- Volume 60 , Number 3
- Page: 404–9
Delayed clearance of circulating immune complexes in mice following administration of antileprosy drugs
ABSTRACT
In this report we describe an animal experiment which showed delayed clearance of preformed 125I-HSA-anti-HSA immune complexes (with five times excess HSA) f rom the circulation of mice treated with antileprosy drugs (dapsone, clofazimine, and rifampin-multidrug therapy for 7 days) in comparison with normal (untreated) mice. The results also showed delayed retention of the preformed immune complexes in the spleen and kidneys of the antileprosy-drug treated animals. The exact mechanism of the delayed handling of preformed immune complexes in mice fed antileprosy drugs could not be ascertained. However, in light of the anticomplementary effects of clofazimine and dapsonc, as reported earlier, and in light of the large accumulation of clofazimine and rifampin in macrophages, it has been postulated that in the drug-fed animals either the immune complexes could not be phagocytosed by macrophages, through the avenue of their C3b receptors, or the immune complexes could not be downgraded easily within the macrophages overloaded with clofazimine and rifampin. These results might have clinical significance and might throw some light on the prolonged persistence of circulating immune complexes in the vascular bed of lepromatous patients even after clinical remission of erythema nodosum leprosum.RÉSUMÉ
Dans ce rapport nous décrivons une expérimentation animale qui a montré une disparition retardée de complexes immuns préformés 125I-HSA-anti-HSA (avec 5 fois plus d'HSA) de la circulation de souris traitées avec des médicaments anti-lépreux (dapsone, clofazimine, et une polychimiothérapie à la rifampicine pour 7 jours) en comparaison avec des souris normales (non traitées). Les résultats ont aussi montré une rétention retardée de complexes immuns préformés dans la rate et les reins des animaux traités avec les médicaments anti-lépreux. Le mécanisme exact de la gestion retardée des complexes immuns préformés chez les souris ayant reçu des médicaments anti-lépreux n'a pas pu être déterminé. Cependant, au vu des effets anti-complémentaires de la clofazimine et de la dapsonc, ainsi que rapportés précédemment, et au vu de la grande accumulation de clofazimine et de rifampicine au niveau des macrophages, il a été supposé que chez les animaux traités soit les complexes immuns ne pouvaient être phagocytés par les macrophages par la voie des récepteurs C3b, soit les complexes immuns ne pouvaient pas être facilement dégradés à l'intérieur des macrophages surchargés de clofazimine et rifampicine. Ces résultats pourraient avoir une signification clinique et pourraient jeter quelque lumière sur la persistance prolongée de complexes immuns circulant dans le lit vasculaire de patients lépromateux même après rémission clinique de l'érythème noueux lépreux.RESUMEN
Comparados con los ratones no tratados, aquellos tratados con una combinación de drogas antileprosas (dapsona, clofazimina y rifampina) por 7 días, muestran un retardo en su velocidad de depuración sanguínea de complejos inmunes (125I-HSA-anti-HSA) preparados en exceso de antígeno. El bazo y los riñones de los animales tratados con las drogas también mostraron una retención retardada de los complejos inmunes. No se pudo establecer el mecanismo exacto del manejo retardado de los complejos inmunes por los animales tratados. Sin embargo, sobre la base de los efectos anticomplementarios de la clofazimina y de la dapsona reportados antes y de la gran acumulación de clofazimina y rifampina en los macrófagos de los animales tratados, es posible que la fagocitosis de los complejos inmunes (vía receptores para C3b) o su degradación, no se lleven a cabo eficientemente en los macrófagos saturados con las drogas. Estos resultados podrían tener implicaciones clínicas y podrían explicar la prolongada persistencia de complejos inmunes circulantes en la red vascular de los pacientes lepromatosos, aún después de la remisión clínica de la reacción leprosa tipo eritema nodoso leproso.Most leprologists now accept that immune complexes (ICs) play a key role in the immunopathogenesis of erythema nodosum leprosum (ENL) (22,29,31). Mycobacterial antigen-antibody complexes have been demonstrated in the circulation of lepromatous leprosy patients (21). Earlier, Chakrabarty, et al. (5) identified several components present in leprosy patients. They detected IgG, C1q, C1s, C3, C-reactive protein and mycobacterial antigens of 67, 20, and 14 kDa. But, most surprisingly, the ICs were not eliminated from the circulation of ENL patients even 3 years after the subsidence of the clinical severity of the reaction(20). Furthermore, ICs are solubilized in fresh normal human sera, and this is mediated by C3 and factor B (4). These authors further showed that during an ENL reaction the patients' sera tend to lose their capacity for complement-mediated solubilization (CMS) of the ICs. Most astonishingly, this CMS of the ICs is not restored even after clinical remission of ENL. This, they suggested, is due to circulating mycobacterial antigens which consume complement by the alternative pathway. Earlier, Saha, et al.showed that at the onset of an ENL reaction, factor B and C3 are cleaved; following clinical improvement ofENL, there is elevation of serum C3 levels. These results perhaps indicated that after the subsidence of a lepra reaction, there is no further breakdown of complement component and no more activation of the complement cascade (30), although the circulating immune complexes (CICs) remain in the vascular bed (24).
