- Volume 60 , Number 1
- Page: 28–35
Histopathological monitoring of an immunotherapeutic trial with Mycobacterium w
ABSTRACT
Immunotherapeutic trials with Mycobacterium w (M. w.) on multibacillary patients are in progress at two large hospitals in New Delhi. A total of 380 patients so far have been inducted into the trial. The histopathological profile of the initial 87 patients (52 in the vaccine group, 35 in the control group) who have now completed 2 years of treatment are presented in this report. The vaccine group received multidrug therapy (MDT) and eight intradermal injections of M. w. every 3 months; the control group had MDT with starch injections as a placebo. Skin biopsies were taken at induction and thereafter at every 6 months. The results show a significantly higher proportion of biopsies with histopathological upgrading and/or clearance of dermal granuloma among the vaccinated cases. The number of patients becoming bacteriologically negative was higher in the vaccine group. There was no increase in the degree of neural inflammation in the biopsies showing upgrading. The lepromin site biopsy in patients who converted to positivily after vaccination showed epithelioid cell granulomas as did the biopsies f rom the nodules developing at the vaccination sites. The histopathological observations confirm the additional immunotherapeutic effect of M. w. used along with standard MDT therapy.RÉSUMÉ
Des essais immunothérapeutiques avec le Mycobacterium w (M. w.) sont en route chez des patients multibacillaires dans deux grands hôpitaux de New-Delhi. Jusqu'à présente un total de 380 patients ont été inclus dans l'expérimentation. Le profil histopathologique des 87 patients de début (52 dans le groupe vacciné, 35 dans le groupe témoins) qui ont maintenant achevé deux années de traitement est présenté dans cet article. Le groupe des vaccinés à reçu une polychimiothérapie (PCT) et 8 injections intradermiques de M. w. à intervalles de trois mois; le groupe témoin recevait une polychimiothérapie avec des injections d'amidon comme placebo. Des biopsies cutanées furent prises au début de l'étude et dans la suite tous les 6 mois. Les résultats montrent une proportion significativement plus élevée de biopsies d'un grade histopathologique plus élevé, et/ou une disparition des granulomes dermiques parmi les cas vaccinés. Le nombre de patients devenant négatifs du point de vue bactériologique était plus élevédans le groupe des vaccinés. Il n'y avait pas d'augmentation dans le degré d'inflammation des nerfs dans les biopsies montrant une inversion. La biopsie du site d'injection de la léprominc chez les patients qui ont viré à la positivité après vaccination a montré des granulomes cellulaires épithélioides tout comme les biopsies des nodules s'étant développés au niveau des sites de vaccination. Les observations histopathologiques confirment l'effet immunothérapeutique traditionnel de M. w. avec une polychimiothérapie standard.RESUMEN
En dos grandes hospitales de Nueva Delhi se llevan a cabo ensayos terapéuticos con Mycobacterium w (M. w) en pacientes multibacilares. Hasta ahora se han incluido 380 pacientes en el ensayo. En este reporte se presenta el perfil histopatológico de los 87 pacientes iniciales (52 en el grupo vacunado, 35 en el grupo control) quiénes ahora han completado 2 años de tratamiento. El grupo vacunado recibió tratamiento con múltiples drogas (MDT) y 8 inyecciones intradérmicas de M.w, cada 3 meses; el grupo control recibió MDT e inyecciones de almidón como placebo. Al inicio del ensayo, y cada 6 meses después, se tomaron biopsias de piel. Los resultados muestran una proporción significativamente mayor de biopsias con histopatologia de granulomas en remisión (upgrading) en la mayoría de los casos vacunados. El número de pacientes bacteriológicamente negativos ha sido mayor en el grupo vacunado. No ha habido incremento en el grado de inflamación neural en las biopsias que muestran remisión. La biopsia del sitio de la lepromina en los pacientes que se convirtieron a positivos después de la vacunación, mostró granulomas con células epitelioides, como lo hicieron los nodulos que se desarrollaron en los sitios de vacunación. Las observaciones histopatológicas confirman el efecto inmunoterapéutico adicional del M.w, usado junto con la quimioterapia estándar con múltiples drogas.Mycobacterium w is a cultivable, saprophytic mycobacterium on trial as a candidate vaccine against leprosy (16). Initial animal experiments have confirmed the organism's immunogenicity and its ability to evoke delayed-type hypersensitivity (DTH) reactions on challenge with M. leprae (4,11,12,15). Subsequent studies have also shown that Mycobacterium w gives a very good correlative DTH reaction with M. leprae in tuberculoid leprosy patients ( 5,6,8,13,14). The first phase of immunotherapeutic trials brought out the fact that Mycobacterium w was able to convert the negative Mitsuda lepromin reaction in lepromatous leprosy (LL) patients to a stable positivity in a significant number of individuals (2). The second phase of the immunotherapeutic trials with this organism on multib'acillary patients is in progress at two large hospitals in Delhi, India.
