- Volume 60 , Number 3
- Page: 485–7
Clarithromycin at very low levels and on intermittent administration inhibits the growth of M. leprae in mice
To The Editor:
Clarithromycin was found previously to inhibit the metabolic activity of Mycobacterium leprae in cell-free and tissue culture systems (2). Furthermore, it had been determined that when M. leprae-infected mice were fed a diet containing clarithromycin [0.01% (4) and 0.1% (5) and 25 mg/kg by gavage five times weekly (7)], bactericidal activity for M. leprae was consistently observed. In none of these studies was clarithromycin administered at a dietary concentration which was found ineffective and, therefore, we initiated these current studies to define clarithromycin's minimal inhibitory dietary, serum, and tissue concentrations for M. leprae in infected mice. The World Health Organization (14) has for the past decade advocated monthly rifampin in the treatment of leprosy. Since a companion antimicrobial agent which has proven activity on intermittent administration might offer particular utility in the practical delivery of effective therapy to leprosy patients in endemic countries, in these studies we also determined the efficacy of intermittent clarithromycin administration in this mouse model system.
Eight-week-old, female, BALB/c weanling mice (Jackson Laboratories, Bar Harbor, Maine, U.S.A.) were infected in both hind foot pads with 5000 M. leprae. The M. leprae isolate utilized in this study originally had been obtained from a lepromatous leprosy patient, maintained in mouse passage in our laboratory for the previous 10 years, and was harvested from the foot pads of mice near the time of its peak multiplication. From the time of infection, groups of mice were continuously fed diet containing various concentrations of clarithromycin [0% (control), 0.0001%, 0.001%, 0.005%, 0.01%, 0.05%, 0.1%]. Also, groups of mice were treated with 0.1 % in diet 3 days weekly (M, W, F), 1 day weekly, and 1 day monthly. At 7, 9, and 11 months after infection, the number of M. leprae from the foot pad pools of two mice (four feet) from each group were determined by standard microscopic procedures(12).When the number of M. leprae per foot pad was found to be > 10 5 , bacillary growth was considered to have occurred (13).
At 7 months, M. leprae grew to 2 × 105 foot pad in the untreated control mice, and remained at approximately that level at the 9- and 11-month harvest intervals (The Table). Clarithromycin 0.0001 % in the diet did not inhibit M. leprae multiplication (The Table). However, clarithromycin 0.001%, 0.005%, 0.01%, 0.05%, and 0.1% each prevented M. leprae multiplication at all three harvest intervals (The Table). Clarithromycin 0.1% in the diet 3 days weekly and even 1 day weekly, also, consistently inhibited the growth of M. leprae, while multiplication of M. leprae in mice treated only once monthly was inhibited both at 7 and 9 months, but not at the 11-month interval (The Table).
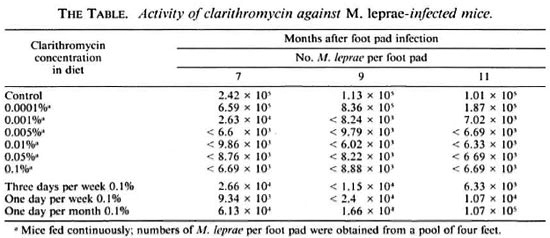
Also, in these studies, mice were fed 2 weeks of each of the test diets, and clarithromycin concentrations in the mouse serum (five mice) and foot pads (one mouse) were determined by an agar disk diffusion method employing Micrococcus luteus ATCC 93418 (3) (Abbott Laboratories, North Chicago, Illinois, U.S.A.). In the untreated control mice and those treated with 0.0001%, 0.001%, 0.005%, and 0.01%, clarithromycin scrum levels were consistently < 0.025 µg per ml and foot pad concentrations < 0.7 µg/g. Clarithromycin 0.05% in the diet resulted in serum levels of 0.16 ± 0.11µg/ml and a foot pad level of 1.7 µg/g, while clarithromycin 0.1% in diet resulted in serum levels of 0.36 ± 0.23 µg/ml and foot pad level of 3.3 µg/g.
These studies have demonstrated that low dietary, serum, and tissue levels of clarithromycin inhibit the growth of M. leprae in mice. Previously, it was found that 0.01% and 0.1% dietary clarithromycin prevented the multiplication of M. leprae in mouse foot pads. In this study, 0.001% dietary clarithromycin and all higher tested concentrations (0.005%, 0.01%, 0.05% and 0.1%) consistently prevented the growth of M. leprae in infected mice. Furthermore, clarithromycin was found effective on intermittent administration, consistently when administered in the diet 1 day and 3 days weekly and even partially active when administered 1 day monthly. Of the three new candidate antimicrobials that appear promising for the therapy of leprosy, minocycline (6) and now clarithromycin, but not fluoroquinolones (10), are active against M. leprae-infected mice when administered less frequently than three times per week.
Previously we found that against M. leprae-infected mice, dietary erythromycin (0.06%) and azithromycin (0.1%) were consistently inactive. On the other hand, clarithromycin (0.1%) and roxithromycin (0.1%) were consistently active and, in fact, bactericidal, clarithromycin being found more active than roxithromycin. Franzblau and Hastings (4) found that while clarithromycin (0.01%) was bactericidal for M. leprae, erythromycin (0.01%) and roxithromycin (0.01%) were inactive. These current studies demonstrate that a tenfold lower concentration of clarithromycin than had been studied previously, 0.001% clarithromycin, has activity for M. leprae. Thus, it would appear that, as had been found previously for both M. avium (2,9) and M. chelonae(1), clarithromycin is superior to other macrolides in its activity for M. leprae.
