- Volume 60 , Number 1
- Page: 13–7
Multidrug therapy in multibacillary leprosy; experience in an urban leprosy center
ABSTRACT
The article records the experience of treating multibacillary (BB, BL and LL) leprosy with multidrug therapy (MDT) in an urban leprosy center. The problem of leprosy is to be properly assessed throughout the Indian subcontinent because most of the epidemiological data f rom the areas labeled low-endemic have to be updated. The regularity of therapy must be ensured and monitored constantly, but in spite of our efforts to do so some factors were beyond our control, such as providing a means of livelihood for the migrants f rom other places. In addition, the intake of drugs also has to be periodically checked f rom the history and discoloration of skin and, most importantly, confirmed by performing random spot tests for dapsone in the urine. The main problems discussed are the difficulty in demonstrating acid-fast bacilli in slit-skin smears f rom the macular form of borderline leprosy (also called dimorphous macular) and, secondly, whether the duration of multibacillary therapy was adequate since only approximately 50% of our patients achieved smear negativity after taking MDT for the stipulated period of 24 months. Experiences f rom other centers have suggested that the duration of MDT should be prolonged in multibacillary patients to achieve smear negative status. Yet anothergroup notes that smear negativity is gradually achieved during the period of surveillance following stoppage of MDT after 24 months. Thèse questions await more information f rom good centers with controlled field studies.RÉSUMÉ
L'article rapporte le traitement de la lèpre multibacillaire (BP, BL et LL), par la polychimiothérapie (PCT) dans un centre urbain de traitement de la lèpre. Le problème de la lèpre doit être évalué de manière adéquate à travers le sous-continent Indien parce que la majorité des données épidémiologiques provenant des régions dites à basse endémicité doivent être remises à jour. La régularité du traitement doit être assurée et surveillée de manière constante, mais en dépit de nos efforts pour agir en ce sens, certains facteurs n'ont pas pu être contrôlés, tels que la fourniture de moyens de subsistance pour les émigrants d'autres régions. De plus, la prise effective des médicaments doit également être contrôlée périodiquement par l'anamnèse ainsi que par la coloration cutanée, et, ce qui est très important, confirmée en réalisant de manière aléatoire des tests pour la recherche de dapsone dans les urines. Les principaux problèmes discutés sont premièrement la difficulté de démontrer des bacilles acido-résistants dans les frottis cutanés, dans les formes maculaires de la lèpre borderline (également appelée forme maculaire dymorphe), et deuxièmement de savoir si la durée du traitement de la lèpre multibacillaire était adéquate puisque seulement environ 50% de nos patients ont atteint une négativation de leur frottis cutané après avoir pris la PCT pour la durée recommandée de 24 mois. Des expériences réalisées dans d'autres centres ont suggéré que la durée de la PCT devrait être prolongée chez les patients multibacillaires pour obtenir la négativation des frottis. Cependant un autre groupe rapporte que la négativation des frottis est progressivement atteinte durant la période de surveillance suivant l'arrêt de la PCT après 24 mois. Ces questions nécessitent de plus amples informations en provenance de centres bien organisés qui réalisent des essais contrôlés sur le terrain.RESUMEN
En este articulo se registra la experiencia adquirida en un centro dermatológico urbano en relación con el tratamiento de pacientes multibacilares (BB, BL y LL) con la terapia multidrogas (MDT). El problema de la lepra debe valorarse apropiadamente en el subcontinente Indio porque la mayoría de los datos epidemiológicos en las áreas llamadas de baja endemia deben ser actualizados. Algunos factores fuera de control, tales como el proporcionar un sitio de habitación a los inmigrantes de otros lugares, entorpecen la vigilancia y el registro de la regularidad de la terapia. La ingestión de las drogas tiene que corroborarse periódicamente en función de la historia y la decoloración de la piel y, más importantemente, debe confirmarse haciendo muéstreos al azar para demostrar la presencia de dapsona en la orina de los pacientes. Los principales problemas que se discuten son la dificultad para demostar bacilos ácido resistentes en los extendidos de linfa cutánea de los pacientes con lepra dimorfa macular y, segundo, si la duración de la terapia multidrogas fue adecuada, puesto que sólo aproximadamente-el 50% de los pacientes de este centro alcanzaron la negatividad bacilar después de tomar la MDT durante el periodo estipulado de 24 meses. Las experiencias de otros centros han sugerido que la duración de la MDT debe prolongarse en los pacientes multibacilares hasta que se logre la negatividad bacilar en los extendidos de linfa cutánea. Otros grupos, sin embargo, indican que la negatividad bacilar se logra gradualmente después de terminar los 24 meses de MDT. Estos aspectos requieren más información por parte de buenos centros dermatológicos con estudios de campo controlados.Multibacillary (MB) leprosy patients constitute the most important group epidemiological because in the absence of effective therapy they can spread the disease to healthy contacts. Thus, the most important component of any leprosy control program should aim at early detection of such cases to make them noninfectious by multidrug therapy (MDT). Cure must be ensured by regular therapy, bacteriological investigations, and surveillance for the recommended period of 5 years (19,20). The present paper is a status report covering the various aspects of multibacillary (MB) leprosy, namely, the therapy, regularity of treatment, and management of complications. The Discussion also highlights the problems of ensuring regularity in patients and the criteria for declaring the patient cured.
