- Volume 60 , Number 3
- Page: 396–403
Analysis of circulating immune complexes f rom leprosy patients for Mycobacterium leprae antigens
ABSTRACT
Circulating immune complexes (CICs) f rom 31 leprosy patients (16 tuberculoid, 15 lepromatous) and 12 healthy volunteers, precipitated by 3.5% polyethylene glycol, were individually subjected to SDS-PAGE and immunoblotting using a variety of monoclonal and polyclonal antibodies against Mycobacterium leprae. A common mycobacterial antigen of an apparent molecular size of 65 kDa was identified in CICs f rom about 40% of the patients. No correlation was observed between the positivity for this antigen and any of the following parameters: bacterial index, M. leprae-specific antibody titers, motor nerve involvement, duration of disease or treatment. Nevertheless, patients with a relatively recent and massive infection were more frequently positive for antigen than the others.RÉSUMÉ
Des complexes immuns circulants (CIC) de 31 patients lépreux ( 16 tuberculoides. 15 lépromateux) et 12 volontaires en bonne santé, précipités par du polyethylene glycol à 3.5%, ont été soumis individuellement au SDS-PAGE et à l'immunoblotting en utilisant une variété d'anticorps monoclonaux et polyclonaux vis-àvis de Mycobacterium leprae. Un antigène mycobacterien commun d'une taille moléculaire apparente de 65 kDa a été identifiée dans les CIC chez environ 40% des patients. Aucune corrélation n'a été observée entre la positivité pour cet antigène et l'un quelconque des paramètres suivants: l'index bactérien, les titres d'anticorps spécifiques vis-à-vis de M. leprae, l'envahissement des nerfs moteurs, la durée de la maladie ou le traitement. Néanmoins, les patients avec une infection relativement récente et massive étaient plus fréquemment positifs pour l'antigène que les autres.RESUMEN
Los complejos inmunes circulantes (CIC) separados por precipitación con polietilén glicol al 3.5% del suero de pacientes con lepra (16 tuberculoides y 15 lepromatosos) y del suero de 12 voluntarios sanos, se analizaron por PAGE-SDS e inmuno-electrotransferencia usando una variedad de anticuerpos monoclonales y policlonales contra el Mycobacterium leprae. Aunque en el 40% de los pacientes se identificó un antígeno micobacteríano común de 65 kDa, no hubo correlación entre la positividad por este antígeno y cualquiera de los siguientes parámetros: índice bacteriano, niveles de anticuerpos específicos para M. leprae, afección de los nervios motores, duración de la enfermedad, o tratameinto. Sin embargo, los pacientes con una infección masiva relativamente reciente fueron más frecuentemente positivos para este antígeno que otros pacientes.There is considerable evidence implicating immune complexes (ICs) in an array of pathogenic and immunomodulatory mechanisms in a variety of diseases (28). In leprosy also, ICs have been demonstrated along the clinico-immunopathological spectrum of the disease (1,16,19,21). Apart from being held responsible for the pathogenesis of reactions-the acute inflammatory episodes often seen after the initiation of antileprosy chemotherapy (19,27) - these ICs are suspected of playing a large immunomodulatory role in leprosy.
Antigens of Mycobacterium leprae (and corresponding antibodies) are regarded as the obvious constituents of ICs in leprosy patients (4,16). Thus, the analysis of these complexes is expected to throw light on the identities of immunopathologically important antigens of M. leprae which have largely remained elusive (9). The present study, aiming at the demonstration and characterization of M. leprae antigens in circulating ICs (CICs), was conducted in leprosy patients suffering from the disease at both ends of the leprosy spectrum, i.e., tuberculoid and lepromatous (21)- In addition to two polyclonal antibodies, pooled lepromatous (LL) serum and anti-BCG antibody, a set of presently available monoclonal antibodies against M. leprae (10,11) was also used for this purpose. Sera were investigated individually, and a correlation of the results was sought with the clinico-bacteriological status and circulating M. leprae-specific antibody levels (5,22) of the patients.
