- Volume 60 , Number 3
- Page: 436–44
Duration of multidrug therapy in paucibacillary leprosy patients; experience in the leprosy control program of the all africa leprosy and rehabilitation training center (ALERT) in Ethiopia
ABSTRACT
Multidrug therapy (MDT), according to the recommendations of a WHO Study Group of 1982, was introduced in the leprosy control program of the All Africa Leprosy and Rehabilitation Training Center (ALERT), Ethiopia, in January 1983. Of 6042 paucibacillary patients who were put on MDT during a period of 7 years, 5485 patients (90.8%) completed the course of MDT; 437 patients (7.2%) did not fulfill the requirement for clinic attendance and cither discontinued MDT themselves or the treatment was discontinued by the service. The remaining 120 patients (2.0%) either died, were transferred, left the control area or continued MDT after 9 months. The urine spot test for the presence of dapsone showed a significantly higher proportion of positive results for patients on MDT than for patients on dapsone. The analysis of the compliance with the prescribed doses of MDT showed that of 963 patients, 81.9% received six doses of MDT and 18.1%, more than six doses; 82.6% of these 963 patients attended with 100% regularity, 12.7%, 3.6%, and 1.1% missed one, two, or three clinic appointments, respectively, while fulfilling the requirement for overall clinic attendance. Of the 429 patients who had not been treated with dapsone before MDT, the skin lesions were clinically active at the time of stopping MDT in 130 patients (30.3%). In all, except one of the 114 patients (0.9%) who attended for follow-up examinations, the skin lesions had become clinically inactive within 2 years after stopping MDT. The recommended duration of MDT isdiscussed based on findings in thc ALERT leprosy control programs and observations by others.RÉSUMÉ
La polychimiothèrapie (PCT), selon les recommandations d'un groupe d'étude de l'OMS en 1982, a été introduite dans le programme de lutte contre la lèpre du "All África Leprosy and Réhabilitation Training Center" (ALERT), en Ethiopie, en janvier 1983. Des 6042 patients paucibacillaires qui furent mis sous polychimiothèrapie durant une période de 7 ans, 5485 patients (90.8%) ont complété leur traitement de PCT; 437 patients (7.2%) n'ont pas rempli la condition de présence aux cliniques et soit ont arrêté la PCT euxmêmes, soit le traitement fut arrêté par le service. Les 120 patients restants (2.0%) soit sont morts soit furent transférés, ont quitté la région ou ont poursuivi la PCT après 9 mois. Le test urinaire pour détecter la présence de dapsone a montré une proportion significativement plus élevée de résultats positifs pour les patients sous PCT que pour les patients sous dapsone. L'analyse du respect des doses prescrites de PCT a montre que des 963 patients, 81.9% ont reçu 6 doses du PCT et 18.1% plus de 6 doses; 82.6% de ces 963 patients ont eu une régularité de 100%; 12.7%, 3.6% et 1.1% ont manqué respectivement un, deux ou trois rendez-vous, bien qu'ils aient rempli les critères de régularité. Parmi les 429 patients qui n'avaient pas été traités par dapsone avant la PCT, les lésions cutanées étaient cliniquement actives au moment de l'arrêt de la PCT chez 130 patients (30.3%). Chez tous à l'exception de l'un des 114 patients (0.9%) qui se sont présentés aux examens de suivi, les lésions cutanées étaient devenues cliniquement inactives dans les 2 ans qui ont suivi l'arrêt de la PCT. La durée de la PCT à recommander est discutée sur la base des observations réalisées dans le programme de lutte contre la lèpre d'ALERT et d'observations faites par d'autres.RESUMEN
En enero de 1983 se introdujo en el programa de control de la lepra del All África Leprosy and Réhabilitation Training Center (ALERT), Etiopía, la terapia con múltiples drogas (MDT) recomendado por la OMS en 1982. De los 6042 pacientes paucibacilares sometidos a la MDT durante un periodo de 7 años, 5485 pacientes (90.8%) completaron el tratamiento; 437 pacientes (7.2%) no asistieron cumplidamente a la clínica de control y desertaron del tratamiento o éste fue suspendido por el servicio. Los 120 pacientes restantes (2.0), o fallecieron, o fueron transferidos, o dejaron el área de control, o continuaron con la MDT después de 9 meses. La prueba en orina para detectar la presencia de