Very recently, we reported that even some antileprosy drugs, such as dapsone and clofazimine, as well as antireactional drugs, such as chloroquine, aspirin and, to a lesser extent, prednisolone, are able to inhibit the CMS of preformed l25 I-HSA-anti-HSA immune complexes (15). Thus, both circulating mycobacterial antigens as well as antileprosy drugs, given to patients with an ENL reaction, prevent the CMS of ICs. These observations raise questions about the handling of CICs in leprosy patients treated with antileprosy multidrug therapy (MDT). The main aim of the present investigation was to study the kinetics of the elimination of preformed ICs from the circulation of normal mice and how it is modulated in normal animals given MDT.
MATERIALS AND METHODS
Preparation and labeling of ICs
Preparation of 125 I-HSA-anti-HSA ICs (at five times the antigen excess) has been described earlier (4). In brief, anti-HSA antiserum was raised in rabbits (3). HSA was labeled with l25 I by the chloramine-T method ( l7 ). A quantitative precipitin curve was constructed using 125 I-HSA and anti-HSA antibody. IC at five times HSA excess as compared to the equivalence point was prepared by mixing 0.35 ml l25I-HSA (1 mg/ ml) with 0.1 ml anti-HSA antiserum. The resulting insoluble precipitates were washed three times with cold phosphate buffered saline (PBS) and then suspended in PBS (13).
Drugs
MDT against leprosy included three drugs: dapsone (Wellcome, India); clofazimine (Surhind Geigy Chemicals, India); and rifampin (Lupin Laboratories, India).
Animal experiments
Animals were divided into two groups. Group A mice were control animals; Group B mice were test animals treated with MDT.
Group A animals. Tissue distribution of radioactivity following intravenous injection of labeled 125I-HSA-anti-IISA at five times antigen excess (ICxs) in normal mice: The method described by Bareelli, et al. was followed (2). Twenty-four healthy, male, Swiss albino mice each weighing approximately 20 g were divided into six groups with four animals in each group. Two-hundred µg labeled ICxs in 0.2 ml with a total radioactivity of 1.1-1.7 × 106 counts per minute (cpm) was injected into the tail vein of each mouse. Thereafter the mice in each group were sacrificed at intervals of 15, 30, 60, 120, 240, and 720 min. Blood samples were taken from the heart of each animal immediately after sacrifice, and 0.1-ml blood samples were taken in a heparinized tube. The liver, spleen, and both kidneys were collected from each animal, rinsed with 0.9% saline, blotted with filter paper, weighed, and kept in separate test tubes. The radioactivity of the whole organs was counted in a gamma counter. The radioactive counts of blood samples were also taken. The total blood volume of the animals was considered as 7% of their body weight (14). The total radioactive counts recovered in the whole blood volume from the individual animals at the various time intervals was expressed as the percentage of the initial radioactive counts in the ICxs samples injected into the animals.
Group B animals. Tissue distribution of radioactivity following intravenous injection of labeled soluble ICxs in MDT-treated mice: Another 24 healthy, adult, normal Swiss albino mice, each weighing about 20 g, were divided into six groups with four animals in each group. On the first day they were fed orally with rifampin (12 mg/kg body weight), clofazimine (1 mg/kg), and dapsone (2 mg/kg) suspended in ground nut oil (1 mg/kg). From day 2 the animals were fed daily with dapsone (2 mg/kg) and clofazimine (1 mg/kg) suspended in ground nut oil (1 ml/kg) for 6 days (26). On the eighth day the animals received 0.2 ml labeled ICxs into the tail vein. The animals were sacrificed at intervals of 15, 30, 60, 120, 240, and 720 min. The remaining procedures were the same as described for Group A.