This report presents the histopathological profile of patients who have now completed 2 years of treatment under the trial. The clinical, bacteriological and other observations on this group of patients at the end of the first year of treatment have been reported earlier (17,18).
MATERIALS AND METHODS
The trial is being carried out at the Departments of Dermatology of Safdarjang Hospital and of Ram Manohar Lohia Hospital, New Delhi. Patients inducted into the trial were randomly allocated into one of two groups. The vaccine group patients received eight doses of a suspension of killed Mycobacterium w by intradermal injection at 3 monthly intervals along with standard multidrug therapy (MDT) for a period of 2 years. Patients in the control group received a similar dose of starch suspension in addition to MDT.
All patients underwent a clinical examination, a skin-smear test and a lepromin test with armadillo lepromin containing 40 x 106 killed bacilli per ml every 3 months and a skin biopsy every 6 months. Repeat skin biopsies were taken from the same lesion as far as possible. In addition, biopsies were taken from vaccination and positive lepromin sites. All biopsies were fixed in 10% Formalin in phosphate buffer, pH 7.2, processed, embedded in paraffin, and 5- µ m sections were cut and stained with hematoxylin and eosin (H&E) and File's stain.
Of a total of 380 patients so far inducted into the trial, the initial 96 were on a singleblind trial where the reporting histopathologist (AM) was not informed to which group each patient belonged; the remaining patients are on a double-blind trial in which coded samples of vaccine and placebo are being used. This initial report is based on the changes seen in 87 patients from the single-blind trial who have completed 2 years of treatment. Of these, 52 belonged to the vaccine group and 35 to the placebo group. The Ridley-Jopling five-group classification scale was used to grade all biopsies in this study. Diagnostic terms that were used in addition to the standard grades of lepromatous (LL), borderline lepromatous (BL), borderline (BB), borderline tuberculoid (BT) and tuberculoid (TT) leprosy are as follows: 1. Indeterminate-This term was used for a biopsy showing perineural or intraneural lymphocytic infiltration with or without the presence of acid-fast bacilli (AFB) in the nerve twigs. Granulomas were absent and no AFB were seen in other dermal structures. 2. Nonspecific dermatitis- This term indicated a biopsy showing only a mild-to-moderate degree of lymphocytic infiltration in the dermis. No histological evidence of leprosy was present. The lymphocytes were either randomly scattered or found around blood vessels and appendages. Nerve twigs did not show any inflammatory cells. No AFB were identified even on the staining of multiple sections. 3. Normal-Skin with dermis and epidermis both within normal limits and with no inflammatory infiltrate.
The granuloma fraction (GF) and a bacterial index (BI) (Ridley scale) were recorded for each biopsy.
RESULTS
Tables 1 to 3 depict the comparative histological grading at induction and after 1 and 2 years of treatment. Thus, 41 out of a total of 52 patients (78.8%) in the vaccine group demonstrated cither an upgrading (Figs. 1,2 and 3) or a clearance of granuloma from the lesion (Fig. 4), while the same was seen in 18 out of 35 (51.4%) patients in the placebo group. This was found to be statistically significant using the test of proportion (p < 0.01). The difference was more marked in the LL and BL groups than in the BB and BT groups. Clearance of granuloma from the dermis without upgrading was seen in 24 patients in the group receiving immunotherapy and MDT as against 12 in the control group (Table 4). Histological upgrading with or without subsequent granuloma clearance was observed in 17 and 6 patients in the two groups, respectively. Both sets of data were statistically significant (p < 0.05). Type 1 reactions accompanied the upgrading in 13 patients in the former group and 6 patients in the latter (Table 5).