dapsona, tuvo una frecuencia de positividad significantivamente mayor en los pacientes tratados con la MDT que en aquellos tratados con dapsona. El análisis del cumplimiento con en el tratamiento, indicó que de 963 pacientes, el 81.9% recibieron 6 dosis de la MDT y 18.1% más de 6 dosis; el 82.6% de estos 963 pacientes asistieron con 100% de regularidad; 12.7%, 3.6% y 1.1%, faltaron a una, dos, o tres consultas, respectivamente. En 130 (30.3%) de los 429 pacientes que no habían sido tratados con dapsona antes de la MDT, las lesiones en piel fueron clínicamente activas al momento de suspender la MDT. En todos las pacientes, excepto uno (0.9%), que asistieron a los exámenes de seguimiento, las lesiones en piel fueron clínicamente inactivas dentro de los 2 años siguientes a la suspensión de la MDT. En función de los resultados de este estudio y de las observaciones por otros, se analiza cual podría ser la duración más apropiada de la MDT.The duration of treatment with multidrug therapy (MDT) is defined in terms of the number of doses of the drugs and the maximum period during which the patients should complete these doses. The usual recommendation is that paucibacillary (PB) patients should complete six monthly supervised doses of rifampin and 6 months of daily self-administered dapsone within a period of 9 months (42, 44). Of major importance is the compliance of the patients with the supervised doses of rifampin. Because patients with PB leprosy are usually not expected to harbor rifampin-resistant Mycobacterium leprae mutants, rifampin alone should be sufficient to cure these patients (43). The rationale for adding dapsone is to avoid the risk of development of rifampin resistance in patients who are wrongly classified as PB.
A marked increase in compliance of patients with MDT as compared with dapsone monotherapy has been observed in many leprosy control programs (19,32,35,37,40,42,45-48). Although the regimen is usually considered adequate to cure PB patients, several investigators have expressed concern about the recommended duration of treatment. Because a proportion of the patients still show signs of clinical activity after the six doses of MDT, they are of the opinion that the treatment period is too short (3, 6,10, 16-18, 20-23. 27, 29-31, 34, 4, 48).
This paper reports on the experience with MDT for PB patients under routine field conditions in the leprosy control program of the All Africa Leprosy and Rehabilitation Training Center (ALERT) in Ethiopia. Data are presented on the compliance of the patients with clinic attendance, and with the unsupervised intake of dapsone, and on the compliance of the services with the prescribed duration of MDT. In addition, findings on clinical activity at the time of stopping MDT and on resolution of activity in skin lesions after stopping treatment are presented. The occurrence of reversal reactions and relapses after stopping MDT and problems distinguishing between a relapse and a reversal reaction are discussed in other papers (4,5).
MATERIALS AND METHODS
Multidrug therapy, according to the 1981 recommendations of the World Health Organization (WHO), was introduced in the ALERT leprosy control program in January 1983. By January 1988, MDT had been implemented in the whole control area. Prior to introduction of MDT, the registered patients were clinically and bacteriologically examined. Indeterminate, tuberculoid (TT), and borderline tuberculoid (BT) patients, who were clinically inactive, had negative skin smears, and had attended regularly for at least 5 years or had been on treatment for over 10 years, regardless of the regularity of attendance, were released from dapsone monotherapy (15). Patients who did not fulfill the criteria for release from dapsone were treated with MDT. In addition, newly diagnosed patients, patients diagnosed with a relapse of leprosy, and patients who returned with active disease after defaulting from dapsone were given a course of MDT.
PB patients were clinically indeterminate, TT, or BT patients who had a bacterial index (BI) of not more than 1 + at any site. In January 1990 the 1988 recommendation of a WHO Expert Committee on Leprosy was introduced. This recommendation was to include patients who are clinically PB but show skin-smear positivity, for the purpose of MDT, in the multibacillary (MB) category (42).