RESULTS
The clearance kinetics of ICxs from the vascular compartment of the Group A and B animals are illustrated in Figure 1. About 40%-50% of the total radioactivity injected intravenously could be recovered from the blood of the animals at 15 min. After 720 min, about one third (15% of the total radioactivity injected) was still present in the peripheral blood of Group A mice. On the other hand, in Group B mice there was delayed clearance of ICxs (Fig. 1). Thus, 720 min after injection of ICxs about half (40%) of the total radioactive IC injected persisted in the circulation.
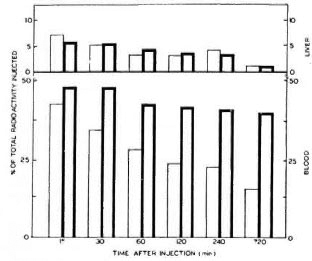
Fig. 1. Relative blood and hepatic distribution of'125I-HSA-anti-HSA immune complexes (1Cxs). Results of Group A and Group B mice are shown by thin outlined bars and thick outlined bars, respectively.There was delayed clearance of the immune complexesin Group B mice.
The retention kinetics and hepatic distribution of ICxs were by and large comparable in Group A and Group B mice (Fig. 1). At 15 min the radioactive counts were 7% and 6% in the livers of Groups A and B mice, respectively (Fig. 1); 720 min after injection, only 1.3% of the radioactivity could be detected in the livers of both groups of animals.
Figure 2 illustrates the delayed rate of retention of radioactivity in the spleen of Group B mice from 60 min onward. The initial rate of uptake of ICxs was, however, slower in Group B than in Group A animals. The percentage of uptake of the total radioactivity in the spleen was very meager in comparison to the hepatic uptake in both groups of animals. Thus, the maximum radioactivity was 0.35% in the spleen of Group A mice 15 min after injection. On the other hand, the maximum uptake in the spleen of Group B mice was 0.54%, which was very delayed (240 min) (Fig. 2).
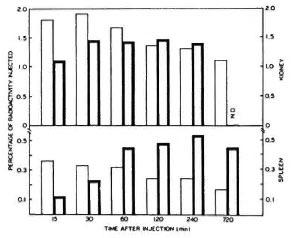
Fig. 2. Relative spleen and kidney distribution of the immune complexes. Results of Group A and Group B mice are shown by the thin outlined bars and thick outlined bars, respectively. There was increased retention in spleen and kidneys of MDT-treated mice. Each bar is the mean value obtained from 4 different mice; ND = not done.
The uptake of ICxs in the kidneys was more in comparison to the spleen but less in comparison to the liver. Thus, in Group A animals the maximum uptake of ICxs, which was 1.8% of the total radioactivity injected, was observed at 30 min. Thereafter, the radioactivity count declined to about 1.2% at hour 12. On the other hand, the rate of uptake of radioactivity in the kidneys of Group B mice was initially slower, but its retention in their kidneys remained persistently as high as 1.4% in comparison to that in Group A mice even after 240 min.
DISCUSSION
The persistence of circulating immune complexes (CICs) in the peripheral blood of Iepromatous leprosy patients is of major clinical importance because it reportedly may lead to ENL episodes (20). In the present study, we attempted to explore the mechanism of the delayed elimination of CICs from the blood of leprosy patients with ENL, even after clinical remission of the reaction (24). We thought that antileprosy drugs might have some role for this persistence of ICs in the circulation of leprosy patients because earlier we had shown that dapsone and clofazimine were anticomplementary (15,26), and it also is well known that complement plays a key role in phagocytosis (19). Here we have been able to document the delayed clearance of preformed immune complexes injected intravenously into mice fed with MDT for 7 days.
The selection of a suitable size IC is of utmost importance because the biological properties of antigen-antibody complexes as well as their FcR-mediated clearance vary significantly with their molecular composition (23). When antigen excess was moderate (five times) in HSA-anti-HSA complexes, their sedimentation pattern showed three well-defined peaks for Ag(45), Ag Ab/ Ag2 Ab(125) and AgAb2(175), of which AgAb2 was the dominant complex (18).
