- Volume 60 , Number 3
- Page: 445–50
One-carbon requirements of Mycobacterium leprae: need for the folate pathway
Editorial opinions expressed are those of the writers.
Why another article on DDS?
The mode of action of dapsone (DDS) has been shown using classical methods; enzymes for folate biosynthesis have been demonstrated in Mycobacterium leprae. Yet, from time to time, doubts are still expressed on how DDS works. The argument goes: M. leprae is a parasite, and can fulfill its metabolic requirements by scavenging the products of its host's biosynthetic pathways. One-carbon (C-l) units, which are dependent on folate, are required essentially for biosynthetic reactions. Therefore, M. leprae should not have any metabolic requirement for C-l, for folate, and thus DDS cannot be acting (at least principally) on folate biosynthesis. However, I will argue in this editorial that M. leprae must indeed have C-l metabolism, although its requirements for C-l are likely to be far less than they would be for a free-living microbe.
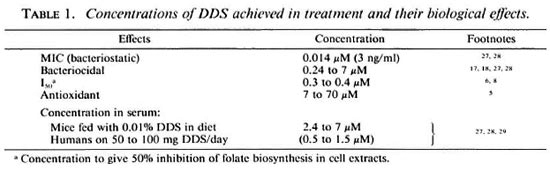
I also wish to address a paradox: DDS kills M. leprae exceptionally slowly, or is bacteriostatic (Table 1), yet its effect on essentially nongrowing M. leprae in "viability tests" or drug screens is relatively rapid.
Alternative ideas for the mode of action of DDS1
Apart from its effect on folate biosynthesis, DDS has immunomodulatory effects-mainly suppressive-and is an antioxidant. Thus, it has been suggested that DDS acts against M. leprae by perturbing the nutritional and immunological relationship between host and M. leprae or even by simply acting on the nutritional status of the host. There is a grain of truth in the concept which underlies these alternative ideas-that leprosy is a nutritional disease. The nutritional status of the host affects the working of the immune system. Then, availability of nutrients inside host cells determines the ability of intracellular parasites to grow and multiply. Sequestration of nutrients vital to intracellular parasites by the host, mediated by protective immune responses, plays a part in controlling the parasites.2-4 However, I view the foregoing concept almost as a truism, certainly applicable to a wide range of parasitic and microbial diseases, and not helping to explain the unique antileprosy effect of DDS at low concentrations. Direct action as an antioxidant is extremely unlikely since the concentration at which antioxidant effects are evident with DDS is 1000 to 10,000 times its minimal inhibitory concentration (MIC).5
Action of DDS on folate biosynthesis
DDS is a competitive inhibitor of dihydropteroate synthetase in mycobacteria (The Figure). In contrast to the action of sulfoncs on Escherichia coli, no dihydropteroate analog is formed in mycobacteria. Thus, the enzyme-substrate, para-aminobenzoic acid (PABA), is able to antagonize the action of DDS.6 However, DDS has a much higher affinity for dihydropteroate synthetase from mycobacteria than from E. coli.6 This high affinity for its target explains the relatively high sensitivity of M. lufu to DDS - M. lufu is a slow-growing but axcnically cultivable microbe with a dihydropteroate synthetase for which DDS has a very similar affinity to the synthase of M. leprae. The extraordinary sensitivity of M. leprae to DDS is probably due to factors additional to the sensitivity of the target enzyme. It is possible that the concentration of PABA is low in M. leprae.6 Furthermore, it is likely that host cells concentrate DDS.7
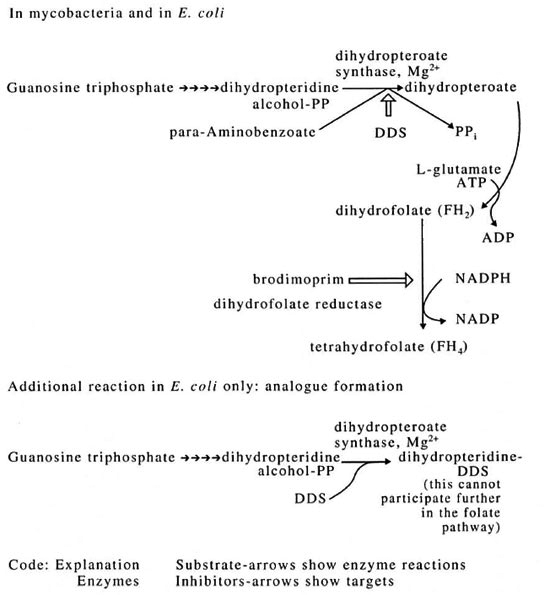
The figure. Pathway of folate biosynthesis.