PB patients were treated with 6 fourweekly, supervised doses of 600 mg rifampin and 100 mg dapsone daily, sclfadministered for 24 weeks. For patients younger than 15 years the dosages of the drugs were adapted according to age. The six doses of MDT had to be collected within a maximum period of 9 months. The doses of rifampin were swallowed under strict supervision by the leprosy control field staff. Since 1987 blister packs of the drugs were, for a maximum period of 3 months, given to patients who were unable to attend a clinic, e.g., during the rainy season.
MDT was discontinued in patients who failed to collect the six doses of the treatment within 9 months. Exceptions were patients who, because of a reversal reaction, were on prednisolone treatment at the time of finishing the six doses of MDT. For these patients MDT was continued until they had completed the course of prednisolone. In addition, patients who were pregnant at the time of completing the six doses of MDT, or had delivered less than 3 months earlier, had to continue the treatment until 3 months after delivery. The background for this was that studies at ALERT had shown that pregnant patients who had been released from dapsone monotherapy had a high risk (about 30%) of developing a relapse or a reversal reaction during the third trimester of pregnancy or during the first 3 months of lactation. (11,12).
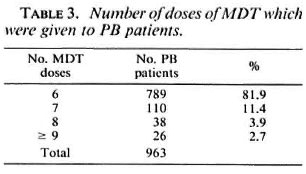
The results of MDT, which were expressed as the percentage of patients who completed the prescribed course of treatment, and the percentage of patients who, for various reasons, did not complete MDT, were monitored for 6 months' cohorts of patients. The findings are presented for all cohorts of patients who started MDT during the period January 1983 to December 1989. These cohorts included 6042 patients, of whom 70% had been treated with dapsone before MDT, 28% were new patients, and 2% were diagnosed with a relapse or with active disease after defaulting from dapsone.
In four rural Ethiopian districts and Addis Ababa, the compliance of patients with self-administration of dapsone was studied with the urine spot test for the presence of dapsone. The urine of the patients who were put on MDT during the first months of implementation ofthe treatment in these areas was examined during the fourth and sixth supervised treatment rounds. The urine was also examined in a sample of the patients who were on dapsone monotherapy in four districts where MDT had not yet been introduced. The findings in the two groups of patients were compared.
During the initial years of MDT implementation, only supervisors were responsible for the delivery ofthe supervised treatment to the patients. They attended all clinics every 4 weeks, examined the patients at the end of the treatment, and released them from MDT. Due to the steep decline in the numbers of patients on chemotherapy, it was decided in 1987 that supervisors should visit clinics with less than seven patients on MDT at 3-month intervals only. The leprosy field workers were made responsible for the delivery ofthe treatment. However, the supervisors remained responsible for the examination ofthe patients before their release from MDT. A clinical assessment at the time of release from MDT, made by experienced staff, was considered important for a later judgment on whether or not a patient had developed new activity of the disease. The practical consequence of this policy was that part ofthe patients had to continue MDT after six doses because they had not yet been examined by a supervisor.
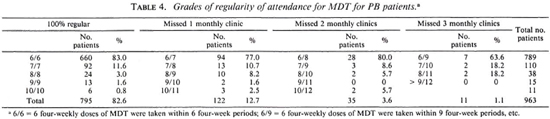
The doses of MDT which have been given to the 963 patients who started the treatment during the period July 1987 to July 1989, and who completed it, are presented. For the same group of patients, the compliance with clinic attendance was analyzed. Of the 963 patients, 429 (44.5%) had not been treated with dapsone before MDT. From the clinical records of these patients an analysis was made ofthe activity of the skin lesions at the time of stopping MDT. Skin lesions were considered inactive if they were no longer raised and erythematous. The regression of clinical activity in the skin lesions after stopping MDT was studied from the patient records.
RESULTS
Of the 6042 patients who were put on MDT during a period of 7 years, 5485 patients (90.8%) completed the treatment within the prescribed period (Table 1). The 5485 patients included those who received seven, eight, or nine doses of MDT within 9 months. These patients were, at the time of finishing six doses of MDT, cither on prednisolone, had not completed 3 months of MDT after delivery, or had not yet been examined by a supervisor. For similar reasons, the 41 patients (0.7%) who were recorded as "continued MDT" had to continue the treatment more than 9 months after they started it. The 437 patients (7.2%) who did not fulfill the requirement for clinic attendance cither discontinued MDT themselves or had their treatment discontinued by the service. The records of these patients snowed that about 60% of them received four or five doses of MDT.