In a separate experiment, we had isolated soluble CICs from the sera of two lepromatous patients at the onset of ENL, as well as after clinical remission of the reaction, using 4% polyethylene glycol (6000) (24), and thereafter their sedimentation constants were determined with a Model E Analytical Ultracentrifuge (Beckman Instruments, Inc., Fullerton, California, U.S.A.). At the onset of ENL, the Svedbcrg constants were 13.2S and 15.4S; after clinical remission of the ENL episode their constants increased to 24S and 20.9S, respectively. Admittedly, the ratio ofantigen to antibody was not known. In the present experiment, therefore, we used preformed HSA-anti-HSA (five times antigen excess) of comparable Svcdberg constants (18).
The present results of delayed clearance of ICxs from the MDT-treated mice might be explained by one of the three following mechanisms:
a) The clearance of ICxs from the Group A drug-untreated mice might be facilitated by complement-mediated solubilization (CMS) of ICs following covalcnt binding of C3b, a complement component fragment of C3 with the amino-acid residue (tyrosine) ofantigen and antibody present in ICs (32). Also, solubilization of ICxs could be mediated by factor B of the alternative pathway, but the involvement of the classical pathway made solubilization faster (4). The delayed clearance of HSA-anti-HSA ICxs from the circulation of Group B MDT-fed mice (Fig. 1) was in keeping with the above notion, because recently we have shown that dapsonc and clofazimine could inhibit CMS of 125 I-HSA-anti-HSA (xs) in vitro (15). Admittedly, we have not demonstrated the in vivo impairment of CMS of IC following administration of MDT in mice for 7 days. However, if this notion is true, then 125I-HSA-anti-HSA ICxs would not be easily solubilized in MDT-fed animals and, thus, its excretion would be delayed.
b) The macrophage plays a significant role in the phagocytosis and clearance of CICs from the circulation (11). In vivo IC-macrophage interaction brings about a blockade of its Fc-receptor-mediated uptake and stimulates C3b-receptor-mediatcd ingestion of ICs (10). Thus, serum complement might facilitate the uptake of ICs by macrophages. It is believed that ICs could activate the complement system (33), leading to the deposition of C3b on the ICs, which might open up an avenue for C3b-receptor-mediated uptake (7). This mechanism might possibly be operative in the handling of serum ICs in health and disease. We have earlier shown the anticomplementary activities of dapsonc and clofazimine by both the hemolytic technique (26) and the CMS technique (15). Therefore, in Group B MT-treated mice, dapsone and clofazimine might interfere with the phagocytosis of l25I-HSAanti-HSA IC via C3b receptors of macrophages. Our observation that in vitro addition of anti-C3b antiserum to fresh human sera remarkably inhibited CMS of 125I-HSA-anti-HSA (xs) also lends support to this notion (unpublished observations). Thus, it clearly indicates that C3b helps in the solubilization of IC, perhaps by deposition of C3b on IC. Moreover, receptors for C3b are also present on red blood cells which are likely to use the receptors to pick up the complement-coated immune complexes and deliver them to Kupffer cells in the liver and spleen macrophages, blood monocytes and kidney mcsangial phagocytes. All these processes of handling immune complexes may be interfered with in the absence of the formation of C3b.
c) An alternative explanation of delayed handling of l25 I-HSA-anti-HSA ICxs from MDT-treated mice might be the large accumulation of clofazimine and rifampin in macrophages (6). Johnson, et al. (12) suggested that antibiotics perhaps either interfered with C3b receptors on the cell surface, and thus caused delayed phagocytosis, or inactivated the various hydrolytic enzymes of macrophages, leading to a loss of the ability to degrade ingested ICs within the macrophages. It has been shown that inhibitors of respiration, such as NaN 3 and KCN, reduced ingestion of ICs by 20º/o-40%, while α deoxyglucose, an inhibitor of glycolysis, led to a 30% decrease of digestion (16).
All of the blood and tissue phagocytes arise from bone marrow and other hemopoietic tissue, and they have a common progenitor (27). The delayed uptake and prolonged persistence of 125 I-HSA-anti-HSA ICxs in the spleens and kidneys of Group B MDT mice (Fig. 2) were consistent with the delayed clearance of ICs from the vascular bed (Fig. 1). The retention of 125 I-HSA-anti-HSA ICxs in the kidneys was maximum at 30 min, which was six times more than that in the spleens perhaps due to the fact that the uptake of iodine by the kidney is next to that in the thyroid (9). The uptake of soluble IC was maximum in the liver, as compared to the spleen and kidney, because soluble ICs are removed from the circulation mainly by the hepatic Kupffer cells (8). The hepatic handling of l25I-HSA-anti-HSA ICxs was similar in both Groups A and B mice (Fig. 1). This needs exploration. Perhaps the excretion of immune complexes through the bile was not affected by the antileprosy drugs.
