimiothérapie ou davantage ne montraient aucune réactivité en comparaison avec ceux des patients qui n'avaient reçu que trois à six mois de chimiothérapie. Les protéines de 12-kDa étaient également reconnues par de l'antisérum M. leprae de lapin hyperimmun.RESUMEN
Se obtuvo una proteína de bajo peso molecular a partir de un sonicado de Mycobacterium leprae derivado de armadillo y a partirde un lisado de Escherichia coli transformada por el fago gt11 portador del gene que codifica para la proteína de 12 kDa del M. leprae. Ambas proteínas tuvieron un peso molecular aproximado de 12 kDa (por PAGE-SDS) y fueron reconocidas por el anticuerpo monoclonal ML06. Con estos antígenos, utilizando un inmunoensayo enzimático (ELISA), se investigó la reactividad de los sueros de 79 pacientes representantes de todo el espectro de la lepra, de 17 contactos, y de 12 individuos normales. Se detectaron anticuerpos contra la proteína de 12 kDa (tanto del lisado como del sonicado) en los sueros de los pacientes (positividad del 44%-100%), pero no en los sueros de los contactos o de los controles sanos. A diferencia de los pacientes con 3-6 meses de quimioterapia, aquellos con un año o más de tratamiento, no mostraron reactividad con la proteina. Las proteínas de 12 kDa también fueron reconocidas por un suero hiperinmunc de conejo anti-M. leprae.Human hosts respond to Mycobacterium leprae, the causative agent of leprosy, by developing both humoral and cell-mediated immune responses. Although the humoral response does not play a significant role in combating the infection, it may be useful for diagnosing the infection. The currently used serodiagnostic assays are based on the detection of antibodies which are considered to be specific against surface components of Mycobacterium leprae (2). The availability of armadillo-derived M. leprae organisms (21) and the cloning of M. leprae proteins in Escherichia coli (26) have provided an opportunity to characterize, isolate and purify M. leprae antigens. The molecular identity of antigenic components recognized by antibodies produced by leprosy patients needs to be determined if specific M. leprae antigens are to be used as the basis of a clinically useful sérodiagnostic test for the disease. A number of investigators have reported recognition of M. leprae-specific 12 kDa by leprosy sera (5,6,14,23). The stimulation of M. leprae-responsive T-cell clones by the 12-kDa protein has been reported by Ottenhoff, et al.(17).
We report the partial purification of a 12-kDa protein (from M. leprae sonicate and from an E. coli λgt11 recombinant DNA clone, Y3184, expressing the M. leprae 12kDa gene) by a single ultrafiltration step, and its reactivity with sera from leprosy patients across the clinical spectrum. The clone Y3184 isolated by Young, et al. (26) recently has been reported to express a biotinylated protein as a β-galactosidasc fusion product(8). In our study, however, it expresses the 12-kDa protein without a fusion partner. Another aspect of the present study was to investigate whether the E. coli-expressed recombinant protein would be recognized by leprosy sera to the same extent as the native 12-kDa protein from M. leprae.
MATERIALS AND METHODS
Sera. Sera from 79 leprosy patients with various types of the disease (TT-tuberculoid: 13, BT -borderline tuberculoid: 24, BL-borderline lepromatous: 17, LL-lepromatous: 25), 17 contacts (of both multibacillary and paucibacillary patients) and 12 normal controls were used for the study. All test and control sera were obtained from Madras, India. In the leprosy sera, four patients from each group had undergone chemotherapy (two each for 3-6 months and the others for a year or more). The patients had been clinically and histologically classified according to the Ridley-Jopling scheme (19) prior to the collection of the sera. All the serum samples were identified according to their sources and stored in aliquots at -20ºC.
M. leprae sonicate. Purified irradiated armadillo-derived M. leprae organisms (obtained from Drs. P. Brennan and S. Hunter, Colorado State University, Fort Collins, Colorado, U.S.A.) were suspended in saline and sonicated at 8ºC in three continuous cycles of 10 min each, using a W-375 sonicator (Heat Systems-Ultrasonics, Plainview, N.Y., U.S.A.) with a 100-W energy output. The supernatant was aliquoted and stored at - 20ºC after the addition of phenylmethylsulfonyl fluoride (PMSF; Sigma Chemical Company, St. Louis, Missouri, U.S.A.).