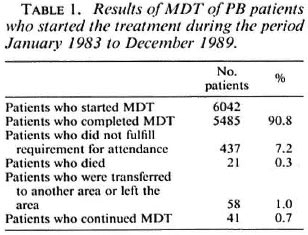
The results of the urine spot test for the presence of dapsone are given in Table 2. The difference between the percentage of positive urine during the fourth and sixth MDT treatment rounds reaches statistical significance at the 5% level (χ 2 = 4.0, p = 0.05). The percentage of positive urine is very significantly higher for the patients on MDT, the two treatment rounds combined, than for the patients on dapsone monotherapy (χ 2 = 62.5, p < 0.001).
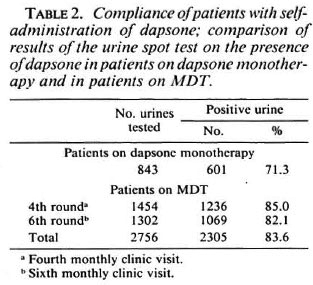
Table 3 presents the number of doses of MDT which have been given to the 963 patients. Not included are 26 patients who had to continue MDT after six doses because they were on prednisolone treatment, pregnant, or had not yet completed 3 months of MDT after delivery. In addition, 92 patients did not complete the course of MDT because of noncompliance with clinic attendance (56 patients), death (6 patients),transfer out or left the control area (30 patients).
The regularity of attendance of the 963 patients is given in Table 4. The clinical activity, at the time of release from MDT, for the 429 patients who had not been treated with dapsone before MDT is presented in Table 5. The percentage of patients whose skin lesions were clinically active is significantly higher for BT patients than for TT patients (χ 2 = 14.3, p < 0.001).
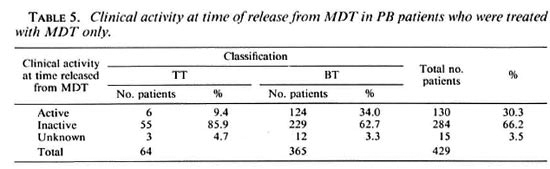
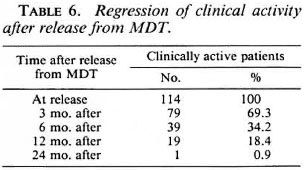
Table 6 gives the regression of clinical activity in the skin lesions of 114 patients after stopping MDT. These patients represent 87.7% of the 130 patients who were recorded as clinically active at the time of their release from MDT (Table 5). The remaining 16 patients either did not attend for follow-up examinations (10 patients) or were diagnosed with a reversal reaction after stopping MDT (6 patients). In all except one patient (0.9%), the skin lesions became clinically inactive within 24 months after stopping MDT.
DISCUSSION
A major advantage of MDT for PB patients is the limited and fixed duration of the treatment. When dapsone monotherapy was the only treatment available for routine field use, a substantial proportion of the patients discontinued the treatment prematurely. It was estimated that during the 10 years before implementation of MDT, at most 60% of the patients who were on treatment in the ALERT leprosy control program completed 5 years of dapsone (1). In a multicenter study in India, it was found that only 53% of the patients were still on dapsone 3 years after they started the treatment and 40% after 5 years (9).
Completion of the course of MDT during the prescribed period by just over 90% of the patients is a very satisfactory result. In several programs a similar result and even over 95% completion of MDT by PB patients has been observed (7,46,48). Others reported that 70% (19) and 80% (32) of the patients completed the course of MDT. Noncompliance with clinic attendance by a little over 7% of the patients is a good performance for a field program. Reports from other programs showed that 20% (32) and 30% (19) of the patients failed to collect the six doses of MDT within the prescribed period. The finding that most of the patients attended with 100% regularity has also been observed by others (48).
Whether the 437 patients who did not complete the course of MDT within the prescribed period were cured with fewer doses of the drugs is not known. Probably less than six doses of rifampin were sufficient for cure, particularly for the patients who had been treated with dapsone before MDT. Rather strict rules for discontinuation of MDT were applied to avoid the possibility that patients would remain on irregular treatment and to ensure that the main emphasis would be given to the education of the patients and staff concerning the importance of regular treatment. Based on the experience that the proportion of patients who did not comply with clinic attendance was small, perhaps they should be offered a second chance to complete a full course of MDT. If patients have to be examined by experienced staff before they are released from MDT, it is unavoidable that some of them will be treated with more than six doses of MDT. The option of continuing treatment with dapsone alone after the six doses of MDT was considered but, for practical reasons, was not pursued.