DDS resistance is probably principally a result of ovcrcxpression of the gene for dihydropteroate synthetase since slightly increased enzyme is detected in resistant mycobacteria.6 There is no difference in the affinity of the enzyme for DDS between enzymes from sensitive or resistant strains of the same mycobactcrium. The decreased permeability to DDS may be a possible resistance mechanism, but this is unlikely in the strains investigated so far since brodimoprim (The Figure) is synergistic with DDS in its antimycobacterial action. 8 This latter observation depends upon dihydropteroate synthetase remaining sensitive to DDS (as established above) and DDS being able to enter the mycobactcrium. The observations on DDS resistance were made in greatest detail with M. lufu, then shown to hold for M. leprae.
One-carbon metabolism in M. leprae
For inhibitors of the folate pathway to work against M. leprae, the bacteria must have to synthesize products using the C-l donors based on FH4 (tetrahydrofolate) which are shown in Table 2.
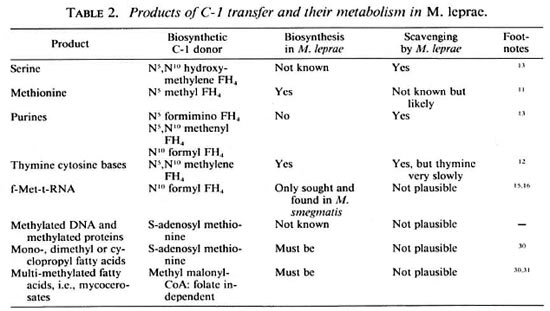
The first clue that M. leprae carries out such biosynthetic reactions is that it contains the enzymes in the folate biosynthetic pathway that have been sought (The Figure). Both dihydrofolate reductase (DHFR)9 and dihydropteroate synthetase6 have been detected, the latter at 9% to 18% of the specific activity found in other mycobacteria studied. This relative activity is quite high considering the slow growth rate of M. leprae, the high proportion of dead and moribund organisms, and in light of the experience with other enzyme assays.10 Generally, biosynthetic activities that are not used by parasites are selected against, yet M. leprae has the capability not only of synthesizing FH4 but also is known to synthesize methionine" and the cytosine and thymine bases12 which depend on FH4 (Table 2).
Could M. leprae manage without folate nevertheless? It takes up serine13 and would be expected to take up methionine since this latter activity is enhanced fourfold in M. avium when it is grown in vivo compared with when it isgrown in Sauton's medium.10 As well as apparently depending upon its host for a supply of purines,13 M. leprae can scavenge pyrimidines.12 However, thymine and thymidine are taken up only very slowly compared with other pyrimidines, uracil and uridine, and thus C-l units are needed for the conversion of the uracil base to the thymine base, a methylation reaction catalyzed by thymidilate synthase. This enzyme has been shown indirectly.12 Intriguingly, all of the enzymes for pyrimidine biosynthesis de novo can be detected in M. leprae even though intact M. leprae organisms do not synthesize pyrimidines.14 Thus, the pathway must be subject to feedback inhibition. Then it is ready to synthesize pyrimidines when the concentration of nucleotides inside the M. leprae falls, which could occur when pyrimidines are not available in the host cell for M. leprae to scavenge.14 Perhaps by having not all but the vital biosynthetic capabilities, M. leprae is able to withstand attempts by the host to prevent its growth which arise from nutritional immunity-and the folate pathway is one of the vital biosynthetic capabilities.
Even when M. leprae is scavenging all of the products that are possible to scavenge (Table 2), using methionine from the host to carry out the methylations to make products which themselves are not available directly from the host, there is one product that M. leprae must still make itself which requires FH4 . This is formyl-methionyl-t-RNA (f-met-t-RNA). f-Met-t-RNA is the product of a FH4 -dependent transformylasc. This t-RNA species is required for the initiation of translation (peptide synthesis), and is universal among bacteria. Both the transformylase and f-met-t-RNA have been confirmed in M. smegmatis.15,16
Rapid effect in DDS in in vitro viability assays
In the host, DDS acts slowly. At bactericidal concentrations around 3 µM it takes 4 months to clear 99.9% of the live leprosy bacilli in man (assessed by the kinetic method 17 ) or it kills 78% of M. leprae in mice as judged by the more quantitative proportional bactericidal method.18 In contrast, in in vitro testing systems effects are observed in less than 3 weeks, usually with concentrations which have only a bacteriostatic effect. The most illustrative examples are the inhibition of thymidine incorporation into nucleic acids of M. leprae inside macrophages in 2 weeks by 0.14 µM DDS,19 and the inhibition of hypoxanthine incorporation into nucleic acids of M. leprae in just 24 hours by 0.5µM DDS.20
How can this difference between the speeds of effects of DDS in vivo and in vitro be explained? Firstly, the measurements are not comparing like with like. The mouse footpad measurements are direct measurements of viability or of infectivity, and require growth of the leprosy bacilli. The in vitro measurements are of correlates of viability at best, are observations of metabolic status, and are judged by their ability to show, for instance, drug susceptibility. Usually, in the in vitro tests it is the statistically significant inhibition of the activity being measured that is being sought,21 and such inhibition may represent an early step in the eventual death or stasis of the microbe that is observed much later, in vivo.