Polyclonal antiserum to M. leprae sonicate. New Zealand white male rabbits were used for raising a hyperimmune antiserum to M. leprae sonicate as described earlier (16). The sera, from rabbits showing an identical response, were pooled, aliquoted and stored at -20ºC.
Preparation of crude lysate from lysogens. The recombinant clone Y3184 of the λgt11 recombinant encoding M. leprae 12-kDa antigen maintained in E. coli Y1089 was obtained from Dr. Thomas Shinnick (Centers for Disease Control, Atlanta, Georgia, U.S.A.). Lambda gt11 phage (without insert) maintained in E. coli Y1090 was obtained from Dr. J. Clark-Curtiss (Washington University, St. Louis, Missouri, U.S.A.). A crude protein lysate was obtained from the clones as described by Huynh, et al. (11). The lysate was checked for the presence of M. leprae 12-kDa protein by immunoblotting (with the use of monoclonal antibody ML06) and subsequently used for further work. Control lysate (from E. coli Y1090) was similarly prepared.
Partial purification of 12-kDa protein from sonicate and lysate. M. leprae sonicate and the crude lysate containing the ML06-reactive antigen were used for purification. A Centriprep ultrafiltration concentrator (Ccntriprep-10 with molecular weight cut off of 10,000; Amicon, Lexington, Massachusetts, U.S.A.) was used for this purpose. The sonicate or lysate was placed in the outer sample container of the Centriprep concentrator and centrifuged in a fixed-angle rotor at 3000 × g × 15 min at 15ºC in an IEC-DPR 6000 centrifuge (Damon, IEC Division, Needham Heights, Massachusetts, U.S.A.). The filtrate from the (inner) filtrate collector was collected and concentrated by lyophilization. The filtrate and the retentates were subjected to SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting to determine the purity and the presence of the ML06-reactive antigen. The control lysate was similarly treated, and the filtrate obtained was referred to as the control filtrate. Protein concentration was determined by using the Bio-Rad protein assay system (Bio-Rad Laboratories, Richmond, California, U.S.A.).
SDS-PAGE. The Centriprep retentates and filtrates were subjected to 15% SDS-PAGE (Mini-Protean II ready gels; Bio-Rad) at a constant voltage of 200 volts for 1 hr under reducing conditions. The gels were stained with 0.25% Coomassie brilliant blue R-250 (CBB; Bio-Rad). Molecular weight markers (MW 12,300 to 66,500; LKB, Bromma, Sweden) were used for estimating the molecular weight by comparing the migration distance.
Western blotting. The antigens separated by SDS-PAGE were electrophoretically transferred from the gel to nitrocellulose membranes (0.2-µm pore size; Bio-Rad) by the method of Towbin, et al. (22) using a Trans-blot apparatus (Bio-Rad) and LKB 2002 power supply. Prestained Bio-Rad molecular weight markers (MW 18,500 to 106,000) were used for the estimation of the molecular weight of the blotted antigens and to detect the efficiency of transfer. The membrane was washed in 50 mM Tris-HC1 buffer (pH 7.4) containing 200 mM NaCl (TBS). It was then incubated in 3% bovine serum albumin (BSA) for 1 hr and subsequently incubated for 2 hr at 25ºC with the monoclonal antibody (MAb) ML06. ML06 was obtained from WHO IMMLEP, Geneva, through Dr. T. Shinnick. Washing in TBS (6 × 5 min) was followed by incubation in 1:1000-diluted peroxidase-conjugated rabbit anti-mouse polyvalent immunoglobulin (Bio-Rad) for 1 hr at 25ºC. After washing, the reaction was visualized using substrate solution (60 nig of 4-choloro-1-naphthol in 20 ml cold methanol mixed with 100 ml TBS containing 60µ1 of 30% hydrogen peroxide), and was stopped by washing the membrane in distilled water. For digestion with protease, the membrane with the blotted antigens was incubated immediately after blotting in a solution of 50 µg/ml trypsin (Sigma) in TBS at 25ºC for 30 min before performing the immunoassay.