The results of the urine spot test for the presence of dapsone in patients on MDT are very encouraging. The findings are similar to those reported earlier from a few hundred patients on MDT in a small area of the ALERT leprosy control program (36). Over 80% of positive urines from patients on MDT was also reported by others (38). The significantly higher percentage of positive urines in patients on MDT compared with patients on dapsone monotherapy underlines the motivation of the patients for the treatment. Monitoring of the intake of dapsone was, however, only done with patients who started MDT during the initial months of its introduction after extensive health education campaigns. The high compliance of patients with the intake of dapsone could be a temporary effect of the introduction of new treatment. Further studies may confirm whether or not this is the case.
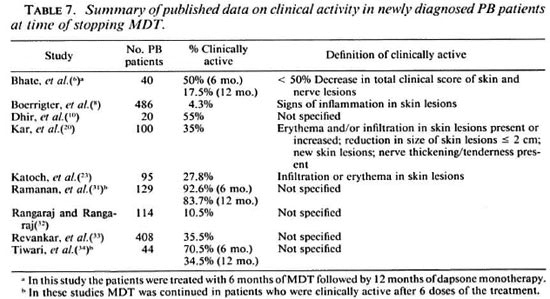
Clinical activity after 6 months of MDT has been studied by several investigators, and data from the literature are summarized in Table 7. The percentage of patients who are reported as clinically active after 6 months of MDT ranges from 4.3% to 92.6%. Possible reasons for this wide range in findings may be: a) differences in the proportion of TT and BT patients. The finding that persistent clinical activity is seen particularly in BT patients has also been reported by others (20,33). In addition, studies have shown that in the majority of TT cases (70% and more) the lesions subsided spontaneously (14,24,25). While in programs which apply large-scale, active case-finding, the portion of TT cases may be quite substantial, many of these patients will remain undiagnosed in programs which rely mainly on self-reporting by patients. This is most probably a reason for the difference between the finding in Malawi, where active casefinding was carried out and only 4.3% of the patients showed signs of clinical activity (7), and the ALERT leprosy control program, where case-finding was almost exclusively passive, b) use of different criteria for clinical activity. In several of the publications, criteria for clinical activity were not given; some investigators considered skin lesions only, while others also took nerve enlargement and tenderness into account.
The observation that clinical activity resolved after stopping MDT has also been reported by others (8,26,33,45,48). Although different criteria may have been used, this suggests that the clinical status after six doses of MDT is not an appropriate indicator for deciding whether or not the treatment period is adequate. Activity in skin lesions indicates the presence of M. leprae antigens, whether related to viable bacilli or to antigens released from dead bacilli. Bacterial antigens may persist for months or years before they are broken down and removed. This is supported by some studies on the resolution of tuberculoid granuloma in skin lesions after stopping treatment. Warndorff, et al. reported that in patients treated with eight weekly doses of rifampin, skin granuloma were still present 2 years after the start of treatment, while these had resolved 1 year later (39). Pattyn, et al. found that 40% of 60 PB patients, who were treated for 6 months with the MDT regimen recommended by the WHO, still had tuberculoid granuloma in their skin lesion biopsies 2 years after the start of MDT, 10% at 4 years, and none at 5 years. They also found that there is no difference in the resolution ofgranuloma in patients treated with dapsone compared with patients treated with regimens which included rifampin (28). Furthermore, on clinical grounds resolution of activity in skin lesions does not appear to be faster with MDT than with dapsone monotherapy (40).