MATERIALS AND METHODS
Study subjects. Thirty-one leprosy patients attending the clinics of the Central JALMA Institute of Leprosy (CJIL), Agra, India, and classified as lepromatous (LL/ BL, N = 15) or tuberculoid (TT/BT, N = 16) according to Ridley and Jopling criteria (21) were selected for the study. Thirteen patients were untreated and 18(13 LL/BL and 5 TT/BT) were under multidrug therapy (27) for periods ranging from 2 weeks to 18 months. Some of the patients were experiencing rcactional episodes [7 borderline-type reactions (type 1) and 2 erythema nodosum leprosum (ENL, type 2)] at the time of the study. All patients were subjected to routine clinical investigations.
Twelve healthy volunteers (8 Indian and 4 European) served as controls.
Bacterial index (BI). Four slit-skin smears from each patient (from both earlobes and at least two active sites, generally on the arm and back) were used for determination of the average BI, according to Ridley's logarithmic scale (20).
Antigens and antibodies. Soluble M. leprae antigen, prepared by sonication and ultracentrifugation of armadillo-derived M. leprae, was supplied by WHO-IMMLEP (courtesy of Dr. R. J. W. Rees, National Institute for Medical Research, London) at a concentration of 1 mg protein/ml. Natural disaccharide (epitope of phenolic glycolipid-I) conjugated with BSA (ND-O-BSA) was provided by Dr. D. Chatterjee (Colorado State University, Fort Collins, Colorado, U.S.A.). Monoclonal antibodies (MAbs) against M. leprae-ML04, ML06, ML30, and ML34 (10,11) -were provided by Dr. J. Ivanyi (Medical Research Council TB and RI Unit, London) in the form of ascites globulins. Pooled serum from five lepromatous leprosy patients showing high titers for M. leprae-specific antibodies (22) and anti-BCG antibody (Dakopatts, Denmark) served as sources for polyclonal antibodies against M. leprae. Peroxidase-conjugated antibodies to mouse, rabbit, and human immunoglobulins (Igs) were obtained, respectively, from Sigma Chemical Co. (St. Louis, Missouri, U.S.A.), Sera Lab (U.K.) and Dakopatts.
In one experiment, optimal absorption of anti-BCG antibodies was obtained as follows: To 10 ml of diluted antibody (1:250 in dilution buffer, described below) 500 µl of BCG sonicate (equivalent to 50 mg wet weight of BCG) was added, and the mixture was rotated gently (5-7 rpm) for 2 hr at room temperature.
Isolation of CICs. Freshly collected sera were subjected to IC precipitation with 3.5% PEG-6000 (16,29)- Briefly, 3-4 ml serum was diluted 1:5 with phosphate buffered saline (0.15 M, pH 7.4) (PBS) containing 0.02 M disodium EDTA (PBS-EDTA). An equal volume of 7% PEG (in PBS-EDTA) was added to it (while stirring), and the mixture was kept at 4ºC overnight. The precipitate was collected by cold centrifugation and washed once with chilled 3.5% PEG. Finally, the pellet (enriched CICs) was reconstituted in PBS up to a volume which was 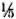 of the original volume of scrum and stored at -20ºC in aliquots.
of the original volume of scrum and stored at -20ºC in aliquots.