Indirect ELISA. Polystyrene, 96-well, flat-bottom microtiter plates (Falcon; Becton-Dickinson Labware, Lincoln Park, New Jersey, U.S.A.) were coated with 50µ1 (0.5 µg protein) of filtrate (containing the 12-kDa protein) per well for 3 hr at 37ºC. The plates were then washed six times with PBS containing 0.05% Tween 20 (PBST) and incubated with 70 µl of 15% BSA in PBS at 37ºC for 1 hr to block nonspecific binding. The plates were washed once in PBST and stored overnight at 4ºC. On the following day, the wells were incubated for 2 hr at 25ºC'with 50 µl of test or control (NHS) sera diluted 1:200 in TBS. After washing six times with PBST, they were incubated for 1 hr at 25ºC with 50 µl of secondary antibody (peroxidase-conjugated rabbit anti-human polyvalent immunoglobulin; Bio-Rad) diluted 1:1000 at room temperature for 1 hr. Subsequently, after washing six times with PBST, each well in the plate was incubated with 50µl of substrate solution (10 mg ortho-phenylene diamine dihydrochloride in 1 ml methanol mixed with 99 ml distilled water and 0.1 ml of 3% hydrogen peroxide) at room temperature for 30 min. The color development was stopped by the addition of 20 µl of 4 N H2S04 per well, and the extinction (E) was measured after 15 min in a Titertek Multiskan reader using a 492nm filter against a blank made up of substrate and stop solutions. Each serum sample was tested in duplicate and mean E was recorded. All the serum samples were similarly tested with E. coli filtrate as the antigen. Results were expressed as ∆E = E(coat with 12-kDa antigen) minus E(coat with E. coli control filtrate). The cut-off value for a positive reaction was defined for each serum sample as the mean value of the control group (NHS) plus two times the standard deviation. This criterion was adopted to ensure the exclusion of doubtful or false-positive results in the test. The results were analyzed by analysis of variance; levels of significant difference were determined by Newman-Keuls multiple range test.
The reactivity of the 12-kDa protein (from sonicate and lysate) with polyclonal rabbit M. leprae antiserum was similarly determined using peroxidase-conjugated goat anti-rabbit polyvalent immunoglobulin (Bio-Rad) as the secondary antibody.
RESULTS
Purification of the ML06-reactive protein. A low molecular weight protein was partially purified from M. leprae sonicate and the crude control and recombinant lysate by a single step of ultrafiltration. Concentration of the filtrate to 1 ml by lyophilization and subsequent SDS-PAGE under reducing conditions and CBB staining indicated the presence of a single band with an apparent molecular weight of about 12,000 (Fig. 1; lanes b, c, d). Immunoblotting indicated that the 12-kDa proteins from sonicate and recombinant lysate were recognized by MAb ML06 and were susceptible to trypsin digestion, while the protein from control lysate was not recognized by ML06 (Fig. 2). Although the retentatcs obtained after filtration of M. leprae sonicate and the recombinant lysate also showed the presence of weak ML06-reactive 12-kDa bands, it appeared that most of the 12-kDa protein was present in the filtrate.
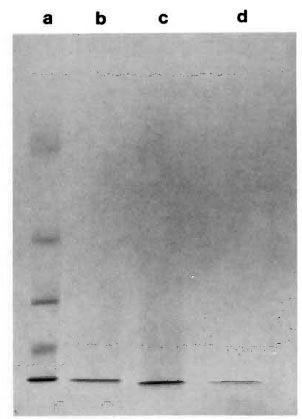
Fig. 1. SDS-PAGE (15%) of Centriprep-10 filtrates. a = molecular weight markers (top to bottom): bovine serum albumin = 66, 250, ovalbumin = 43,000,carbonic anhydrase = 30,000, myoglobin = 17, 200,cytochromc c = 12, 300; b = M. leprae sonicate; c =lysate of E. coli containing M. leprae 12-kDa gene; d = E. coli control lysate.