That bacterial antigens may persist for some time is also illustrated by the fact that reversal reactions, which occur as a result of increased cell-mediated immunity to M. leprae antigen, can occur in PB patients 2 or more years after the start of antilcprosy treatment. These reactions do occur despite continuing treatment (40,41), as well as after stopping MDT, in the absence of evidence for a relapse of the disease (5,8). Continuation of MDT, or dapsone alone, after 6 months of MDT, until cessation of signs of clinical activity of the disease, or extension of the treatment period to 12 months, is strongly recommended by several investigators (6,10,16,18,20-23,27,29-31,34,48) and is practiced in some countries. A judgment on the effectiveness of 6 months of MDT should, however, be based on the relapse rate after stopping treatment.
As I have discussed in another paper (4), it has been observed in several leprosy control programs that the relapse rate after 6 months of MDT is low in PB leprosy, less than 1% per year, during the first years after stopping the treatment. Continuation of treatment until cessation of signs of clinical activity may be justified if it can be shown that this will decrease the risk of relapse after stopping treatment. So far, this has not been proved.
In a study in India, which included 5366 patients who had been treated with 6 months of MDT and 8861 patients who received from 7 to 12 months of MDT, no significant difference in the relapse rate (0.39% and 0.30%, respectively) was found (13). However, the period of follow up after stopping MDT, which was only 1 year, was too short to draw final conclusions.
In conclusion, there is so far no justification to deviate from the recommendation to treat PB patients with 6 months of MDT: a) The short and, certainly, the fixed duration of the treatment facilitates field operations enormously, b) There is no indication that extension of the treatment period will lead to a decrease in the relapse rate which is, in any case, low during the first years after stopping MDT. c) The findings in the ALERT leprosy control program and in other programs show that clinical activity, which is present after 6 months of MDT, resolves spontaneously after stopping treatment, d) If the duration of MDT were to be based on clinical criteria, which are apparently difficult to standardize, this would result in an extension of the treatment period beyond 6 months in a (substantial) proportion of the patients, e) This is unjustifiable on the available data and, almost inevitably, likely to be associated with poor compliance.
Acknowledgment. I would like to express my thanks to the ALERT leprosy control supervisors who collected the information on the results of completion of MDT, to the staff of the leprosy control computer department who computerized the data on attendance, and to the Dutch medical students who did most of the urine spot tests for the presence of dapsone. My thanks also go to the ALERT/AHRI Research Committee which approved my study on the operational aspects of MDT, and to Prof. Dr. A. S. Muller for his comments and suggestions.
REFERENCES
1. ALL AFRICA LEPROSY AND REHABILITATION TRAINING CENTRE. Annual reports, 1973-1982. Addis Ababa: ALERT.
2. ALL AFRICA LEPROSY AND REHABILITATION TRAINING CENTRE. Manual for implementation of multidrug therapy. 3rd rev. Addis Ababa: ALERT, 1989.
3. BECX-BLEUMINK, M. Operational aspects of multidrug therapy. Int. J. Lepr. 57(1989)540-551.
4. BECX-BLEUMINK, M. Relapses in leprosy patients after release from dapsone monotherapy; experience in the leprosy control program of the All Africa Leprosy and Rehabilitation Training Center (ALERT) in Ethiopia. Int. J. Lepr. 60(1992)161-172.
5. BECX-BLEUMINK, M. and BERHE, D. Occurrence of reactions, their diagnosis and management in leprosy patients treated with multidrug therapy; experience in the leprosy control program of the All Africa Leprosy and Rehabilitation Training Center (ALERT) in Ethiopia. Int. J. Lepr. 60(1992)173-184.
6. BHATE, R. D., GUPTA, C. M., CHATTOPADHYAY, S. P. and SINGH, I. Experience with multidrug therapy in paucibacillary leprosy. Indian J. Lepr. 58(1986)244-250.
7. BOERRIGTER, G., PONNIGHAUS, J. M. and FINE, P. E. M. Preliminary appraisal of WHO-recommended multiple drug regimen in paucibacillary leprosy patients in Malawi. Int. J. Lepr. 56(1988)408-417.
8. BOERRIGTER, G., PONNIGHAUS, J. M., FINE, P. E. M. and WILSON, R. J. Four-year follow-up results of a WHO-recommended multiple drug regimen in paucibacillary leprosy patients in Malawi. Int. J. Lepr. 59(1991)255-261.
9. COLLIER, P. J. A study of case-holding in leprosy in Asia based on duration of treatment. Lepr. Rev. 54(1983)89-94.
10. DHIR, R., GUHA, P. K. and SINGH, G. Short term chemotherapy of paucibacillary leprosy. Indian J. Lepr. 58(1986)549-554.