Acknowledgment. The authors are grateful to the Indian Council of Medical Research, New Delhi, India, for financial grants to A. Kashyap and A. Sahu.
REFERENCES
1. AGUADO, M. T. and MANNIK, M. Clearance kinetics and organ uptake of complement-solubilized immune complexes in mice. Immunology 60(1987)255-260.
2. BareELLI, U., RADEMACHER, R., OOI, Y. M. and OOI, B. S. Modification of glomerular immune complex deposition in mice by activation of the reticuloendothelial system. J. Clin. Invest. 67(1981)20-27.
3. CAMPBELL, D., H., GARVEY, J. S., CREMER, N. E. and SUSSDORF, D. H. Methods in Immunology. New York: W. A. Benjamin. Inc., 1964.
4. CHAKRABARTY, A. K., KASHYAP, A., SEHGAL, V. N. and SAHA, K. Solubilization of preformed immune complexes in sera of patients with type 1 and type 2 lepra reaction. Int. J. Lepr. 56(1988)559-565.
5. CHAKRABARTY, A. K., MAIRE, M., SAHA, K. and LAMBERT, R. H. Identification of components of IC purified from human sera. Demonstration of mycobacterial antigens in immune complexes isolated from sera of lepromatous patients. Clin. Exp. Immunol. 51(1983)225-231.
6. CONALLY, M. L. and JACKSON, R. D. Uptake of reticuloendothelial cells of the remophenazone B 663. Br. J. Exp. Pathol. 43(1962)650-654.
7. DAHA, M. R. and VAN Es, L. A. The role of cellular Fc and C3 receptors on the complement-dependent degradation of stable soluble immunoglobulin aggregates by normal and trypsin-treated peritoneal macrophages. Immunology 47(1982)203-209.
8. DAHA, M. R., VEERHUIS, R. and VAN ES, L. A. Activation of complement by soluble IgG aggregates and their subsequent interaction with macrophage. Immunol. Lett. 14(1987)217-224.
9. DIEM, K. and LENTNER, J. R. Document Geigy Scientific Tables. Basle: J. R. Geigy, S.A., 1970.
10. GRIFFIN, F. M., JR. Effects of soluble immunecomplexes on Fc receptor- and C3b receptor-mediated phagocytosis by peritoneal macrophages. J. Exp. Med. 152(1980)905-919.
11. GRIFFIN, F. M., JR. and MULLINAX, P. J. ln-vivo activation of macrophage C3 receptors for phagocytosis. J. Exp. Med. 162(1985)352-357.
12. JOHNSON, J. D., HAND, W. L., FRANCIA, J. B., KING-THOMPSON, N. and CORWIN, R. W. Antibiotic uptake by alveolar macrophages. J. Lab. Clin. Med. 95(1980)429-439.
13. KABAT, E. A. and MAYER, M. M. Experimental Immunochemistry. Springfield, Illinois, U.S.A.: Charles C. Thomas, 1958, pp. 76-77.
14. KAPOOR, R. K., TRIPATHI, A. K., CHAKRABARTY, A. K. and SEN, P. Effects of preformed immune complexes on liver enzymes and their serum clearance in mice. Indian J. Med. Res. [B] 94(1991)222-227.
15. KASHYAP, A., SEHGAL, V. N., SAHU, A. and SAHA, K. Anti-leprosy drugs inhibit the complementmediated solubilization of preformed HSA-anti-HSA immune complexes in normal fresh human sera. Int. J. Immunopharmacol. 14(1991)269-273.
16. LESLIE. R. G. Macrophage handlings of solubleimmune complexes. Use of specific inhibitors to study the biochemical events involved in complex calabolism. Eur. J. Immunol. 10(1980)799-802.
17. MCCONAHEY, P. J. and DIXON, F. J. A method of trace iodination of proteins for immunologic studies. Int. areh. Allerg. Appl. Immunol. 29(1966)185-189.