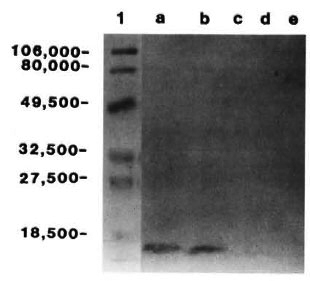
Fig. 2. Immunoblots of Centriprep- 10 filtrates of a = M. leprae sonicate; b = E. coli lysate containing M. leprae 12-kDa gene; c and d = trypsin digestionsof a and b; e = control E. coli lysate (with no insert).Lane 1 contains the prestained markers; numbers on left indicate molecular weight.
ELISA. ELISA was performed using the 12-kDa protein preparations as antigen. The results are shown in The Table and in Figure 3. Leprosy sera showed a significantly higher response (p < 0.01) than the controls. Sera from the eight patients who had undergone a year or more of chemotherapy showed no reactivity when compared to those from patients with 3-6 months of chemotherapy. Sera from contacts as well as from healthy individuals did not exhibit positive reactions. None of the sera showed any reactions when E. coli control filtrate was used as the antigen.
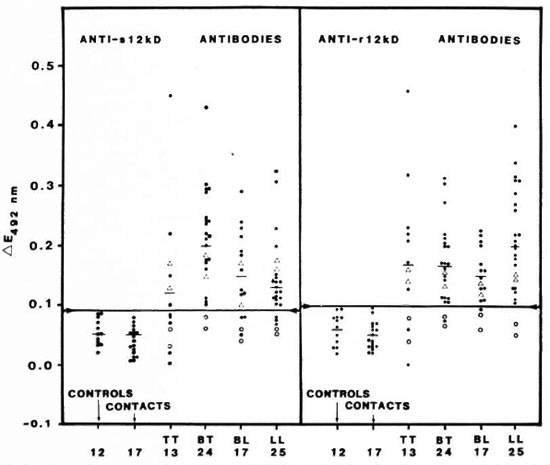
Fig. 3. Comparison of seroreactivity of sonicate 12-kDa (s12kD, left panel) and recombinant 12-kDa (r12kD, right panel) proteins in leprosy patients (TT, BT, BL and LL), in contacts of leprosy patients, and in normalcontrols. Sera diluted 1/200 in both cases. Each point = one individual; horizontal bars = mean ∆E values; horizontal line joining arrows in each panel = corresponding mean ∆E + 2 S.D. of the control group; ∆ = patients who had undergone 3-6 months of chemotherapy; ○ = 1 or more years of chemotherapy.
Strong reactivity was also observed between the 12-kDa protein preparations and the rabbit anti-M. leprae hyperimmune serum. The antibody titer was 1:20,000 when M. leprae sonicate was used as the antigen. When the sonicate 12-kDa protein was used as the antigen, the titer was 1:10,000. On the other hand, the titer with cloned 12-kDa antigen was only 1:500. No reactivity was observed with E. coli filtrate nor was any binding observed with normal rabbit serum.