SDS-PAGE and immunoblotting. SDS-PAGE (under reducing conditions) on minislabs of 12.5% analytical gel was performed according to Laemmli (13) in a Mini Protean-II apparatus (Bio-Rad Laboratories, Richmond, California, U.S.A.). After electrophoresis, the gel slabs were either stained for proteins (with PAGE-blue 83, BDH) or subjected to clectroblotting (24) in Bio-Rad's Trans-Blot apparatus (60 v × 4 hr) in a cold room using S&S nitrocellulose paper (NCP; 0.45-µm pore size). After the transfer, the NCP was blocked with 3% skimmed milk powder (Anikspray; Lipton India Ltd.) prepared in Tris-buffered saline (TBS; 0.01 M Tris, pH 7.4) containing 0.1% Tween 20 for 2 hr at room temperature (RT) with gentle (5-7 rpm) rocking (Rockomat II; Technomora, Switzerland). Later the paper (either as such or after cutting into 5-mm-wide strips) was incubated (2 hr at RT with gentle rocking) serially with primary and/or peroxidase-conjugated secondary antibodies. Antibody dilutions (1:1000 for MAbs, 1:250 for anti-BCG Ab, 1:10 for pooled LL serum, and 1:500 for peroxidase conjugates) were made in 1% milk-TBS containing 0.05% Tween 20 (dilution buffer). Five washes of 5 min each with TBS-0.05% Tween 20 were given after each antibody reaction and before adding substrate (0.05% 4-chloronaphthol, 0.02% H2O2 in TBS); the final wash was given with TBS alone. Color was developed by gentle rocking, and the reaction was stopped by washing the strips extensively with distilled water. The NCP strips containing electroblotted molecular weight markers (BDH) were stained separately with Amido black.
Assays for M. leprae-specific antibodies. Species-specific antibodies to the epitopes on the 35-kDa protein and phenolic glycolipid-I (PGL-I) of M. leprae were assayed in the sera using standardized ELISA procedures. Antibodies to the 35-kDa protein were assayed by an antibody competition ELISA (serum antibody competition test, SACT) (22,23). Briefly, the ELISA plates coated with a sonic extract of M. leprae were incubated with peroxidase-conjugated MAb ML04 (P-ML04, reactive to an epitope on 35-kDa protein) in the presence or absence of serial tenfold dilutions of a serum; o-phenylenediamine was used as substrate, and the percent MAb binding for each dilution of serum (100% = mean OD 492 for P-ML04 binding in the absence of serum) was calculated. The results are expressed as ID50 values (serum dilution causing 50% inhibition of ML04 binding). Based on the data obtained from nonleprosy control subjects (22 and this study), a scrum with an ID50 value of > 10 was regarded as positive.
Anti-PGL-I antibodies were assayed by indirect ELISA (5,22). Sera, diluted 1:300, were incubated with ND-O-BSA coated to the wells of an ELISA plate. Binding of the IgM type of antibodies was detected by serial incubations with peroxidase-conjugated anti-human IgM Ab and o-phenylenediamine substrate. On the basis of the results obtained from nonleprosy controls (22 and this study), a scrum showing an OD 492 value of > 0.20 was regarded as positive for antibody.
RESULTS
Proteins in PEG precipitates
SDS-PAGE and staining with PAGE-blue revealed a range of proteins (mol. wts. > 12-kDa) which were precipitated from serum at 3.5% PEG concentration (Fig. 1). The presence of these proteins was noticed consistently, although with variable intensities, in all CIC precipitates prepared from either healthy controls or leprosy patients (tuberculoid or lepromatous). Upon immunoblotting, many of these proteins (mol. wts. > 40-kDa) reacted directly with peroxidase-conjugatcd anti-human Ig antibodies (Fig. 2), indicating an immunoglobulin origin for such constituents.
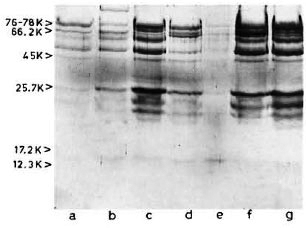
Fig. 1 . SDS-PAGE (under reducing conditions) and protein staining of representative CICs from healthy subjects (lanes a and e) and tuberculoid (lanes b to d)or lepromatous (lanes land g) patients. Molecular weight markers are indicated on left.