Secondly, M. leprae organisms may be exquisitely vulnerable in the in vitro tests. They are stressed, as evinced by their elaboration of copious heat-shock22 and ironregulated proteins.23 Moreover-and this seems to have been overlooked-3',5' nucleotides characteristic of the stringent response are also detected in M. leprae.24 All of these separate but related responses are consistent with growth and survival in vitro.25 A major consequence of these responses is a metabolism which conserves resources and nutrients-thus RNA and protein biosynthesis are both limited. These stressed M. leprae organisms are then further stressed even in the controls in the viability assays by being transferred to an environment in which they cannot grow and - at least when the assays are done with M. leprae in macrophages-by being exposed to oxygen radicals. With no prospect of recovery, exposure of the leprosy bacilli to antileprosy agents may show rapid effects. In the case of DDS, starvation of M. leprae organisms in vitro may result in a decreased level of PABA inside the bacteria and, consequently, increased susceptibility to DDS.
Conclusions and future directions
There is no doubt that DDS is a powerful inhibitor of folate biosynthesis, and that M. leprae possess biosynthetic pathways that require folate (in its final, reduced form, FH4 ). One product of folate-based (C-1) metabolism that M. leprae cannot scavenge from its environment is formyl-methionyl-t-RNA, the universal t-RNA for initiation of translation in bacteria. No doubt because of its universality f-met-t-RNA has never been sought in M. leprae: perhaps this editorial will stimulate an examination. My idea that M. leprae in vitro is particularly vulnerable to DDS and other antilcprosy agents is based on much Research. But it is an idea which is, to some extent, an hypothesis and thus makes predictions which are testable. The rates of macromolccular biosynthesis should fall after isolation of M. leprae-they must be measured in systems from disrupted A4. leprae since levels of intracellular pools of intermediates are likely to fall. Intracellular PABA should fall. It is possible that molecules related to stress, such as 3',5' nucleotides and heat-shock proteins, may be synthesized and the latter should soon be known since it is possible to follow synthesis ofmRNA for heat-shock proteins in mycobacteria.26 Intriguingly, by following some of these parameters it may eventually be possible to assess one of the oldest of all problems in leprosy bacteriology: when, if, or how the bacteria are in an environment in which they are close to axenic growth.
- Paul R. Wheeler, Ph.D.
London School of Hygiene and Tropical Medicine
Keppel Street
London WCIE 7 HT, U .K.
1. No references are attributed to the hypotheses; they were advanced imaginatively and in good faith to try to rationalize a puzzling disease. It is not the author's intent to set up sincere contributors just to knock them down. Nevertheless, there is little experimental evidence.
2. Byrne, G. I., Lehmann, L. K. and Landry, G. J. Induction of tryptophan catabolism is the mechanism for gamma-inteferon-mediated inhibition of intracellular Chlamydia psittari replication in T24 cells. Infect. Immun. 53(1986)347-351.
3. Lepper, A. W. D. and Wilks, C. R. Intracellular iron storage and the pathogenesis of paratuberculosis. Comparative studies with other mycobacterial, parasitic or infectious conditions of veterinary importance. J. Comp. Pathol. 98(1988)31-53.
4. Pfefferkorn, E. R. Interferon gamma blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc. Nat . Acad. Sci. U.S.A. 81(1984)908-912.
5. Bergel, M. Investigaciones farmacologías y toxocologias sobre las sulfonas. Acta Leprol. 57(1974)5-49.
6. Kulkarni, V. M. and Seydel, J. K. Inhibitory activity and mode of action of diaminodiphcnylsulphone in cell-free folate synthesising systems prepared from Mycobacterium lufu and Mycobacterium leprae. Chemotherapy 29(1983)58-67.
7. Jagannathan, R. and Mahadevan, P. R. Minimum inhibitory concentration of drugs against Mycobacterium leprae as determined by an in vitro assay. J. Biosci. 10(1986)137-144.
8. Coates. E. A., Cordes, H.-P., Kulkarni, V. M., Richter, M., Schaper, K.-J., Weise, M. and Scydel, J. K. Multiple regression and principal component analysis of antibacterial activities of sulfoncs and sulfonamides in whole cell and cell-free systems of various DDS sensitive and resistant bacterial strains. Quant. Struct. Act. Relat. 4(1985)99-109.