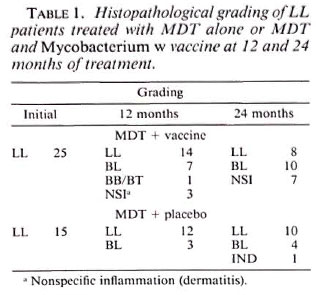
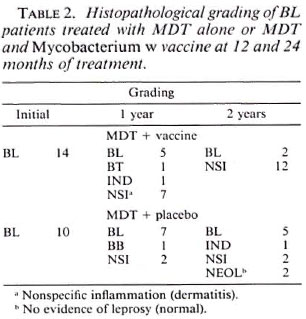
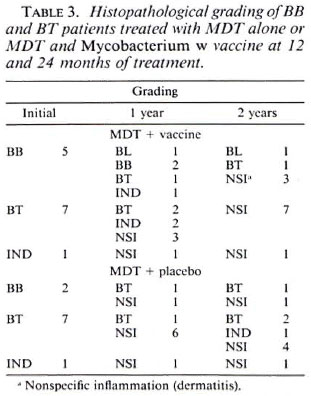
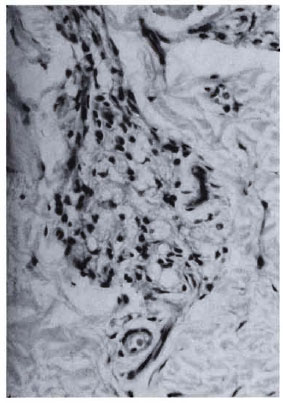
Fig. 1a. Dermal granuloma from biopsy taken atinduction, showing histiocytes with a few scattered lymphocytes. Histological BI 3+; patient classified asLL (H&E x 400).
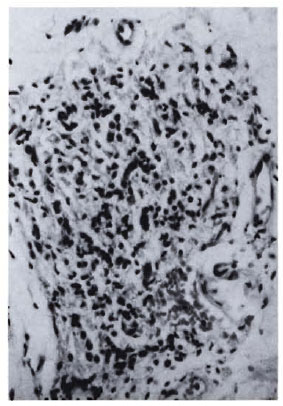
Fig. 1b. Dermal granuloma from repeat biopsy of same patient taken after completion of 24 mo. of chemotherapy and immunotherapy with Mycobacterium w . Histiocytes have lost their vaculoated character, and there is a heavy influx of lymphocytes into the granuloma. Histological BI 0+; patient classified BL at thisstage (H&E x 400).
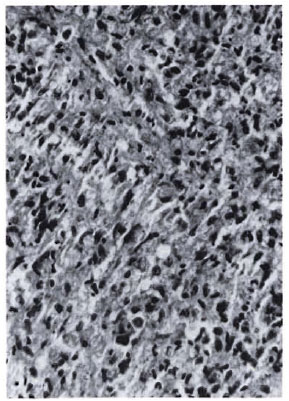
Fig 2a. Dermal granuloma from biopsy taken at induction, showing histiocytes with a scattering of lymphocytes. Histological BI 6+; patient classified LL (H&E x 400).
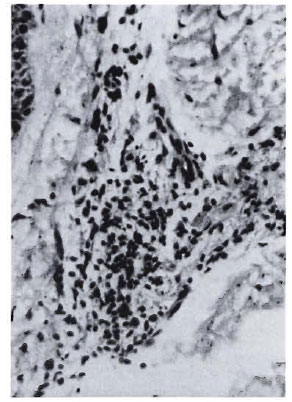
Fig. 2b. Dermal granuloma from biopsy of samepatient taken after completion of 24 mo. of chemotherapy and immunotherapy with Mycobacterium w ,showing focal collection of histiocytes with heavy influx of lymphocytes. Histological BI 1 +; patient classified BL (H&E x 400).
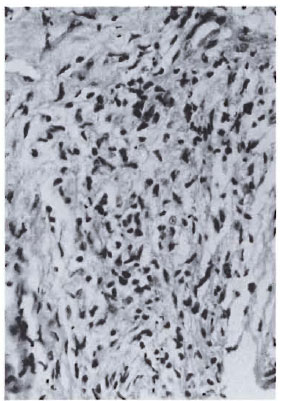
Fig. 3a. Dermal granuloma from biopsy taken atinduction, showing histiocytes with equal proportionof scattered lymphocytes. Histological BI 4+; patient classified BL (H&E x 400).