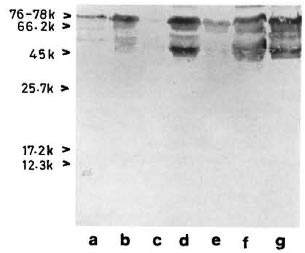
Fig 2. Reaction of representative CIC-electroblot with peroxidase-labeled anti-human Ig antibody. Lane a = healthy; lanes b to d = tuberculoid; lanes e to g = lepromatous.
Reaction with monoclonal antibodies to M. leprae
The reactivities of ML06, ML30, and ML34 Mabs with the corresponding M. leprae antigens (12-kDa protein, 65-kDa protein and its degradation products, and lipoarabinomannan or LAM), as reported previously (3,10,11,15), were reconfirmed in the present study (results not shown). No reaction could be seen with ML04 since the corresponding epitope on the 35-kDa protein is known to become denatured in the process of immunoblotting (10,11). None of these MAbs reacted with the constituents of any of the CICs upon immunoblotting.
Reaction with polyclonal antimycobacterial antibodies
With pooled LL serum. As an antibody source, pooled LL scrum reacted with an array of antigens resolved from the sonic extract of M. leprae (Fig. 3, lane a). However, when electroblotted CICs were treated with pooled LL serum, the resultant picture (Fig. 4) was difficult to distinguish from that of a direct reaction between CICs and secondary antibody (peroxidase-conjugated anti-human Ig: Fig. 2). A faint reaction at about the 26-kDa position was seen with some of the CICs (Fig. 4, arrow). The fact that this reaction was also seen with CICs prepared from the control sera suggests that it could have been caused by one of the several autoantibodies demonstrable in lepromatous leprosy patients (19).
Fig 3. Reaction of M. leprae electroblots with apooled LL serum (lane a) or anti-BCG antibody (lane b). Arrow on right = prominent 65-kDa band stainedby both polyclonal antibodies.
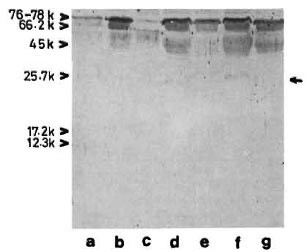
Fig. 4. Reaction of representative CIC-electroblots
with a pooled LL serum. Lane a = healthy; lanes b to d = tuberculoid; lanes e to g = lepromatous; arrow on right = faint  26-kDa band seen in some of the CICs.
26-kDa band seen in some of the CICs.
With anti-BCG antibody. The commercially available rabbit anti-BCG antibody stained M. leprae antigens in a manner similar to that of pooled LL serum except for a few differences, particularly in the low molecular weight region (Fig. 3, lane b).
The reaction of the clcctroblotted CICs with anti-BCG antibody revealed the presence of an antigen with an apparent molecular weight of 65 kDa (Fig. 5) in 12 (about 40%) of the CIC-prccipitates from leprosy patients, irrespective of the type of leprosy (Table 1). In some instances, another faint and diffused reaction (mol. wt. < 65 kDa) was observed in association with the main 65-kDa band (Fig. 5, arrow). The mycobacterial origin of this antigen was confirmed in two ways: a) incubation of the CIC-blots directly with secondary antibody (peroxidase-labeled anti-rabbit Ig) did not show any reaction, and b) optimal absorption of anti-BCG antibodies with BCG sonicate (as described under Materials and Methods) abolished the reaction with 65kDa antigen of CICs (as also with all M. leprae antigens visible in Fig. 3, lane b).
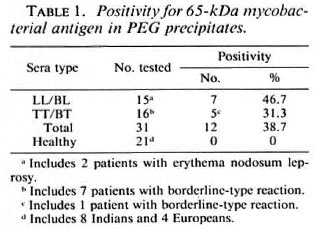
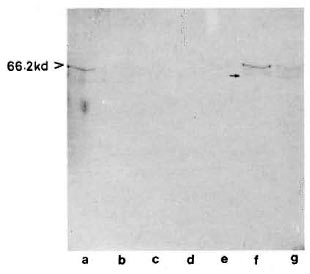
Fig. 5. Reaction of representative CIC-electroblots with anti-BCG antibody. Lanes a to c tuberculoid; lane d = healthy; lanes e to g = lepromatous. Some-times a diffused band (arrow, lane f) was seen in association with the main 65-kDa band.
