- Volume 60 , Number 3
- Page: 410–6
An experimental study to evaluate the bactericidal activity of ofloxacin against an established Mycobacterium leprae infection
ABSTRACT
The bactericidal effect of a new quinolone, ofloxacin (OFLO), was determined on an established Mycobacterium leprae infection in nude mice. Various drug regimens, including combinations of drugs, were examined for different treatment periods. OFLO and rifampin (RMP) individually failed to produce significant killing after treatment with a single large dose. However, when single large doses of OFLO and RMP were given in combination, a 100-fold reduction in viability was achieved. For a longer period of treatment both of these drugs, at lower doses, produced a moderate reduction in viability. The addition of dapsone to the lower dose of OFLO resulted in a significant reduction in viability, while lower doses of RMP and OFLO together produced a moderate reduction in viability.RÉSUMÉ
L'eífet bactericide d'une nouvelle quinolone, rofloxacine (OFLO), a été evalué vis-á-vis d'une infection confirmée à Mycobacterium leprae chez la souris nue. Différents régimes thérapeutiques, incluant des combinaisons de médicaments, ont été testés pour des durées diverses. L'olloxacine et la rifampicine (RMP) administrées individuellement n'ont pu produire un effet bactéricide significatif après une administration unique à forte dose. Cependant, quand de fortes doses uniques d'ofloxacine et de rifampicine étaient administrées en combinaison, la viabilité a été réduite de 100 fois. Quand ils étaient administrés pour des périodes plus longues à doses plus faibles, chacun de ces médicaments a produit une réduction modérée de la viabilité. L'addition de dapsone à l'olloxacine administrée à faible dosage a résulté en une réduction significative de la viabilité tandis que des doses faibles de rifampicine et de l'olloxacine ensemble ont produit une réduction modérée de la viabilité.RESUMEN
Se determinó el efecto de la ofloxacina (OFLO), una nueva quinolona, sobre la infección establecida por el Mycobacterium leprae en el ratón desnudo. Se analizaron varios esquemas de tratamiento, incluyendo combinaciones de drogas y diferentes periodos de tratamiento. La OFLO y la rifampina (RMP), administradas individualmente en una dosis grande y única, no tuvieron un efecto bactericida importante. Sin embargo, una dosis grande y única de las drogas administradas simultáneamente, condujo a una reducción importante (aproximadamente 100 veces) de la viabilidad bacilar. Administradas solas en dosis más bajas y durante un período de tratamiento más largo, estas drogas produjeron una moderada reducción de la viabilidad. Mientras que la adición de dapsona a la dosis baja de OFLO dió como resultado una significante reducción de la viabilidad del M. leprae, las dosis bajas de RMP y OFLO, combinadas, produjeron sólo una moderada reducción de la misma.Although multidrug (dapsone, rifampin and clofazimine) therapy (MDT) has now become well established in the treatment of leprosy, the high incidence of dapsonc resistance, both primary and secondary, and the emergence of resistance to rifampin, make it imperative to continue searehing for new drugs with activity against the leprosy bacillus. Of the new candidate antimicrobial agents, the fluoroquinolones offer interesting possibilities. One of the earlier fluoroquinolones, ciprofloxacin, although very effective against a wide range of common gram-positive and gram-negative organisms, failed to show any activity against Mycobacterium leprae in mouse foot pad studies (1,7). One of the new fluoroquinolones, ofloxacin, has recently been shown to be extremely effective in experimental leprosy infection in mice (5,12,13), whereas pefloxacin was only partly effective (7).
In preliminary clinical trials both Pefloxacin and ofloxacin have shown rapid bactericidal activity against M. leprae, in previously untreated patients, and are currently undergoing further clinical trials (6). In view of the promising results obtained with ofloxacin in preliminary studies, we examined the effect of this compound cither as a single agent or in combination with proven antileprosy drugs, such as dapsonc and rifampin, on an established M. leprae infection in nude mice in order to determine the rate of killing of this organism by different regimens.