DISCUSSION
During the last decade, attempts have been made by several investigators to standardize a specific immunoassay for leprosy using various M. leprae antigens (7,13,20,24 ). The fluorescent leprosy antibody absorption test (1) has been used by several investigators and has shown about 90%-100% and 70%-80% positivity in LL and TT cases, respectively (20). The availability of cloned M. leprae 12-kDa antigen (26), the prominent presence of the 12-kDa protein in cell sonicate, the availability of a MAb specifically recognizing it (12) and the reports on its immunoreactivity (5,6,14,23) were some of the reasons for initiating this study to determine its seroreactivity in leprosy patients. Clone Y3184 was subsequently shown to express a biotinylated protein, as a β-galactosidase fusion product (8). However, in our studies with Y3184, we detected the expression of a ML06-reactive 12-kDa protein without a fusion partner. We were able to obtain the protein in substantial quantities in partially purified form by a single step of ultrafiltration and use it in a simple ELISA to detect antibodies in sera from leprosy patients. The presence of other proteins with a molecular weight within the range of 12,000 in this preparation cannot be ruled out at this time. The immuno-purification and seroreactivity of the 12-kDa antigen with LL sera has been previously reported by Britton, el at. (5). Since the serum samples showed no detectable binding to the control E. coli filtrate, no previous absorption of the sera to E. coli was performed. Our results suggest that the cloned 12-kDa protein contains the ML06-rccognition epitope. We have also shown that this protein was recognized by leprosy sera as well as polyclonal rabbit hyperimmune anti-M. leprae serum. We observed, in the latter instance, that reactivity to the sonicate protein was higher as compared to the cloned protein. It is possible that antibodies in the rabbit serum recognized epitopes in the sonicate protein that may not be present on the cloned protein. Antibodies in the sera of patients, however, recognized both the cloned and sonicate protein to a similar extent. Since the E. coli 12-kDa protein by itself showed no seroreactivity, its probable presence in the partially purified, cloned 12kDa protein preparation from lysate was not considered critical in the seroreactivity results.
Chakrabarty, et at. (6) have previously reported that specific antibody activity in sera from LL patients, infected armadillos, and hyperimmune rabbits are mainly directed against the 12- and the 33-kDa antigens. We have observed reactivity of the 12-kDa protein not only with LL sera but also with sera from tuberculoid as well as borderline patients. This is in contrast to the observations made by Klatser, et al. (14) and Patil, et al. (18), who were unable to demonstrate a positive serological response in sera from TT patients when screened with the 12-kDa antigen. The relationship of the 12-kDa antigens reported by these and other investigators (6,14, 18,25) with the 12-kDa antigen purified in this study is currently unknown. A purified native and recombinant 10-kDa M. leprae protein which bears 44% homology with the hsp 10 (Gro ES) of E. coli has been reported by Mehra, et al. to be a major T-cell antigen (15). One cannot rule out the presence of this protein in the 12-kDa protein preparation in this study.
Patients who had undergone a year or more of chemotherapy showed no 12-kDa reactivity. It is likely that the destruction of bacilli caused by prolonged chemotherapy could release antigens of a different nature, thus inducing antibodies that may not be the same as those seen in untreated patients. It also appears that the duration of treatment could be a contributing factor since the serum samples from patients who had undergone only 3-6 months of chemotherapy still showed the presence of antibodies to 12-kDa, although at a lower level. None of the contacts' sera reacted with either the cloned or the sonicate protein. The reactivity of sera from contacts with recombinant M. leprae antigens has been reported earlier by Hartskeerl, et al. (10). However, these antigens were distinct from the well-characterized M. leprae antigens including the 12kDa.
Hartskeerl, et al. (9) have also reported the nucleotide and amino acid sequence of a 12-kDa M. leprae protein. Britton, et al. (3) had shown a band of 12-kDa to be a major protein in M. leprae resonicate (3), and this band was the major M. leprae protein radiolabeled by Chakrabarty, et al. (6). This observation was confirmed by Britton, et al. (4) who also showed that the 12-kDa expressed human, B-cell-reactive epitopes since it was strongly precipitated by human leprosy serum. The 12-kDa protein has been implicated in the immune response to M. leprae since it stimulated the T-cell clones isolated from TT patients (17). These reports and studies from our laboratory point toward the immunoreactivity of the 12-kDa protein of M. leprae.
Acknowledgment. This work was supported by the U.S. Leprosy Panel of the U.S.-Japan Cooperative Medical Science Program administered by the National Institute of Allergy and Infectious Diseases (Grant 127189).