None of the CICs obtained from healthy subjects showed the presence of 65-kDa or any other mycobacterial antigen.
Association of antigen positivity with different parameters
Possible associations between positivity for the apparent 65-kDa M. leprae antigen (in CICs) and the following parameters were investigated: type of leprosy (tuberculoid or lepromatous, with or without reactions); serum levels of M. leprae-specific antibodies (as determined by PGL-I-ELISA and SACT-ELISA); BI; duration of disease or treatment; and motor nerve involvement. No statistically significant association was noted with any of these parameters individually. However, we noticed a greater tendency for patients with recent (< 3 years) disease and motor nerve involvement to show a positivity for the apparent M. leprae antigen than the rest of the group. There were 5 such patients, and 4 of them (3 briefly treated LL patients with high BI values and M. leprae specific antibodies and 1 untreated TT patient with massive motor damage resulting in drop foot) were antigen positive (Table 2). Out of the remaining 26 patients, only eight were antigen positive.
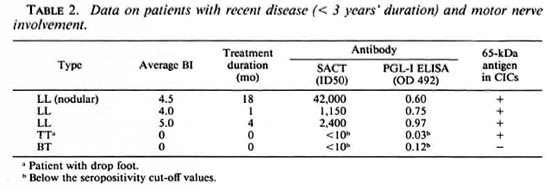
The observed lack of association between antigen positivity and the reactional states was of particular significance; neither of the 2 lepromatous patients in reaction (ENL) and only 1 out of 7 tuberculoid patients in reaction were positive.
DISCUSSION
Lepromatous leprosy patients synthesize high titers of antibodies against a variety of M. leprae antigens (9,19). Given that they also harbor a high bacterial load, one would expect the circulating immune complexes (CICs) from a majority of them to show positivity for many M. leprae antigens. Nevertheless, in our study CICs from less that 50% of the lepromatous patients turned out to be positive and, at that, for only one antigen of M. leprae origin. These observations partly corroborate the findings of Ramanathan, et al. (16) who reported positivity for anti-M leprae antibodies in CICs from only 40% of lepromatous leprosy patients (with or without reaction). The CIC-associated antigen demonstrated by us (65 kDa, a common mycobacterial antigen) is most probably identical to the one (65-69kDa antigen) reported by Chakrabarty, el al. (4) in PEG-precipitated CICs of pooled lepromatous sera, using anti-BCG antibodies.
Of considerable significance is the observed positivity for the CIC-associated M. leprae antigen in about 30% of the tuberculoid leprosy patients as well, which probably indicates that the true extent of infection in these patients may exceed the externally noticeable lesions (12). The failure to detect anti-M. leprae antibody in the CICs of such patients by Ramanathan, et al. (4) could be due to the inability of a majority of them to mount a good antibody response to M. leprae antigens (9).
In view of the known ubiquitous distribution of the 65-kDa antigen, a stress protein, across the genera and species of organisms including humans (7), the mycobacterial origin of the detected antigen was ascertained. The reaction between apparent 65kDa M. leprae antigen of CICs and anti-BCG antibody could be abolished by prior absorption of the antibody with BCG sonicate. The efficacy of this treatment was evident from the fact that it also abolished the reaction with a whole range of M. leprae antigens in parallel experiments. The 65kDa antigen is also known to exist in the form of degradation products (3,11), which most probably explains the existence of a faint and diffused band in some of the CICs investigated by us.