MATERIALS AND METHODS
Animals. It is now well established that nude mice allow extensive progressive growth of M. leprae, leading to a lepromatous type of disease in these animals (11). These mice were therefore used in the study to produce bacillifcrous disease so that the effect of treatment could be examined more accurately. Nude mice of an outbred strain (CD1) with microbiologically defined gut flora were obtained from a commercial source (Charles River, Margate, Kent, U.K.); the y were maintained in an otherwise germfree condition in isolators. Subinoculations were performed in a normal CD1 strain of mice obtained from the same commercial source (Charles River).
Experimental protocol. Human leproma-derived M. leprae maintained in nude mice were used as inocula; they had previously been found to be sensitive to both dapsone (DDS) and rifampin (RMP). The acid-fast bacilli (AFB) in a foot pad homogenate from an infected mouse were counted (10) and diluted to provide a suspension containing 5 × 10 5 AFB/ml. A 20 µl volume of the suspension was inoculated into each hindfoot pad of the nude mice (10 4 AFB/foot pad).
Infected foot pads were harvested at intervals to monitor the count, and when this had reached over 107/foot pad, which occurred about 10 months after inoculation, the mice were allotted to various groups, three animals per group, for treatment with different drug regimens as shown in Table 1. RMP and ofloxacin (OFLO) were administered by gavage. The drugs were suspended in sterile distilled water containing 0.05% agar in a mortar and pestle and freshly prepared every week. DDS was administered continuously, mixed in powdered mouse food. Groups 2, 3 and 4 had a single large dose of RMP 25 mg/kg, OFLO 450 mg/kg, and a combination of RMP 25 mg/kg plus 450 mg/kg of OFLO, respectively. Group 5 was given RMP 10 mg/kg once every 28 days, group 6 was treated with mogenizer unit (Silverson Ltd., Chesham, OFLO 150 mg/kg daily (5 days a week), and group 7 with a combination of OFLO 150 mg/kg daily plus DDS 0.01% w/w in the diet daily. Treatment of group 8 consisted of a combination of OFLO 150 mg/kg daily and RMP 10 mg/kg once every 28 days.
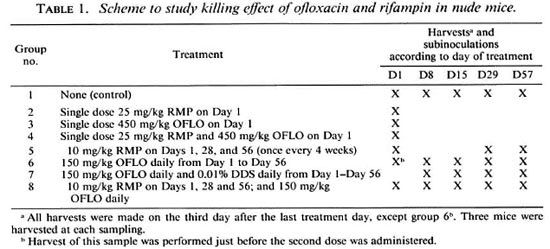
Mice from each treatment group sampled at the time intervals shown in Table 1. The maximum period of treatment was 57 days (2 months). For sampling, foot pads from three mice were dissected out aseptically, homogenized in a sealed ho Buckinghamshire, U.K.), the homogenates pooled, and the number of AFB in the pool counted microscopically as described above.
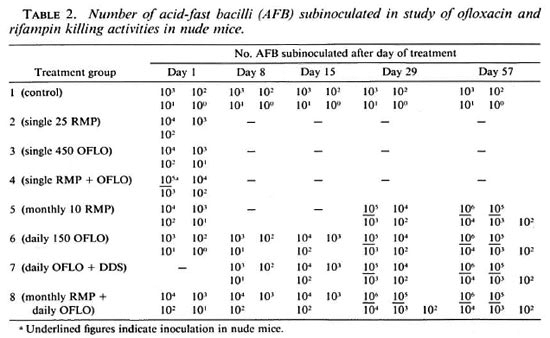
Further study was based on the determination of the bactericidal effect by the proportional bactericidal test (3,9). The bacterial suspensions from individual groups were diluted and subinoculated into groups of normal and, in some cases, nude mice. Subinoculations were carried out in accordance with the plan in Table 2 to determine the viability at the end of each treatment period. Inoculated mice were left for 12 months after which harvests of foot pads of the mice were made and the AFB counted. A positive growth was scored if there was a count of at least 105 AFB per foot pad in normal mice. The counts from the individual groups were analyzed by a computerassisted program based on a statistical analysis method (8) and the most probable number of viable bacilli per 104 inoculum determined. Comparison of mean values were analyzed by Student's t test.