OBSERVATIONS
Table 1 shows that a total of 855 MB patients out of a total of 1363 leprosy cases were included in the study spanning a 2 ½ year period from 1988 to June 1990. Among them, 95 (11.11%) were from the Union Territory of Delhi comprising the indigenous group; the rest of them (760) were mainly from the states of Haryana, Uttar Pradesh, Bihar, and West Bengal, and the neighboring countries such as Bangladesh and Nepal.
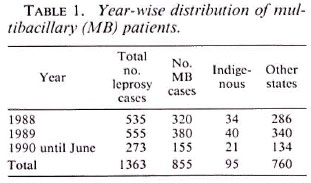
According to the clinical features the patients were categorized, as shown in Table 2, as: 362 borderline leprosy (BB). 202 borderline lepromatous (BL), and 291 lepromatous leprosy (LL). A lepromin test (Mitsuda reaction) done in untreated patients was weakly positive in some BB cases and negative in the rest. A bacterial index (BI) from the standard six sites was done in 693 (81.05%) patients who had not received antiLeprosy therapy before; 292 BB, 176 BL, 225 LL. The BI positivity varied from 2 + to 6+ depending on the position of the disease in the MB leprosy spectrum. Seventy of the 292 BB cases with only macular lesions exceeding 10 in number had a negative BI. The remaining 162 out of the 855 total cases had taken partial or complete treatment elsewhere and, as a result, their smears were negative and they had registered here for either completion of treatment or follow-up during the surveillance period or both. A routine histopathological study was performed in 594 (70%) patients (BB 240. BL 152 and LL 202), and the picture was consistent with the clinical diagnosis. However, in the dimorphous macular (BB) group comprising 50 untreated patients, a biopsy was confirmative in only 20 cases. In the rest a patchy perivascular infiltrate of lymphocytes and a moderate number of histiocytes were seen in the dermis. On serial sections acid-fast bacilli (AFB) could be demonstrated in 30 out of these 50 cases and the BI was 1 +. All of the patients were given MDT as recommended by the World Health Organization (WHO) (19) with the addition of rifampin for the first 15 days as modified by the Government of India (3), i.e., rifampin 600 mg/day for the first 15 days and then 600 mg/month supervised, clofazimine 300 mg/month supervised and 50 mg/day unsupervised, and dapsone (DDS) 100 mg/day unsupervised.
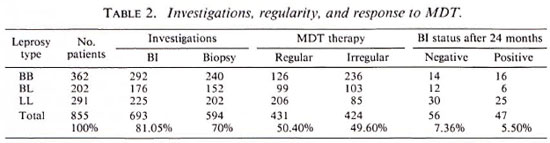
The patients came at monthly intervals for clinical assessment and continuation of therapy. The BI was repeated once in 3 months and the biopsy once in 6 months.
A spot test fordapsone in the urine was done randomly to check if the patient was taking the drugs regularly. Only 431 (50.40%) patients were regular (defined as those who took the drug for not less than 9 months in a year); the rest were irregular with many absconding after the first of the first few visits.
Of the 431 patients who took regular therapy, 103 completed the stipulated period of 24 months of which 56 were declared both clinically and bacteriologically cured with the BI becoming negative. The pretreatment BI of these 56 cases was 1+ to 3+ in 36, 3+ to 4+ in 16, and 4+ to 5+ in 4; However, 47 patients still showed positivity varying from 1+ to 2 + ; their BI before commencement of MDT was 4+ to 6+ in 28 patients and 3+ to 4+ in 19 patients.