An array of proteins were precipitated from individual sera with 3.5% PEG due to the fact that this technique, like other techniques for this purpose, serves only to isolate a "CIC-enriched fraction" and not the CICs alone (4,16,29). Low levels of CICs occur naturally, even in healthy subjects (16,28), which is the reason for our using some PEG precipitates from normal sera also.
The four anti-M. leprae monoclonal antibodies used in this study did not react with any CIC constituent. The MAb ML30 is known to identify an epitope on the 65-kDa antigen of armadillo-derived M. leprae (3). Perhaps this epitope was absent or proteolytically deleted in the antigen present in CICs. It appears unlikely that the other monoclonal antibodies (8), not tested by us, would have provided different results since a potent polyclonal antibody like anti-BCG antibody (which showed strong reactions with a large number of M. leprae antigens) failed to identify anything except the 65kDa antigen and, at that, only in some instances. The apparent inability of a pooled LL serum to distinctly identify the 65-kDa antigen in CICs, despite reacting strongly to it in electroblotted M. leprae extract, could be attributed to either a) the occurrence of direct, overlapping reactions between certain host-serum-derived constituents of CICs and secondary antibody (Fig. 2), or b) inaccessibility of the 65-kDa antigen due to a complex formation with immunoglobulins.
A mycobacterial cell-wall-associated major stress protein like the 65-kDa antigen is a good candidate for predominant release in the blood of leprosy patients harboring substantial quantities of viable M. leprae. Our observations also indicate that the CICs were more frequently positive for the antigen in patients with recent, massive, and largely untreated infection. Further, the antibody level against the 65-kDa antigen has been reported to be relatively low (14), probably due to its partial recognition by the immune system as "self" (7). Such conditions (including low antibody levels) would favor the formation of immune complexes in "antigen excess" facilitating better antigen recognition.
Since the immune complexes have mainly been implicated in pathogenesis (28), their antigenic analysis is likely to offer clues to the pathogenic rather than immunoprotective moieties. Indeed, the 65-kDa stress protein has been held responsible for the pathogenesis of adjuvant arthritis (26). Perhaps PGL-I, an immunosuppressive moiety of the M. leprae cell wall (25), also circulates in the blood of leprosy patients (6) in the form of an immune complex due to its coexistence with antibodies and its ability to activate the complement system (17). The conspicuous absence of lipoarabinomannan (LAM), yet another mycobacterial cell-wall antigen of an immunosuppressive nature (15), in CICs analyzed by us needs to be considered. Although LAM is capable of generating an overwhelming antibody response in leprosy patients, it most probably remains deposited in the host tissue due to its affinity for cell membranes (2), and is less likely to circulate in the free (or antibodybound) form in the blood.
A specific role for M. leprae antigen-positive CICs in the pathogenesis of leprosyassociated reactions could not be established in this or previous studies (16). However, it is the total load of immune complexes, irrespective of the type(s) of constituent antigen(s), which could be more important in this context (18). Further, since the ICs which are formed or get deposited in the skin have been more directly implicated in the pathogenesis of reactions (28), the analysis of such in situ complexes may provide better insight into the pathogenic moieties of M. leprae.
Acknowledgment. We are grateful to Dr. R. J. W. Rees for providing M. leprae antigen; to Dr. J. Ivanyi for monoclonal antibodies to M. leprae; and to Dr. D. Chatterjee for ND-O-BSA. We also thank Mr. P. N. Sharma and Mr. R. Dayal for technical assistance and Mr. H. O. Agarwal for photography. The manuscript was kindly reviewed by Dr. H. Srinivasan. Some materials used in this study were generously donated by LEPRA, U.K.
REFERENCES
1. BJORVATN, B.. BARNETSON, R. ST.C , KRONVALL, G., ZUBLER, R. H. and LAMBERT, P. H. Immune complexes and complement hypercatabolism in patients with leprosy. Clin. Exp. Immunol. 26(1976)388-396.