The levels of clarithromycin found in the mouse serum and foot pad with 0.1% administered in the diet were of the same order of magnitude (4,5), albeit somewhat lower than the levels generated by that same diet in previous studies. Because of the lack of sufficient sensitivity of the bioassay, the minimal inhibitory serum concentration against M. leprae could not be determined. However, it was certainly remarkably low, < 25 ng/ml. Assuming the same linear relationship between dietary concentration and resultant serum levels obtained between dietary concentrations of 0.01% and 0.001% as was found at 0.1% and 0.05%, the minimal inhibitory serum concentration for clarithromycin against M. leprae would be < 2.5 ng/ml, approximately that found previously only for dapsone against M. leprae, 3 ng/ml (8). Because a small 400mg human dose of clarithromycin results in a peak serum level of 1.1 µg per ml and in mice the highest dietary concentration tested, 0.1% dietary clarithromycin (100-fold greater than minimal effective dietary concentration), results in serum levels of approximately threefold less, there is every reason to believe that the pharmacokinetics in man would result in clarithromycin being equally effective in the treatment of human disease.
In conclusion, the very low dietary, serum, and tissue concentrations required to inhibit M. leprae found in these studies, and the fact that clarithromycin has been found consistently bactericidal against M. leprae in mice, lend optimism to the application of clarithromycin in the treatment of leprosy. Because clarithromycin is active on intermittent administration in mice and also at levels easily obtained in man, there are prospects that clarithromycin might, as with rifampin, be applied in some intermittent schedule for the treatment of lepromatous leprosy patients.
- Robert H. Gelber, M.D.
Lydia P. Murray
Patricia Siu
Mabel Tsang
Medical Research Institute of California Pacific Medical Center
2200 Webster Street
San Francisco, California 94115 and
GWL Hansen's Disease Center
Carville, Louisiana 70721, U.S.A.
Acknowledgment. These studies were supported by a grant from Abbott Laboratories and contract #9007- 02 from the GWL Hansen's Disease Center in Carville, Louisiana. We want to thank Roger Hill for his assistance in the preparation of this manuscript.
REFERENCES
1. BROWN, B. A., WALLACE, R. J., JR., ONYI. G. O., DE ROSAS, V. and WALLACE, R. J., III. Activities of four macrolides, including clarithromycin, against Mycobacterium fortuitum, Mycobacterium chelonae, and M. chelonae-like organisms. Antimicrob. Agents Chemother. 36(1992)180-184.
2. FERNANDES, P. B., HARDY. D. J., MCDANIEL. D., HANSON, C. W. and SWANSON, R. N. In vitro and in vivo activities of clarithromycin against Mycobacterium avium. Antimicrob. Agents Chemother. 33(1989)1531-1534.
3. FERNANDES, P. B., RAMER, N., RODE, R. A. and FREIBERG, L. Bioassay for A-56268 (TE-031) and identification of its major metabolite, 14-hydroxy6-O-methyl erythromycin. Eur. J. Clin. Microbiol. Infect. Dis. 7(1988)73-76.
4. FRANZBLAU, S. G. and HASTINGS, R. C. In vitro and in vivo activities of macrolides against Mycobacterium leprae. Antimicrob. Agents Chemother. 32(1988)1758-1762.
5. GELBER, R. H. Activities of various macrolide antibiotics against Mycobacterium leprae infection in mice. Antimicrob. Agents Chemother. 35(1991)760-763.
6. GELBER, R. H., SIU, P., TSANG, M., ALLEY, P. and MURRAY, L. P. Effect of low-level and intermittent minocycline therapy on the growth of Mycobacterium leprae in mice. Antimicrob. Agents Chemother. 35(1991)992-994.
7. Ji, B., PERANI, E. G. and GROSSET, J. H. Effectiveness of clarithromycin and minocycline alone and in combination against experimental Mycobacterium leprae infection in mice. Antimicrob. Agents Chemother. 35(1991)579-581.
8. LEVY, L. and PETERS, J. H. Susceptibility of Mycobacterium leprae to dapsone as a determinant of patient response to accdapsone. Antimicrob. Agents Chemother. 9(1976)102.
9. NAIK, S. and RUCK, R. In vitro activities of several new macrolide antibiotics against Mycobacterium avium complex. Antimicrob. Agents Chemother. 33(1989)1614-1616.
10. PATTYN, S. R. Activity of ofloxacin and pefloxacin against Mycobacterium leprae in mice. Antimicrob. Agents Chemother. 31(1987)671-672.
11. RAMASESH. N., KRAHENBUHL, J. L. and HASTINGS, R. C. in vitro effects of antimicrobial agents on Mycobacterium leprae in mouse peritoneal macrophages. Antimicrob. Agents Chemother. 33(1989)657-662.
12. SHEPARD, C. C. and MCRAE, D. H. A method for counting acid-fast bacteria. Int. J. Lepr. 36(1968)78-82.
13. SHEPARD. C. C. Statistical analysis of results obtained by two methods for testing drug activity against Mycobacterium leprae in mice. Int. J. Lepr. 50(1982)96-101.
14. WHO STUDY GROUP. Chemotherapy of leprosy for control programmes. Geneva: World Health Organization, 1982. Tech. Rep. Ser. 675.