18. MOLLER, N. P. H. Fc mediated precipitation. I. A new role of the Fc portion of IgG. Immunology 38(1979)631-640.
19. MORGAN, E. L. and WEIGLE, W. D. Biological activities residing in the Fc region of immunoglobulin. Adv. Immunol. 40(1987)61-134.
20. RAMANATHAN, V. D., TYAGI, P., RAMANATHAN, U., KATOSH, K., SENGUPTA, U. and RAMU, G. Persistent reduced solubilization of immune complexes in leprosy patients with reactions. Int. J. Lepr. 59(1991)5-11.
21. RIDLEY, D. S. and JOB, C. K. The pathology of leprosy. In: Leprosy. Hastings, R. C, ed. Edinburgh: Churchill Livingstone, 1985, p. 127.
22. RIDLEY, M. J. and RIDLEY, D. S. The immuno pathology of erythema nodosum leprosum: the role of extravascular complex. Lepr. Rev. 54(1983)95-107.
23. SAHA, K., CHAKRABARTY, A. K., SHARMA, V. and SEHGAL, V. N. An appraisal of third complement component (C3) and breakdown product (C3d) in erythema nodosum Icprosum (ENL). Lepr. Rev. 83(1982)253-260.
24. SAHA, K., CHAKRABARTY, A. K., SHARMA, V. K. and SEHGAL, V. N. Polyethylene glycol precipitates in serum during and after crythema-nodosum leprosum - study of their composition and anticomplementary activity. Int. J. Lepr. 52(1984)44-48.
25. SAHA, K., SHARMA, V., CHAKRABARTY, A. K. and SEHGAL, V. N. Breakdown product of factor B as an index of complement activation of lepromatous leprosy and its relation with bacillary load. Scand. J. Immunol. 17(1983)37-43.
26. SAHU, A., SAHA, K., KASHYAP, A. and CHAKRABARTY, A. K. Interaction of anti-leprosy drugs with rat complement systems. Immunopharmacology 15(1988143-150.
27. SEGAL, A. W. and SOOTHIL, J. F. Phagocytosis. In: Pediatric Immunology. Soothill, J. R., Haywood, A. R. and Wood, C. B. S., eds. Oxford: Blackwell Scientific Publications, 1983, pp. 37-47.
28. SEGAL, D., TITUS, J. A. and DOWER, S. K. The FcR-mcdiated endocytosis of mouse immune complexes by cells from the p 338 D, mouse macrophage line II. J. Immunol. 130(1983)138-144.
29. SEHGAL, S. and KUMAR, B. Circulating and tissue immune complexes in leprosy. Int. J. Lepr. 49(1981)294-301.
30. SEHGAL, V. N., SHARMA, V. and SHARMA, V. K. The effect of anti-reactional drugs on complement components in type II erythema nodosum leprosum reaction. Br. J. Dermatol. 119(1988)255-258.
31. SEHGAL, V. N., SRIVASTAVA, G. and SUNDHRAM, J. A. Immunology of reactions in leprosy: current status. Int. J. Dermatol. 27(1988)157-162.
32. TAKATA, Y., TAMARA, N. and FUJITA, T. Interaction of C3 with antigen-antibody complexes in the process of solubilization of immune precipitates. J. Immunol. 132(1984)2531-2537.
33. TYAGI, P., RAMANATHAN, D., GIRDHAR, B. K., KATOSH, K., BHATIA, A. S. and SENGUPTA, U. Activation of complement by circulating immune complexes isolated from leprosy patients. Int. J. Lepr. 58(1990)31-38.
1. Ph.D., Department of Immunology, Vallabhbhai Patel Chest Institute, University of Delhi, P.O. Box 2102, Delhi 110007, India.
2. M.B.B.S., Ph.D., Department of Immunology, Vallabhbhai Patel Chest Institute, University of Delhi, P.O. Box 2102, Delhi 110007, India.
3. Ph.D., Department of Immunology, Vallabhbhai Patel Chest Institute, University of Delhi, P.O. Box 2102, Delhi 110007, India.
4. Ph.D.,Department of Biochemistry, University College of Medical Sciences, Delhi 110095, India.
5. M.D., Assistant Director, Department of Microbiology, National Institute of Communicable Diseases, 22 Sham Nath Marg, Delhi 110054, India.
Present address for Dr. Saha: 45A Seva Bazar Street, Calcutta 700005, India.
No reprints available.
Received for publication on 16 December 1991.
Accepted for publication in revised form on 12 May1992.