2. BRENNAN, P. J. The carbohydrate-containing antigens of Mycobacterium leprae. Lepr. Rev. 57 Suppl. 2(1986)39-51.
3. BUCHANAN, T. M., NOMAGUCHI. H., ANDERSON, D. C, YOUNG, R. A., GILLIS, T. P., BRITTON, W. J., IVANYI, J., KOLK, A. H. J., CLOSS, O., BLOOM, B. R. and MEHRA, V. Characterization of antibody-reactive epitopes on the 65-kilodalton protein of Mycobacterium leprae. Infect. Immun. 55(1987)1000-1003.
4. CHAKRABARTY, A. K., MAIRE, M. A., SAHA, K. and LAMBERT, P. H. Identification of components of ICs purified from human sera. II. Demonstration of mycobacterial antigens in immune-complexes isolated from sera of lepromalous patients. Clin. Exp. Immunol. 51(1983)225-231.
5. CHO, S. N., FUJIWARA. T., HUNTER. S. W., REA, T. H., GELBER. R. H. and BRENNAN, P. J. Use of an artificial antigen containing the 3, 6-di-O-methyl-β-D-glucopyranosyl epitope for the serodiagnosis. J. Infect. Dis. 150(1984)311-322.
6. CHO, S. N.. HUNTER, S. W., GELBER, R. H., REA,T. H. and BRENNAN. P. J. Quantitation of the phenolic glycolipid of Mycobacterium leprae and relevance to glycolipid antigenemia in leprosy. J. Infect. Dis. 153(1986)560-569.
7. DUDANI, A. K. and GUPTA, R. S. Immunological characterization of a human homolog of 65-kilodalton mycobacterial antigen. Infect. Immun. 57(1989)2786-2793.
8. ENGERS, H. D., ABE, M., BLOOM, B. R.. MEHRA, V.. BRITTON, W., BUCHANAN. T. M., KIIANOLKER, S. R., YOUNG, D. B., CLOSS, O., GILLIS, T., HARBOE, M., IVANYI, J., KOLK, A. H. J. and SHEPARD, C. C. Results of a World Health Organization-sponsored workshop on monoclonal antibodies to Mycobacterium leprae. Infect. Immun. 48(1985)603-605.
9. GAYLORD, H. and BRENNAN, P. J. Leprosy and leprosy bacillus: recent development in characterization of antigens and immunology of the disease. Annu. Rev. Microbiol. 41(1987)645-657.
10. IVANYI, J., MORRIS, J. A. and KEEN, M. Studies with monoclonal antibodies to mycobacteria. In: Monoclonal Antibodies Against Bacteria. Macario, A. J. L. and Macario, E. C, eds. New York: Academic Press, 1985, pp. 59-90.
11. IVANYI, J., SINHA. S., ASTON, R., CUSSELL, D., KEEN, M. and SENGUPTA, U. Definition of species specific and cross reactive antigenic determinants of Mycobacterium leprae using monoclonal antibodies. Clin. Exp. Immunol. 52(1983)528-536.
12. KARAT, A. B. A., JOB, C. K. and RAO, P. S. S. Liver in leprosy: histological and biochemical findings. Br. Med. J. 6(1971)307-310.
13. LAEMMLI, U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(1970)680-685.
14. LEVIS, W. R., MEEKER, H. C, SCHULLER-LEVIS, G. B., GILLIS. T. P. and ZABRISKIE. J. Serodiagnosis of leprosy: relationship between antibodies to Mycobacterium leprae phenolic glycolipid I and protein antigens. J. Clin. Microbiol. 24(1986)917-921.
15. MORENO, C, MEHLERT, A. and LAMB, J. The in hibitory effect of myco-bacterial lipoarabinomannan and polysaccharides upon polyclonal and monoclonal human T cell proliferation. Clin. Exp. Immunol. 74(1988)206-210.
16. RAMANATHAN, V. D., PARKASH, O., RAMU, G., PARKER, D., CURTIS. J., SENGUPTA, U. and TURK, J. L. Isolation and analysis of circulating immune complexes in leprosy. Clin. Immunol. Immuno pathol. 32(1984)261-268.