We express our gratitude to Dr. Thomas Gillis and Dr. Frank Collins for the valuable suggestions given during the preparation of this manuscript. Our sincere thanks to Dr. P. J. Brcnnan, Dr. T. Shinnick and Dr. J. Clark-Curtiss for providing the M. leprae cells, monoclonal antibody ML06, and the lysogens. We appreciate the help provided by Dr. W. Kirlin Xor the statistical evaluation of the data and Ms. Y. Z. Windham for her technical assistance. We extend our thanks to Ms. S. Walton for typing this manuscript and to Mr. J. Perry for the illustrations.
REFERENCES
1. ABE, M., MINAGAWA, F., YOSHINO, Y., OZAWA, T., SAIKAWA, K. and SAITO, T. Fluorescent leprosy antibody absorption (FLA-ABS) test for detecting subclinical infection with Mycobacterium leprae. Int. J. Lepr. 48(1980)109-119.
2. ANONYMOUS. Serological tests for leprosy. (Editorial) Lancet 1(1986)533-535.
3. BRITTON, W. J., HELLQVIST, L., BASTEN, A. and RAISON, R. L. Mycobacterium leprae antigens in volved in human immune responses; identification of four antigens by monoclonal antibodies. J. Immunol. 135(1985)4171-4177.
4. BRITTON, W. J., HELLQVIST, L., GARSIA, R. J. and BASTEN, A. Dominant cell wall proteins of My cobacterium leprae recognized by monoclonal antibodies. Clin. Exp. Immunol. 67(1987)31-42.
5. BRITTON, W. J., HELLQVIST, L., IVANYI, J. and BASTEN, A. Immunopurification of radiolabeled antigens of Mycobacterium leprae and Mycobacterium bovis (Bacillus Calmette-Guérin) with monoclonal antibodies. Scand. J. Immunol. 26(1987)149-159.
6. CHAKRABARTY, A. K., MAIRE, M. A. and LAMBERT.P. H. SDS-PAGE analysis of M. leprae protein antigens reacting with antibodies from sera from lepromatous patients and infected armadillos. Clin. Exp. Immunol. 49(1982)523-531.
7. CHO, S.-N., YANAGIHARA, D. L., HUNTER, S. W., GELBER, R. H. and BRENNAN, P. J. Serological specificity of phenolic glycolipid I from Mycobacterium leprae and use in scrodiagnosis of leprosy. Infect. Immun. 41(1983)1077-1083.
8. DALE, J. W. and PATKI, A. Mycobacterial gene expression and regulation. In: Molecular Biology of the Mycobacteria. McFadden, J., cd. London: University Press, 1990.
9. HARTSKEERL, R. A., STABEL, L. F. E. M., HERMANS, C. J., KLATSER, P. R. and THOLE, J. E. R. Nucle otide and deduced amino acid sequence of a My cobacterium leprae 12K protein. Nucl. Acids Res. 18(1990)1294.
10. HARTSKEERL, R. A., VAN RENS, R. M., STABEL, L. F. E. M., DE WIT, M. Y. L. and KLATSER, P. R. Selection and characterization of recombinant clones that produce Mycobacterium leprae antigens recognized by antibodies in sera from household contacts of leprosy patients. Infect. Immun. 58(1990)2821-2827.
11. HUYNH, T. V., YOUNG, R. A. and DAVIS, R. W. Constructing and screening cDNA libraries in λgt10 and λgt11. In: DNA Cloning Techniques: a Practical Approach. Vol. 1. Glover, D., ed. Oxford: IRL Press, 1985, pp. 49-78.
12. IVANYI, J., SINHA, S., ASTON, R., CUSSELL, D., KEEN, M. and SENGUPTA, U. Definition of species specific and cross-reactive antigenic determinants of Mycobacterium leprae using monoclonal antibod ies. Clin. Exp. Immunol. 52(1983)528-536.
13. KLATSER, P. R., DE WIT, M. Y. L. and KOLK, A. H. J. An ELISA-inhibition test using monoclonal antibody for the serology of leprosy. Clin. Exp. Immunol. 62(1985)468-473.