RESULTS
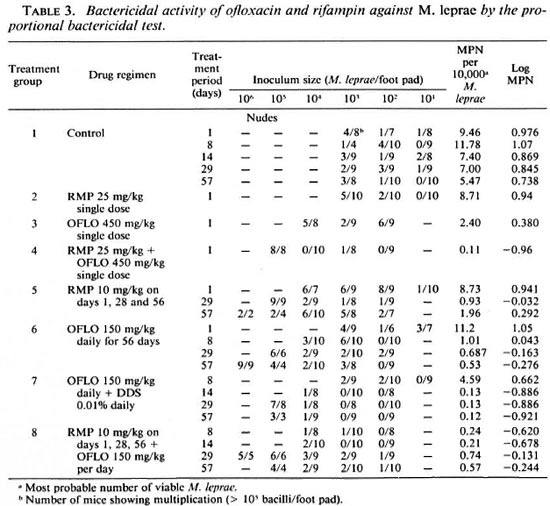
The counts from individual groups are shown in Table 3. These figures were then converted into log numbers and were plotted as shown in The Figure. It can be seen from The Figure that the control mice (group 1) showed a low initial viable count and produced a moderate reduction in viability at the end of the 57-day experimental period, i.e., 12 months after actual inoculation. This was probably because the bacilli had reached the stationary phase of growth in the nude mice, when loss of viability may be expected to begin. However when the treated groups were compared with the control, several interesting observations could be made.
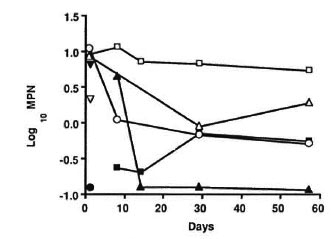
The figure. Effect of different drug regimens onthe viability of M. leprae. □ = Control; ∇ = OFLO once; ∆ = RMD × 3; ▲ = OFLO + DDS daily; ▼ =RMP once; ● = RMP + OFLO once; ○ = OFLO daily; ■ = RMP × 3 + OFLO daily.
A single large dose of RMP (group 2) failed to show any significant effect compared to the control (p > 0.05). However, a single large dose of 450 mg/kg OFLO (group 3) produced a significant reduction in viability (p < 0.05). The combination of a single large dose of RMP and OFLO (group 4) produced a 100-fold reduction in viability, much more than that produced by cither drug alone with a significance value of p < 0.001.
Both RMP 10 mg/kg once a month (group 5) and OFLO 150 mg/kg daily (group 6) produced a moderate reduction in viability but the effect became more marked as the treatment period progressed. Infact, the viability was reduced by 10-fold only after 8 days of treatment with the OFLO regimen.
The combination of OFLO 150 mg/kg daily and DDS 0.01% daily for 57 days (group 7) was found to be most effective and produced a significant reduction in viability compared to the control (p < 0.001) and also maintained the effect during the course of treatment. The viability was reduced by about 100-fold after 14 days of treatment. An enhancement of the bactericidal effect was also observed when RMP 10 mg/kg once a month was added to the OFLO 150 mg/ kg daily (group 8) regimen, but although the difference between group 7 and 8 was not statistically significant, a difference of about 1 log between these counts was evident from The Figure. A lack of a statistically significant difference was due to the fact that very low numbers of surviving bacilli were found after treatment, which gave high standard error figures. It was also interesting to note that there was no difference in the counts between group 6 and group 8, indicating that the addition of RMP to OFLO failed to produce any demonstrable additional bactericidal effect in this experiment.