17. RAMANATHAN, V. D., PARKASH, O., TYAGI, P., SENGUPTA, U. and RAMU, G. Activation of the human complement system by phenolic glycolipid I of Mycobacterium leprae. Microb. Pathogen. 8(1990)403-410.
18. RAMANATHAN, V. D, SHARMA, P., RAMU. G. and SENGUPTA, U. Reduced complement-mediated immune complex solubilization in leprosy patients. Clin. Exp. Immunol. 60(1985)553-558.
19. REA, T. H. and LEVAN, N. E. Current concepts in immunology of leprosy. areh. Dermatol. 113(1977)345-352.
20. RIDLEY, D. S. Bacterial indices. In: Leprosy in Theory and Practice. Cochrane, R. G. and Davey,T. F..eds. Baltimore: Williams and Wilkins, 1964, pp. 620-622.
21. RIDLEY, D. S. and JOPLING, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
22. SINHA, S., MCENTEGART, A., GIRDHAR, B. K., BHATIA, A. S. and SENGUPTA, U. Appraisal of two Mycobacterium leprae specific serological assays for monitoring chemotherapy in lepromatous(LL/ BL) leprosy patients. Int. J. Lepr. 57(1989)24-32.
23. SINHA, S., SENGUPTA, U., RAMU, G. and IVANYI, J. A serological test for leprosy based on competitive inhibition of monoclonal antibody binding to the MY2a determinant of Mycobacterium leprae. Trans. R. Soc. Trap. Med. Hyg. 77(1983)869-871.
24. TOWBIN, H., STAEHELIN, T. and GORDON, J. Electrophoretic transfer of proteins from Polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Nat. Acad. Sei. U.S.A. 76(1979)4350-4354.
25. VACHULA, M., HOLZER, T. J. and ANDERSON, B. R. Suppression of monocyte oxidative response by phenolic glycolipid I of Mycobacterium leprae. J. Immunol. 142(1989)1696-1701.
26. VAN EDEN, W., THOLE, J. E. R., VAN DER ZEE, R., NOORDZIJ, A., VAN EMBDEN, J. D. A., HENSEN, E. J. and COHEN, I. R. Cloning of the mycobacterial epitope recognized by T lymphocytes in adjuvant arthritis. Nature 331(1988)171-173.
27. WHO EXPERT COMMITTEE ON LEPROSY. Sixth report. Geneva: World Health Organization, 1988. Tech. Rep. Ser. 768.
28. WHO SCIENTIFIC GROUP. The role of immune complexes in disease. Geneva: World Health Organization, 1977. Tech. Rep. Ser. 606.
29. ZUULER, R. H. PERRIN, L. H., CREIGHTON, W. D. and LAMBERT, P. H. The use of polyethylene glycol (PEG) to concentrate immune complexes from serum or plasma samples. Ann. Rheum. Dis. 36 Suppl. 1(1977)23-25.
1. Ph.D., Central JALMA Institute for Leprosy (ICMR), Taj Ganj, Agra 282001, India.
2. Ph.D., Central JALMA Institute for Leprosy (ICMR), Taj Ganj, Agra 282001, India.
3. M.D., Central JALMA Institute for Leprosy (ICMR), Taj Ganj, Agra 282001, India.
4. Ph.D., Central JALMA Institute for Leprosy (ICMR), Taj Ganj, Agra 282001, India.
Reprint requests to Dr. Sinha, Scientist-C, Division of Membrane Biology, Central Drug Research Institute (CSIR), Chattar Manzil Palace, Lucknow 226001, India.
Present address for Dr. Chaturvedi: Division of Microbiology, Central Drug Research Institute (CSIR), Lucknow 226001, India.
Received for publication on 6 Mareh 1991.
Accepted for publication in revised form on 27 May 1992.