9. Seydel, J. K., Wempe, E. G., Rosenheld, M., Jagannathan, R. and Dhople, A. M. In vitro and in vivo experiments with the new inhibitor of Mycobacterium leprae brodimoprim alone and in combination with dapsone. Arzeim. Forsch. 40(1990)69-75.
10. Wheeler, P. R. Enzymes and other metabolically active components of mycobacteria. Lepr. Rev. 57 Suppl. 2(1986)21-32.
11. Sritharan, V., Ratledge, C. and Wheeler, P. R. Aspartate metabolism in Mycobacterium avium grown in host tissue and axenically and in Mycobacterium leprae. J. Gen. Microbiol. 136(1990)203-209.
12. Wheeler, P. R. Pyrimidine scavenging by Mycobacterium leprae. FEMS Microbiol.Lett.57(1989)179-184.
13. Wheeler, P. R. Biosynthesis and scavenging ofpurines by pathogenic mycobacteria including Mycobacterium leprae. J. Gen. Microbiol. 133(1987)2999-3011.
14. Wheeler, P. R. Pyrimidine biosynthesis de novo in Mycobacterium leprae. FEMS Microbiol. Lett.57(1989)185-190.
15. Deobagkar, D. N. and Gopinathan, K. P. Two forms of methionyl-transfer RNA synthetase from Mycobacterium smegmatis. Biochem. Biophys. Res. Commun. 71(1976)939-951.
16. Deobagkar, D. N. and Gopinathan, K. P. Studies on transfer RNA from mycobacteria. Can. J. Microbiol. 24(1978)693-702.
17. Shepard, C. C. A brief review of experiences with short-term clinical trials monitored by mouse foot-pad inoculation. Lcpr. Rev. 52(1981)299-308.
18. Colston, M. J., Hilson, G. R. F. and Banerjee, D. K. The "proportional bactericidal test": a method for assessing bactericidal activity of drugs against Mycobacterium leprae. Lepr. Rev. 49 (1978)7-15.
19. Mittal, A., Sathish, M., Seshadri, P. S. and Nath, I. Rapid, radiolabelled microculture method that uses macrophages for in vitro evaluation of Mycobacterium leprae viability and drug susceptibility. J. Clin. Microbiol. 17(1983)704-707.
20. Wheeler, P. R. Measurement of hypoxanthine incorporation in purified suspensions of Mycobacterium leprae: a suitable method to screen for anti-leprosy agents in J. Med. Microbiol. 25(1988)167-174.
21. Barelay, R. and Wheeler, P. R. Metabolism of mycobacteria in tissues. In: Biology the Mycobacteria. Vol. 3. Ratledge, C, Stanford, J. and Grange, J. M., eds. London: Academic Press, 1989, pp. 37-106.
22. Young, D. B., Mehlert, A., Bal V., Samperio, P. M., Ivanyi, J. and Lamb, J. R. Stress proteins and the immune response to mycobacteria -antigens as virulence factors? Antonie van Leeuwenhoek 54(1988)431-439.
23. Sritharan, M. and Ratledge, C. Iron-regulated proteins of mycobacteria grown vitro and their occurrence in Mycobacterium avium and Mycobacterium leprae grown in vivo. Biol. Metals 2(1990)203-208
24. Lee, Y. N. and Colston, M. J. Measurement of ATP generation and decay in Mycobacterium leprae in vitro. J. Gen. Microbiol. 131(1985)3331-3337.
25. Lindquist, S. The heat shock response. Ann. Rev. Biochem. 55(1986)1151-1192.
26. PATEL, B. K. R., Banerjee, D. K. and Butcher, P. D. Characterization of the heat shock response in Mycobacterium bovis BCG. J. Bacteriol. 173(1991)7982-7987.
27. Ellard, G. A. The chemotherapy of leprosy. Part 1. Int. J. Lepr. 58(1990)704-716.
28. Waters, M. F. R. The chemotherapy of leprosy. In: The Biology of the Mycobacteria. Vol. 3. Ratledge, C, Stanford, J. and Grange, J. M, eds. London: Academic Press, 1989, pp.405-476.
29. Zuidema, J., Hilbers-Modderman, E. S. M. and Merkus, F. W. H. M. Clinical pharmacokinetics of dapsone. Clin. Pharmacokin. 11(1986)299-315.
30. Minnikin, D. E. Lipids: complex lipids, their chemistry, biosynthesis and roles. In:The Biology of the Mycobacteria. Vol. I. Ratledge, C. and Stanford, J., eds. London: Academic Press, 1982, pp. 37-106.
31. Rainwater, D. L. and Kolattukudy, P. E. Fatty acid biosynthesis in Mycobacterium tuberculosis var bovis BCG; purification and characterization of a novel fatty acid synthase, mycocerosate synthase, which elongates n-fatty acyl CoA with methylmalonyl CoA. J. Biol. Chem. 260(1985)616-623.