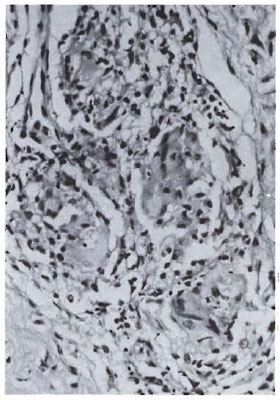
Fig. 3b. Dermal granuloma from biopsy of samepatient taken after completion of 6 mo. of therapy with MDT + Mycobacterium w. Focal epithelioid cell granuloma formation with early giant cells is seen. Lymphocytes are sparse but arranged in periphery of granuloma. Histological BI 0+; patient classified BT (H&E x 400).
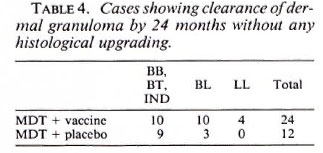
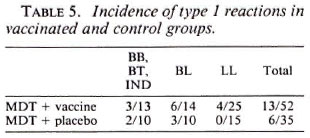
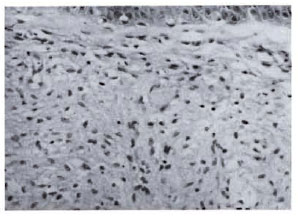
Fig. 4a. Dermal granuloma from biopsy at induction, showing typical foam cell infiltrate with clear subepidermal zone. Histological BI 6+; patient classified LL (H&E x 400).

Fig. 4b. Dermal granuloma from repeat biopsy ofsame patient after 24 mo. of therapy with MDT + Mycobacterium w . Dermis shows only sparse perivascular lymphocytic infiltration in superficial zone. Histological BI 0+; biopsy reported as nonspecific inflammation (H&E x 400).
The fall in the histological BI between the two groups was also different. Twenty-five out of the 41 (61%) patients who were bactcriologically positive at induction did not show any bacilli in the skin biopsy by 2 years in the vaccine group; the same was seen in 13 out of 30 (43.3%) in the placebo group. While this difference fell just short of the level of statistical significance, the rate of fall of the BI as seen in the slit-skin smears was found to be significantly greater in the vaccine group using analysis of variance (ANOVA) (p < 0.01). Analysis of the GF data revealed a gradual fall in both groups with no significant difference being seen between them. The histological data observed so far correlates well with the improvement in clinical scoring, fall in slitskin smear BI and lepromin conversion.
Biopsies from vaccination sites (Fig. 5) showed a granulomatous response with formation of epithelioid cell and giant cell clusters around a central area of necrotic debris, probably representing the injected material.
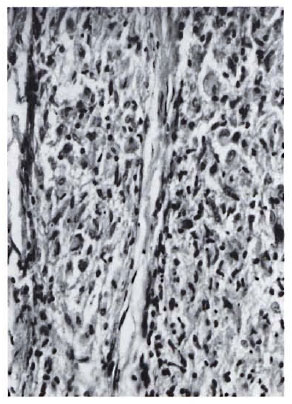
Fig. 5. Section of biopsy of nodule formed at vaccination site 21 days after inoculation with Mycobacterium w . An epithelioid cell granuloma with mild lymphocytic infiltration is seen (H&E x 400).
Biopsies from lepromin-reaction sites in lepromin-converted individuals revealed a variable pattern, with some patients showing typical features of positive DTH reaction in the form of well-developed epithelioid and giant cell granulomas while others showed cellular clusters in the dermis with poorly formed epithelioid cells in the central portions of the clusters.
Particular attention was paid to dermal nerve twigs in vaccinated cases with histological upgrading to check for any associated increase in nerve damage. Increased perineural or intraneural inflammatory cell infiltration, swelling of nerve twigs, or appearance of caseation in the nerve bundles were looked for and found to be absent in all of the biopsies.
DISCUSSION
The present trial with Mycobacterium w was designed to test the immunotherapeutic effect of Mycobacterium w when used in conjunction with a standard chemotherapeutic regimen in multibacillary patients.
For comparison, a group of patients receiving chemotherapy and a placebo was followed as a control. For obvious ethical reasons, a group receiving only immunotherapy could not be included.