As can be seen from Table 3, some groups of nude mice were also inoculated with 10 5 and 106/foot pad on the assumption that some drug combinations might produce a more rapid and complete bactericidal effect (i.e., 0/10 in all groups of normal mice). The presence of fewer than 1 in 10 4 viable organisms might be detected by the use of 10 5 and 10 6 inocula. However, almost all groups of normal mice produced some positive mice with a 10 4 inoculum and would, therefore, be expected to produce growth in almost all of the inoculated nude mice at either 10 5 or 10 6.
DISCUSSION
In the present study, the viability of M. leprae in the initial control harvest and in the subsequent harvests was unexpectedly low (about 0.1%). This resulted in some difficulty in the interpretation of the viability figures in the treated groups. Nevertheless, when the treatments were started, even at 0.1% viability, there were an estimated 10 4 viable bacilli present per 107 M. leprae. Antibiotic treatment reduced the viability by 1 to 2 logs. The low initial viability of the bacilli in this experiment was insufficient to demonstrate the 3-to-4 log reduction of viable organisms reported by others during treatment of experimental infection with RMP or OFLO (5,7). However, within the constraints imposed in the present experiment, a l-to-2 log reduction of viability should be acceptable as evidence of bactericidal action.
Using the proportional bactericidal test, we have found that individually both OFLO at 150 mg/kg daily and RMP 10 mg/kg once a month produced a killing effect, but the effect was not remarkable. There was a reduction of about 1 log after about 28 days of treatment, but there was no further reduction thereafter. When RMP was added to OFLO very little additional effect was observed, indicating that at the given concentrations significant synergy between these two agents does not occur. Nevertheless, a remarkable effect was observed when DDS was added to OFLO. DDS was given at 0.01% in the diet. Unfortunately, since no separate DDS group was included, it was difficult to interpret this 100-fold reduction of viability. However, in previous experiments we observed that DDS alone produced only a slight bactericidal effect when given at 0.01% (2). By extrapolation, it may therefore be possible to assume that this combination has produced a substantial synergy. The bactericidal drugs OFLO and RMP may have failed to synergizc significantly because both of the drugs act at the nucleic-acid level (by inhibiting gyrase and RNA polymerase, respectively) and, thus, may fail to complement each other's activity. On the other hand, DDS and OFLO being functional at two different levels of cell metabolism (i.e., DDS acting on the folic-acid biosynthesis pathway), there may be complementation of their activity resulting in synergistic action. However, although sulfone activity is well characterized in fast-growing organisms, its mechanism of action is still not very clear in mycobacteria.
In clinical practice, lack of synergy between two antimicrobials is unlikely to be of major significance. During treatment of lepromatous leprosy patients by multidrug therapy, spontaneous mutants resistant to one drug or the other are expected to be dealt with by another drug, i.e., spontaneous RMP-rcsistant mutants would be taken care of by clofazimine and DDS and vice versa. But if the organism is also DDS resistant, these spontaneous RMP-resistant mutants are virtually on monotherapy with clofazimine, a most unsatisfactory situation. The addition of a new drug such as OFLO, which is undoubtedly more effective than DDS or clofazimine to M. leprae, is likely to have a much better effect despite the fact that RMP failed to synergize with OFLO. Moreover, if the organism is DDS sensitive, inclusion of OFLO in the treatment regimen may produce a distinct advantage.
A single large dose of RMP 25 mg/kg once did not produce any reduction in viability. The killing effect of a single dose of RMP is extremely variable (unpublished data). Nevertheless, a single dose of 10 mg/kg produced a killing of approximately 50% of the organisms in normal mice (4). A single dose of OFLO at 450 mg/kg resulted in a moderate reduction in viability. Although the failure of a single large dose of RMP was somewhat unexpected, the inability of a single large dose of OFLO to produce a significant reduction in viability was not surprising, in view of the relatively slow rate of killing by the fluoroquinolones compared to RMP (6). More interesting, however, was the observation that a combination ofa large single dose of RMP and OFLO (group 4) produced a 2-log reduction of viability. In contrast, the same combination at a lower but prolonged dosage (group 8) failed to produce a similar effect.