Reactions were seen in a total of 89 (10.40%) patients: 23 in BB, 16 in BL, and 50 in LL. They were cither admitted to the hospital or treated as outpatients depending upon the severity of the reactions. Most reactions subsided on giving aspirin and nonsteroidal antiinflammatory drugs. Oral corticosteroids were given in moderate-to-severe reactions, particularly when neuritis was a predominant feature. In some LL and BL patients with erythema nodosum leprosum (ENL), thalidomide was used with excellent results. Ten patients had recurrent reactions which subsided over time without discontinuation of MDT.
Trophic ulcers were seen in 21 (2.47%); six had bony involvement and had to be hospitalized. Claw hand was seen in 42 (4.94%) and foot drop in 14 (1.65%). These patients were advised physiotherapy and given appropriate splints.
DISCUSSION
It has been adequately stressed that leprosy should be regarded as a problem of the entire country without any epidemiological division into low-or high-endemic zones mainly because the data from these areas are based on obsolete surveys (5) and, secondly, because the increasing intermingling of people from various places giving rise to new settlements is a continuous feature of growing towns and cities. This is evident from a recent report of the prevalence of leprosy in Delhi (11).
The problem of ensuring regular therapy has been the major issue in many centers. In a well-established center where 22% of the patients were irregular, the main reasons recorded for the lack of compliance were inadequate health education, unsuitable timing of the clinic, and intolerance to dapsone(17). However, only 50% ofour patients were regular. The irregular ones were mainly from the neighboring states. The only means of persuading them to come was motivation during the initial visit, writing letters to their place of work and native place, and giving additional benefits such as railway tickets. Some did return and resumed regular treatment with this approach but others did not. This again is because, in contrast to rural areas and towns, a cosmopolitan city like Delhi attracts people who primarily seek temporary jobs as a means of livelihood and patients also seek confirmation of their disease against the impossibility of being told that they do not have leprosy (12).
Apart from collecting the drugs, their ingestion has to be checked periodically also because attendance at the clinic correlates poorly with regularity of intake of these drugs as seen even in reputed centers (8,9,14). The worst consequence of this may be the emergence of drug-resistant bacilli which may be difficult to treat in cases of multiple drug resistance. This can be kept in check only when tests to detect dapsone in the urine are performed in leprosy centers or by devising regimens containing high degrees of supervised drug administration in areas with poor compliance (6).
We had included the macular form of BB, also called dimorphous macular, in the MB group although slit-skin smears and histopathology did not reveal AFB in a significant number of cases. This macular form of BB is peculiar in the sense that AFB are difficult to demonstrate. The clinical evolution and histopathological features of dimorphous macular leprosy indicate that this should be placed in the MB group since careful examination reveals AFB in the dermal nerves (4). Further, AFB also have been demonstrated in nasal mucosal biopsies from such dimorphous macular patients, indicating that they are infective (G. Ramu, personal communication). Thus, in practice when investigations are not forthcoming, it is reasonable to include patients having multiple hypopigmented macules, particularly more than 10 because that is the upper limit for borderline tuberculoid leprosy, in the MB group. This would also put an end to their erroneous inclusion in the indeterminate group which is, unfortunately, still continuing (2).
DURATION OF THERAPY IN MB PATIENTS
The latest WHO Expert Committee's recommendation is that MDT should be given for at least 2 years and, wherever possible, up to smear negativity (19). In our study, out of 103 patients who completed 24 months of MDT, 56 achieved smear negativity but 47 did not. A similar observation has been recorded in another study although the majority became negative after 24 months of MDT (3). Studies from other countries have also shown that 2 years may not be sufficient to achieve smear negativity (10,16), particularly in patients showing a high BI before initiating MDT, and the relapse of MB leprosy after MDT has also been recorded (18). This had prompted workers to increase the duration of MDT to 5 years (10) or 7 years (1) to achieve smear negativity in MB cases. This slow clearance of the BI is a recognized problem related to MDT that has not been satisfactorily resolved (15). The question of stopping therapy has been discussed recently in light of the observations by other workers, and the authors opine that treatment can be stopped after 2 years irrespective of smear negativity since these patients registered a bacterial decline and became smear negative during the period of surveillance without any specific therapy (7). However, this relevant and serious question awaits further clarification on the possibility of relapse which can be answered only by well-organized studies in centers involving large numbers of patients.
REFERENCES
1. ACHENBACH, R. E. Experience on mixed and supervised treatment of leprosy on the basis of multidrug regimen proposed by the World Health Organization. Rev. Argent. Dermatol. 70(1989)58-59.