11. DUNCAN, M. E., MELSOM, R., PEARSON, J. M. H. and RIDLEY, D. S. The association of pregnancy and leprosy. 1. New cases, relapse of cured patient and deterioration in patients on treatment during pregnancy and lactation-results of a prospective study of 154 pregnancies in 147 Ethiopian women. Lepr. Rev. 52(1981)245-262.
12. DUNCAN. M. E., PEARSON. J. M. H., RIDLEY, D. S., MELSOM, R. and BJUNE. G. Pregnancy and leprosy: the consequences of alterations of cellmediated and humoral immunity during pregnancy and lactation. Int. J. Lepr. 50(1982)425-435.
13. EKAMBARAM, V. and RAO, M. K. Relapse rate in paucibacillary leprosy patients after multidrug therapy in North areot District. Indian J. Lepr. 63(1991)34-42.
14. EKAMBARAM, V. and SITHAMBARAM, M. Self-heal ing in non-lepromatous leprosy in the area of ELEP leprosy control project Dharmapuri (Tamil Nadu). Lepr. India 49(1977)387-392.
15. ETHIOPIA MINISTRY OF HEALTH. Manual for implementation of multiple drug therapy in Ethiopia. Addis Ababa: Ministry of Health, 1983.
16. GIRDHAR, B. K. Multi-drug therapy in leprosy. Indian J. Lepr. 59(1987)145-151.
17. GRUGNI. A., NADKARNI, N. J., KINI. M. S. and MEHTA, V. R. Relapses in paucibacillary leprosy after MDT-a clinical study. Int. J. Lepr. 58(1990)19-24.
18. INDIAN ASSOCIATION OF LEPROLOGISTS. Summary of the seminar on "consensus on treatment regimens in leprosy and problems of drug delivery." Indian J. Lepr. 56(1984)158-159.
19. KALTHOFF. P. G. The use of MDT in three western regions of Nepal. Lepr. Rev. 57Suppl. 3(1986) 106-1 14.
20. KAR, P. K., JHA, P. K., PANAYACH, J. S. and SNEHI, P. S. A clinico-pathological study of multidrug regimen in paucibacillary leprosy. Indian J. Lepr. 60(1988)235-241.
21. KATOCH, K. Recent trends in chemotherapy of paucibacillary' leprosy. ICMR Bull. 20(1990).
22. KATOCH, K., RAMANATHAN, U., NATARAJAN, M., BAGGA, A. K., BHATIA, A. S., SAXENA, R. K. and RAMU, G. Relapses in paucibacillary patients after treatment with three short term regimens containing rifampin. Int. J. Lepr. 57(1989)458-464.
23. KATOCH, K., RAMU, G. and RAMANATHAN, U. Chemotherapeutical trials with different regimens containing rifampicin in paucibacillary type of leprosy cases-a preliminary report. Indian J. Lepr. 57(1985)499-506.
24. NOORDEEN, S. K. Regression of disease in tuberculoid and maculo-anaesthetic type of leprosy. Lepr. India 42(1970)143-147.
25. NOORDEEN, S. K. Evolution of tuberculoid leprosy in a community. Lepr. India 47(1975)364-368.
26. OREGE, P. A., OBURA, M., OKELO, C, OKUKU, P., NYAWLO, J. and MOKOKHA, S. Multidrug therapy for treatment of paucibacillary leprosy. The western Kenya experience. Int. J. Lepr. 57 Suppl. 1(1989)341.
27. PALANDE, D. D. and RAMU, G. Report about informal get-together of leprologists involved in MDT. Int. J. Lepr. 56(1988)120-121.
28. PATTYN, S. R., HUSSER, J. A., BAQUILLON, G., MAIGA, M. and JAMET, P. Evaluation of five treatment regimens, using either dapsone monotherapy or several doses of rifampicin in the treatment of paucibacillary leprosy. Lepr. Rev. 61(1990)151- 156.
29. PAVITHRAN, K. Relapse of paucibacillary leprosy after short course multidrug therapy. Indian J. Lepr. 60(1988)225-229.