Acknowledgment. This investigation received financial support from the THELEP component of the UNDP/World Bank/WHO Special Program for Research and Training in Tropical Diseases. The authors are grateful to Hoechst Pharmaceuticals for the provision of ofloxacin for this study. The authors acknowledge with thanks the excellent technical support provided by Mr. John Ford.
REFERENCES
1. BANERJEE, D. K. Ciprofloxacin (4-quinolone) and Mycobacterium leprae. Lepr. Rev. 57(1986)159-162.
2. COLSTON, M. J., ELLARD, G. A. and GAMMON, P. T. Drugs for combined therapy: experimental studies on the antileprosy activity of ethionamide and prothionamide and a general review. Lepr. Rev. 49(1978)115-126.
3. COLSTON, M. J., HILSON, G. R. F. and BANERJEE, D. K. The "proportional bactericidal test," a method for assessing bactericidal activity of drugs against Mycobacterium leprae in mice. Lepr. Rev. 49(1978)7-15.
4. GROSSET, J. H. and GUELPA-LAURAS, C. C. Activity of rifampin in infections of normal mice with Mycobacterium leprae. Int. J. Lepr. 55(1987)847-851.
5. GROSSET, J. H., GUELPA-LAURAS, C. C, PERANI, E. G. and BEOLETTO, C. Activity of ofloxacin against Mycobacterium leprae in the mouse. Int. J. Lepr. 56(1988)12-18.
6. GROSSET, J. H., Jr, B., GUELPA-LAURAS, C. C, PERANI. E. G. and N'DELI, L. Clinical trial of Pefloxacin and ofloxacin in the treatment of lepromatous leprosy. Int. J. Lepr. 58(1990)281-295.
7. GUELPA-LAURAS, C. C, PERANI, E. G., GIROIR, A. M. and GROSSET, J. H. Activities of Pefloxacin and ciprofloxacin against Mycobacterium leprae in the mouse. Int. J. Lepr. 55(1987)70-77.
8. HALVORSON, H . O. and ZIEGLER, N . R. Application of statistics to problems in bacteriology. I. A means of determining bacterial population by the dilution method. J. Bacteriol. 25(1933)101-121.
9. HILSON, G. R. F. and BANERJEE, D. K. The proportional bactericidal test: a method for testing in vivo bactericidal action of a persistent drug. IRCS Med. Sci. 2(1974)1037.
10. HOLMES, I. B. and HILSON, G. R. F. The effect of rifampicin and dapsone in experimental Mycobacterium leprae infections: minimum inhibitory concentrations and bactericidal action. J. Med. Microbiol. 5(1972)251-261.
11. LANCASTER, R. D.. HILSON, G. R. F., MCDOUGALL, A. C. and COLSTON, M. J. Mycobacterium leprae infection in nude mice: bacteriological and histological responses to primary infection and large inocula. Infect. Immun. 39(1983)865-872.
12. PATTYN. S. R. Activity of ofloxacin and Pefloxacin against Mycobacterium leprae in mice. Antimicrob. Agents Chemother. 31(1987)671-672.
13. SAITO, H., TOMIOKA, H. and NAGASHIMA, K. In vitro and in vivo activities of ofloxacin against Mycobacterium leprae infection induced in mice. Int. J . Lepr. 54(1986)560-562.
1. M.D., Ph.D., F.R.C.Path., Department of Medical Microbiology, St. George's Hospital Medical School, CranmerTerrace, London SW17 ORE, U.K.
2. B.Sc, Ph.D., Department of Medical Microbiology, St. George's Hospital Medical School, CranmerTerrace, London SW17 ORE, U.K.
Received for publication on 2 January 1992.
Accepted for publication in revised form on 30 April 1992.