2. AGARWAL, U. S., HANDA, A. K., MATHUR, D., MEHTA, R. D., MITTAL, A., DHAR, N. and MATHUR, N. K. Hypopigmented lesions in early leprosy-clinical and histological study. Indian J. Lepr. 62(1990)416-421.
3. CHOPRA, N. K., AGRAWAL, J. S. and PANDYA, J. S. Impact of multidrug therapy in leprosy in Baroda district (Gujarat). Indian J. Lepr. 61(1989)179-189.
4. COCHRANE, R. G. Signs and symptoms. In: Leprosy in Theory and Practice. Cochrane, R. G. and Davey, T. F., eds. Bristol: John Wright, 1964, p. 257.
5. DHARMENDRA. Strategy of leprosy eradication in India. Indian J. Lepr. 58(1986)511-512.
6. ELLARD, G. A., PANNIKAR, V. K., JESUDASAN, K. and CHRISTIAN, M. Clofazimine and dapsone resistance in leprosy. Lepr. Rev. 59(1988)205-213.
7. GANAPATI, R., PAI, R., GANDEWAR. K. L. and THRESSIA, X. J. For how long should a multibacillary leprosy patient be treated? Indian J. Lepr. 61(1989)467-471.
8. GIRDHAR, A.. MISHRA. B., BAGGA, A. and GIRDHAR, B. K. Drug compliance among self-motivated leprosy patients. Indian J. Lepr. 60(1988)506-509.
9. HUIKESHOVEN, H. C. J.. HONHOFF. C, VAN EYS, G. J. J. M., ANTEN, J. G. F.. MAYER, J. M. A. and VAN HELDEN, H. P. T. Weekly self-medication of leprosy patients monitored by DDS/creatinine ratio in the urine. Lepr. Rev. 47(1976)201-209.
10. Li, W., et al. Effects of multidrug therapy on multibacillary leprosy for 3 years. China Lepr. J. 4(1988)129-131.
11. M ISRA. R. S. and R AMESH, V. Leprosy in the Union Territory of Delhi. Indian J. Lepr. 59(1987)292-299.
12. MISRA, R. S., RAMESH, V. and NIGAM, P. K. Leprosy in low endemic areas of India: an appraisal and suggested methods for control. Indian J. Lepr. 61 (1989) 345-340.
13. Multidrug regimen advised for treatment of leprosy cases. Directorate General of Health Services (Leprosy Division). New Delhi: Ministry of Health and Family Welfare. Government of lndia. 1985.
14. NAIK, S. S. and GANAPATI, R. Regularity of dapsone intake by leprosy patients attending an urban leprosy centre. Lepr. India 49(1977)201-215.
15. NOORDEEN, S. K. Multidrug therapy (MDT) and leprosy control. Indian J. Lepr. 62(1990)448-458.
16. PAKSOY, N. and LEVI, Y. Preliminary assessment of multidrug therapy in leprosy patients in Western Samoa. N. Z. Med. J. 102(1989)580-591.
17. RAGHAVIA, M., BANSAL, R. D., SRINIVASA, D. K., S OUNDARASANANE, M. B. and RAMAN, G. Absenteeism in leprosy patients in a rural area in Tamil Nadu. Indian J. Lepr. 59(1987)322-329.
18. VAN BRAKEL, W., KIST, P., NOBLE, S. and O' TOOLE, L. Relapse after multidrug therapy in leprosy: a preliminary report of 22 cases in west Nepal. Lepr. Rev. 60(1989)45-50.
19. WHO EXPERT COMMITTEE ON LEPROSY. Sixth report. Geneva: World Health Organization, 1988. Tech. Rep. Ser. 768.
20. WHO STUDY GROUP. Chemotherapy of leprosy for control programmes: report of a WHO Study Group. Geneva: World Health Organization, 1982. Tech. Rep. Ser. 675.
1. D.V.&D., M.D.. Dermatologist., Department of Dermatology and Leprology, Safdarjang Hospital, New Delhi 110029, India.
2. D.D., M.D.. Senior Dermatologist., Department of Dermatology and Leprology, Safdarjang Hospital, New Delhi 110029, India.
3. D.V.&D., Chief Medical Officer (NFSG)., Department of Dermatology and Leprology, Safdarjang Hospital, New Delhi 110029, India.
This article is a modification of a presentation at the Workshop on Multidrug Therapy of Leprosy in Low-endemic Areas of India, 19-22 November 1990, New Delhi. India.
Received for publication on 22 March 1991.
Accepted for publication in revised form on 5 November 1991.