The histologic observations bring out three points for consideration: a) there was a significant difference in the histologic response to therapy in the two groups, b) biopsies from lepromin reaction sites from patients who converted to lepromin positivity after vaccination showed features of DTH reaction, and c) Mycobacterium w it'was seen to stimulate a granulomatous reaction in intradermal injection in lepromin-negative patients.
The histologic response occurred in either of two patterns of histological change. Thus, there was a histological upgrading, i.e., a shift from the LL toward the TT end of the leprosy spectrum along with reduction in granuloma size or, alternatively, a clearance of the dermal granuloma with the histological classification remaining the same. Both indicate a positive response to therapy and were seen in a significantly larger proportion of patients in the vaccine group compared to the placebo group. Earlier studies on the histologic responses to immunotherapy and chemotherapy combinations have mainly concentrated on the immunological upgrading achieved. Thus, the retrospective study (9) from Venezuela on patients receiving M. leprae and BCG combinations along with chemotherapy revealed immunological upgrading in 88.3% of patients in the vaccine group. However, no control group was available for comparison of data in this report which was a retrospective analysis of patients treated over a number of years. In another study from Bombay, India (1,3), where killed ICRC bacilli was administered in addition to chemotherapy with dapsone, Deo, et al. noted that reversal reactions developed in 5 out of 46 vaccinated patients with subsequent histopathological examination confirming upgrading of the lesions. Similar observations had earlier been recorded by Hastings and Job (7) in patients treated with "transfer factor."
Granuloma clearance is an interesting histological change seen to occur more frequently and more rapidly in patients receiving immunotherapy along with MDT. This feature was seen particularly in patients in the borderline groups, such as BL, BB and BT. Granuloma clearance is a normal process seen during remission of the disease under therapy. It is, however, pertinent to note that immunotherapy appears to be able to hasten the process of granuloma clearance even in those cases who do not show immunological upgrading. Since the presence of bacterial antigens is the major factor for persistence and growth of the dermal granuloma, quicker granuloma clearance indicates a faster clearance of M. leprae antigens from the lesional areas, even in those patients in the vaccine group who do not show upgrading.
Any attempt at immunotherapy in a leprosy patient must take into consideration the possibility of evoking nerve damage during the process of immunological upgrading. In this respect, the absence of any histological evidence of increases in dermal nerve twig inflammation is certainly a welcome feature of using Mycobacterium w. This correlates well with the clinical observation that no increase in the incidence of neuritis involving the larger nerve trunks was seen among the vaccinated cases. It is possible that Mycobacterium w presents the "right mix" of antigenic determinants which are able to stimulate cell-mediated mechanisms responsible for lepromin conversion, histological upgrading, and granuloma clearance without stimulating hypersensitivity reactions which would lead to nerve damage.
The results of this study thus provide histopathological confirmation of the positive immunotherapcutic effect of Mycobacterium w when used in conjunction with MDT over the use of chemotherapy alone. The trial is still in progress, and the final results from the 300 patients under observation will be available soon.
REFERENCES
1. BHATKI, W. S., CHULAWALA, R. G., BAPAT, C. V. and DEO, M. G. Reversal reaction in lepromatous patients induced by vaccine containing killed ICRC bacilli -a report of five cases. Int. J. Lepr.51(1983)466-472.
2. CHAUDHURI, S., FOTEDAR, A. and TALWAR, G. P. Lepromin conversion in repeatedly lepromin negative BL/LL patients after immunization with autoclaved Mycobacterium w. Int. J. Lepr. 51(1983)159-168.
3. DEO, M. G., BAPAT, C. V., CHULAWALA, R. G. and BHATKI, W. S. Potential anti-leprosy vaccine from killed ICRC bacilli -a clinicopathological study. Indian J. Med. Res. 74(1981)164-177.
4. FOTEDAR, A., MEHRA, N. K., MUSTAFA, A. S. and TALWAR, G. P. Local reaction to intradermal instillation of Mycobacterium w and ICRC bacilli in mice. Lepr. India 50(1978)520-533.
5. GIRDHAR, B. K. and DESIKAN, K. V. Results of skin tests with five different mycobacteria. Lepr. India 50(1978)555-559.
6. GOVIL, D. C. and BHUTANI, L. K. Delayed hypersensitivity skin reaction to lepromin and antigens prepared from four other mycobacteria. Lepr. India 50(1978)550-554.
