- Volume 60 , Number 1
- Page: 36–43
Cellular immune responses to mycobacterial heat shock proteins in nepali leprosy patients and controls
ABSTRACT
Sixty-three leprosy patients, representing the entire leprosy spectrum f rom tuberculoid to lepromatous disease, and 17 healthy Nepali subjects were tested for their T-cell responses to the purified p65 and p70 protein antigens of Mycobacterium bovis BCG using a lymphocyte proliferative assay. There was strong correlation between the responses to BCG and M. leprae and the responses to the two antigens. Patients and controls lacking a response to either of the whole cell preparations failed to respond to the purified antigens, while BCG responders at the lepromatous pole of the disease did mount a response to both antigens. Significant differences in the magnitude of the responses to these antigens were obtained between controls and the disease groups. In individual subjects, responses to the two antigens were significantly correlated to each other.RÉSUMÉ
Soixante-trois patients lépreux, représentant le spectre entier de la lèpre, de la maladie tuberculo´de à la maladie lépromateuse, et 17 sujets népalais en bonne santé ont été testés quant à la réponse de leurs cellules-T vis-à-vis des antigènes protéiques purifiés P65 et P70 du BCG de Mycobacterium bovis utilisant un test de prolifération lymphocitaire. II y avait une forte corrélation entre les réponses au BCG et M. leprae et les réponses aux deux antigènes. Les patients et les contrôles qui présentaient une réponse déficiente à l'une des deux préparations cellulaires ne répondaient pas non plus aux antigènes purifiés, tandis que ceux qui répondaient au BCG alors qu'ils présentaient une maladie lépromateuse développaient une réponse aux deux antigènes. Des différences significatives dans l'ampleur des réponses à ces antigènes ont été observées entre les groupes de témoins et des groupes de personnes malades. Chez les sujets individuels, les réponses aux deux antigènes étaient corrélées l'une à l'autre de manière significative.RESUMEN
Usando un ensayo de linfoproliferación, se probó la reactividad de las células T de 63 pacientes con lepra y de 17 individuos sanos nepaleses, hacia los antigenos p65 y p70 purificados del Mycobacterium bovis, BCG. El grupo de pacientes con lepra incluyó todas las variedades de la enfermedad. Hubo una fuerte correlación entre las respuestas al BCG y al M. leprae y las respuestas hacia los dos antígenos purificados. Los pacientes y controles que no respondieron a ninguno de los microorganismos Íntegros, tampoco respondieron a los antígenos purificados; aquellos pacientes respondedores del extremo lepromatoso si respondieron a los dos antígenos. Se encontraron diferencias muy significativas en la magnitud de respuesta hacia estos antígenos, entre el grupo control y el grupo de enfermos. En los sujetos individuales, las respuestas a los dos antígenos también estuvieron significativamente correlacionadas entre si.The clinical manifestations of leprosy are determined by the host's immune response to Mycobacterium leprae ranging from tuberculoid leprosy, with strong T-lymphocyte-mediated responses and few demonstrable bacilli, to lepromatous leprosy, with no apparent T-lymphocyte response and uncontrolled proliferation of M. leprae (11).The majority of individuals infected with M. leprae develop strong cellular immunity to the bacillus and no clinical disease (6). However, the nature of the mycobacterial antigens stimulating these cellular responses is poorly understood.
Recently, a number of protein antigens of M. leprae have been identified including antigens with apparent molecular weight (Mr) 70, 65, 36, 28, and 18-kDa, whose encoding genes have been isolated (10,22). The 70-kDa protein is common to M. leprae, M. bovis bacillus Calmette-Guérin (BCG) and M. tuberculosis (2,3), and is the mycobacterial homolog of the 70-kDa heat-shock protein of eukaryotcs and prokaryotes (10). The purified antigen elicits a proliferative response and delayed-type hypersensitivity in BCG vaccinees (2,14) and a serological response in tuberculosis and leprosy patients (2). Similar homologs of the 70-kDa heat-shock protein from a range of intracellular parasites stimulate significant host immune responses (21). The genes encoding the 65-kDa protein in M. leprae, M. tuberculosis and M. bovis are almost identical (18) and display a moderate degree of homology with the groEL gene of Escherichia coli (19). This protein is immunodominant in the murine B and T lymphocyte response to mycobacterial preparations (5,12), and BCG vaccinées respond to the antigen (4,14) . T-cell clones reactive with the 65-kDa protein have been isolated from individual leprosy patients (7), but the relative role of the 65-kDa and 70kDa proteins as antigens across the leprosy spectrum is undetermined. We have therefore examined the cellular immune responses of leprosy patients with active disease and control subjects to the 65-kDa and 70-kDa mycobacterial proteins, with a view to examining the following questions: a) Do healthy subjects from endemic areas respond to the proteins and, in particular, are individuals unresponsive to M. leprae or BCG primed to respond to the mycobacterial 65-kDa or 70-kDa proteins by exposure to other parasitic or bacterial infections? b) What is the pattern of reactivity in patients across the leprosy spectrum? and c) Are there differences in responsiveness to the two antigens?