30. PAVITHRAN. K. Multidrug therapy for paucibacillary patients: WHO regimen inadequate? Lepr. Rev. 58(1987)306-308.
31. RAMANAN, R., MANGIANI, P. R., GHORPADE, A. and BHAGOLIWAL, S. K. Follow-up study of paucibacillary leprosy on multidrug regimen. Indian J. Lepr. 59(1987)50-53.
32. RANGARAJ, M. and RANGARAJ, J. Experience with MDT in Sierra Leone; clinical, operational and managerial analysis. Lepr. Rev. 57 Suppl. 3(1986)77-91.
33. REVANKAR, C. R., KARJIVKAR, V. G., GURAV, V. J. and GANAPATI, R. Clinical assessment of paucibacillary leprosy under multidrug therapy-three years followup study. Indian J. Lepr. 61(1989)344-349.
34. TIWARI, V. D., TUTAKNE, M. A., SINGH, G. and DUTTA, R. K. Multidrug therapy in hospitalised leprosy cases. Indian J. Lepr. 60(1988)71-76.
35. UNDP/WORLD BANK/WHO SPECIAL PROGRAMME FOR RESEAERCH AND TRAINING IN TROPICAL DISEASES. Report of the fifth meeting of the Scientific Working Group on the Chemotherapy of Leprosy. Geneva: World Health Organization, 1986. TDR/ THELEP-SWG (57) 86.3.
36. VAN ASBECK-RAAT, A.-M. and BECX-BLEUMINK, M. Monitoring dapsone self-administration in a multidrug therapy programme. Lepr. Rev. 57(1986)121-127.
37. VAN BRAKEL, W., KIST, P., NOBLE, S. and O'TOOLE, L. Relapses after multidrug therapy for leprosy: a preliminary report of 22 cases in West Nepal. Lepr. Rev. 60(1989)45-50.
38. VAN TRIER, Y. D. M. and DE SOLDENHOFF, R. Self administered dapsone compliance of leprosy patients in eastern Nepal. Lepr. Rev. 62(1991)53-57.
39. WARNDORFF, J., BOURLAND, J. and PATTYN, S. R. Follow-up on short-course 2 months' rifampicin treatment of paucibacillary leprosy. Lepr. Rev. 53(1982)9-17.
40. WATERS, M. F. R. The chemotherapy of leprosy. In: The Biology of the Mycobacteria; Volume 3. Clinical Aspects of Mycobacterial Disease. Ratledge, C, Stanford, J. and Grange, J. M., eds. London: Academic Press, 1989, pp. 406-474.
41. WATERS. M. F. R., RIDLEY, D. S. and RIDLEY, M. J. Clinical problems in the initiation of multidrug therapy. Lepr. Rev. 57 Suppl. 3(1986)92-100.
42. WHO EXPERT COMMITTEE ON LEPROSY. Sixth report. Geneva: World Health Organization, 1988. Tech. Rep. Ser. 768.
43. WHO STUDY GROUP. Chemotherapy of leprosy for control programmes. Geneva: World Health Organization, 1982.
44. WORLD HEALTH ORGANIZATION. A Guide to Lep rosy Control. 2nd edn. Geneva: World Health Organization, 1988.
45. WORLD HEALTH ORGANIZATION. Report of a con sultation on implementation of multidrug therapy for leprosy control. Geneva: World Health Organization, 1985. WHO/LEP/85.1.
46. WORLD HEALTH ORGANIZATION. Report of the consultation on technical and operational aspects of leprosy, Male, Maldives. Geneva: World Health Organization, 1990. WHO/CTD/LEP/90.3.
47. WORLD HEALTH ORGANIZATION. Report of the interregional conference on leprosy control in Africa. Brazzaville. Geneva: World Health Organization, 1989. WHO/CDS/LEP/89.1.
48. WORLD HEALTH ORGANIZATION. Report of the second coordinating meeting on implementation of multidrug therapy in leprosy control programmes, Geneva, 1986. Geneva: World Health Organization, 1987. WHO/CDS/LEP/87.2.
M.D., D.T.P.H., Plasweg 15, 3768 AK Soest, The Netherlands.
Received for publication on 11 February 1992.
Accepted for publication in revised form on 24 July 1992.