7. HASTINGS, R. H. and JOB, C. K. Reversal reactions in lepromatous leprosy following transfer factor. Am. J. Trop. Med. Hyg. 27(1978)977-1004.
8. HOGERZEIL, L. M. and PRABHUDAAS, N. Delayed hypersensitivity skin reactions to lepromins prepared from M. leprae and selected cultivable mycobacteria. Lepr. India 50(1978)560-565.
9. MEYERS. W. M., MCDOUGALL, A. C, FLEURY, R. N., NEVES, R., REYES, O. and BINFORD, C. H. Histologic responses in sixty multibacillary leprosy patients inoculated with autoclavcd Mycobacterium leprae and live BCG. Int. J. Lepr. 56(1988)302-309.
10. MUSTAFA, A. S. and TALWAR, G. P. Five cultivable mycobacterial strains giving blast transformation and leukocyte migration inhibition of leukocytes analogous to Mycobacterium leprae. Lepr. India 50(1978)498-508.
11. MUSTAFA, A. S. and TALWAR, G. P. Delayed hypersensitivity skin reaction to homologous and heterologous antigens in guinea pigs immunized with M. leprae and four selected cultivable mycobacterial strains. Lepr. India 50(1978)509-519.
12. MUSTAFA, A. S. and TALWAR. G. P. Enlargement of draining lymph nodes in mice by four selected cultivable strains of mycobacteria. Lepr. India 50(1978)534-538.
13. SAHIB, H. S. M. and VELLUT, C. Some observations on skin reactions induced by lepromin and four other mycobacterial antigens. Lepr. India 50(1978)579-587.
14. SHARMA, R. C. and SINGH, R. Comparative study of skin reactions in leprosy patients to M. leprae lepromin and to antigens from cultivable saprophytic mycobacteria. Lepr. India 50(1978)572-578.
15. SREEVATSA and DESIKAN, K. V. Evaluation of the efficacy of candidate vaccines against M. leprae infection in mice. Indian J. Lepr. 60(1988)252-259.
16. TALWAR, G. P., MUKHERJEE, R., ZAHEER, S. A., SHARMA, A. K., KAR, H. K., MISRA, R. S . and MUKHERJEE, A. Present approaches to immunotherapy and immunoprophylaxis for leprosy. In: Progress in Vaccinology. Vol. 2. G. P. Talwar, ed. New York: Springer-Verlag, 1989, pp.303-313.
17. TALWAR, G. P.. ZAHEER, S. A., MUKHERJEE, R.,WALIA, R., MISRA, R. S., SHARMA, A. K., KAR, H. K., MUKHERJEE, A., PARIDA, S. K., SURESH, N. R., NAIR, S. K. and PANDEY, R. M. Immunothcrapeutic effects of an anti-leprosy vaccine based on a saprophytic cultivable mycobacterium, Mycobacterium w , in multibacillary leprosy patients. Vaccine 8(1990)121-129.
18. TALWAR, G. P., ZAHEER, S. A., SURESH, N. R.,PARIDA, S. K., MUKHERJEE, R., SINGH, I. G., SHARMA, A. K., KAR, H. K., MISRA, R. S . and MUKHERJEE. A. Immunotherapeutic trials with a candidate anti-leprosy vaccine based on Mycobacterium w. Trop. Med. Parasitol. 41(1990)369-370.
1. M.D., Assistant Director, Institute of Pathology-ICMR, Safdarjang Hospital;
2. M.B.B.S., D.V.D., Senior Medical Officer, National Institute of Immunology, Shahid Jeet Singh Marg.
3. D.V., M.D., Senior Specialist and Head;
4. M.D., D.D., Dermatologist and Head. Department of Dermatology and Leprology, Safdarjang Hospital.
5. M.D., Specialist, Department of Dermatology, Venereology and Leprology, Dr. Ram Manohar Lohia Hospital, New Delhi, India.
6. Ph.D.. Staff Scientist V; National Institute of Immunology, Shahid Jeet Singh Marg.
7. D.Sc, F.I.CA.. F.A.Sc, F.N.A., Director, National Institute of Immunology, Shahid Jeet Singh Marg.
Reprint requests to Dr. Ashok Mukherjee, Assistant Director, Institute of Pathology, P.O. Box 4909, New Delhi 110029, India.
Received for publication on 25 June 1991.
Accepted for publication in revised form on 16 October 1991.