14. KLATSER, P. R., VAN RENS, M. M. and EGGELTE, T. A. Immunochemical characterization of Mycobacterium leprae antigens by the SDS-polyacrylamide gel electrophoresis immunoperoxidase technique (SGIP) using patients' sera. Clin. Exp. Immonol. 56(1984)537-544.
15. MEHRA, V., BLOOM, B. R., BAJARDI, C. A., GRISSO, C. J., SIELING, P. A., ALLAND, D., CONVIT, J., FAN, X . -D., HUNTER, S. W., BRENNAN, P. J., REA, T. H. and MODLIN, R. L. A major T cell antigen of Mycobacterium leprae is a 10-kD heat-shock cognate protein. J. Exp. Med. 175(1991) 275-284.
16. NAVALKAR, R. G. Immunologic analysis of My cobacterium leprae antigens by means of diffusion- in-gel methods. Int. J. Lepr. 39(1971)105-112.
17. OTTENHOFF, T. H. M., KLATSER, P. R., IVANYI, J., ELFERINK, G. J., DE WIT, M. Y. L. and DE VRIES, P. R. P. Mycobacterium leprae-specific protein antigens defined by cloned human helper T cells. Nature (London) 319(1986)66-68.
18. PATIL, S. A., GIRDHAR, B. K., SINGH, K. P. and SENGUPTA, U. Detection of Mycobacterium leprae antigens in the sera ofleprosy patients by sandwich immunoradiometric assay using monoclonal antibodies. J. Clin. Microbiol. 28(1990)2792-2796.
19. RIDLEY, D. S. and JOPLING, W. H. Classification of leprosy according to immunity; a live-group system. Int. J. Lepr. 34(1966)255-273.
20. SENGUPTA, U. Mycobacterium leprae antigens and their utility in immunodiagnostics ofleprosy. Trop. Med. Parasitol. 41(1990)361-362.
21. STORRS, E. E . The nine-banded armadillo: a model for leprosy and other biomedical Research. Int. J. Lepr. 39(1971)703-714.
22. TOWDIN, H., STAEHELIN, T. and GORDON, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sei. U.S.A. 76(1979)4350-4354.
23. VEGA-LOPEZ, F., STOKER, N. G., LOCNISKAR. M. F., DOCKRELL, H. M., GRANT, K. A. and McADAM, K. P. W. Recognition of mycobacterial antigens by sera from patients with leprosy. J. Clin. Microbiol. 26(1988)2474-2479.
24. YOUNG, D. B. and BUCHANAN, T. M. A serological test for leprosy with a glycolipid specific for Mycobacterium leprae. Science 221(1983)1057-1059.
25. YOUNG, D. B., KAUFMANN, S. H. E., HERMANS, P. W. M. and THOLE, J. E. R. Mycobacterial protein antigens: a compilation. Molec. Microbiol. 6(1992)133-145.
26. YOUNG. R. A., MEHRA, V., SWEETSER, D., BUCHANAN, T., CLARK-CURTISS, J., DAVIS, R. W. and BLOOM, B. R. Genes for the major protein antigens of the leprosy parasite Mycobacterium leprae. Nature (London) 316(1985)450-452.
1. Ph.D., Department of Microbiology and Immunology, Morehouse School of Medicine. 720 Westview Drive SW, Atlanta, Georgia 30310, U.S.A.
2. Ph.D., Department of Microbiology and Immunology, Morehouse School of Medicine. 720 Westview Drive SW, Atlanta, Georgia 30310, U.S.A.
3. M.S., Department of Microbiology and Immunology, Morehouse School of Medicine. 720 Westview Drive SW, Atlanta, Georgia 30310, U.S.A.
4. M.D., Department of Microbiology and Immunology, Morehouse School of Medicine. 720 Westview Drive SW, Atlanta, Georgia 30310, U.S.A.
5. B.S., Department of Microbiology and Immunology, Morehouse School of Medicine. 720 Westview Drive SW, Atlanta, Georgia 30310, U.S.A.
Received for publication on 29 January 1992.
Accepted for publication in revised form on 21 May 1992.