MATERIALS AND METHODS
Antigens. Whole M. leprae bacilli (WML) and M. leprae sonicate (MLS) were kindly provided by Dr. R. J. W. Rees through the IMMLEP Program of the World Health Organization. BCG sonicate was prepared from freeze-dried M. bovis BCG (CSL, Melbourne) as described (3), sterile filtered, and stored at 1 mg/ml in phosphate buffered saline (PBS), pH 7.2, at -20ºC. The mycobacterial proteins were purified from BCG sonicate by monoclonal antibody (MAb) affinity chromatography using MAb L22 for p65 (4) and MAb L7 for p70 (2). The purity of the preparations was confirmed by SDS-PAGE electrophoresis and immunoblotting. The mycobacterial p70 preparation contained a major band of 70 kDa with a minor band of 32 kDa, both of which reacted with L7 but not L22 on immunoblots. The p65 preparation contained a major band of 65 kDa and smaller bands of 50-65 kDa, all of which reacted with L22 but not L7 (2,4). The proteins were stored freeze-dried at - 20ºC and reconstituted in PBS prior to use. Purified protein derivative (PPD) of M. tuberculosis was supplied by the Serum Statens Institute, Kamstrup, Denmark, and concanavalin A by Sigma (Poole, U.K.).
Subjects. The 63 leprosy patients were diagnosed at Anandaban Leprosy Hospital, Kathmandu, Nepal, on the basis of clinical findings and bacteriological skin smears. There were 12 female and 51 male subjects with an age range of 12-67 years. Two patients had tuberculoid disease (TT), 31 borderline tuberculoid (BT), 4 borderline (BB), 10 borderline lepromatous (BL), and 16 lepromatous (LL) as defined by the Ridley and Jopling classification (17).
At the time of testing, 13 patients were newly diagnosed and untreated, 7 had completed therapy 1-6 years previously, and 43 were under treatment. Fifteen of the current treatment group were receiving dapsone monotherapy and the remainder multidrug therapy. Six of the patients were tested at the onset of reaction (4 reversal reaction; 2 erythema nodosum leprosum). Of the 37 patients skin tested with lepromin, 22 were positive.
Control subjects constituted 17 male health workers who had worked at Anandaban Leprosy Hospital for 1 -19 years. The age range of this group was 20-37 years. All 13 tested with lepromin had a positive skin reaction.
Lymphocyte proliferative assays. Venous blood from the subjects was collected into preservative-free heparin 10 U/ml (Sigma), and peripheral blood mononuclear cells (PBMC) were separated by centrifugation over a Ficoll-Hypaque gradient (specific gravity 1.078). The cells were washed twice in RPMI 1640 (Sigma) containing 0.01 M sodium bicarbonate and 25 raM Hepes buffer and 2 raM L-glutamine plus 50 mg/1 penicillin and 100 mg/1 streptomycin. Cell suspensions were adjusted to 106 cells per ml in RPMI containing 10% heat-inactivated human A serum or autologous heatinactivated serum. Two-hundred-microliter aliquots were dispensed into the wells of a flat-bottom, 96-well microtiter plate (Flow Laboratories, Irvine, Scotland), and antigens diluted in RPMI were added in triplicate. BCG sonicate, M. leprae sonicate (MLS), p65, and p70 were used at a final concentration of 10, 3, 1, 0.3, and 0.1 µ g/ml; PPD at 10 and 1 µ g/ml; whole M. leprae (WML) at 108, 107 and 106 bacilli/ml; and concanavalin A at 10 µ g/ml.
Control wells received media alone. The cultures were incubated for 5 days at 37ºC in 5% CO2 in humidified air, and were pulsed with 3H-thymidine, 0.5 µ Ci/well (BARC, Bombay, India) for a further 16 hr. Incorporation of'H-thymidine into proliferating cells was measured in disintegrations per minute (dpm) by liquid scintillation spectroscopy with a Tricarb 1500 counter (Packard Instrument Co., Downers Grove, Illinois, U.S.A.). The specific dpm value was obtained by subtracting the mean dpm in control triplicate wells from the mean of test wells. The stimulation index (SI) was the ratio of the mean 3H-thymidine incorporation in test wells to that in wells without antigen. A response was considered positive if the SI was  4.
4.
The differences in means between groups were tested by Student's t test, and the differences in the proportions of positives in individual groups were compared by the chisquared test with Yates' correction. The correlations between responses to any two antigens in individual patients were tested with Spearman Rank correlation coefficient.
RESULTS
Each of the mycobacterial antigens tested induced a graded proliferative response in PBMC from leprosy patients and control subjects (Figs. 1, 2). The optimal concentration for comparing responses between individual subjects was 1 µ g/ml for BCG sonicate, p65 and p70, and 107 bacilli/ml for WML. Subjects were grouped according to their responses to the crude mycobacterial antigens (BCG sonicate and WML) for comparison with the responses of the two purified proteins (Table 1). The majority (63 of 80) of the whole group, including both leprosy patients and control subjects, responded to one or more of the crude antigen preparations; 28 responded to both BCG sonicate and WML, 27 to BCG sonicate alone, and 8 to WML alone. Of these responders to crude antigens, 60 of 63 (95%) responded to the p65 antigen and 55 of 60 (92%) responded to p70. By contrast, only one (6%) of the 17 nonresponders to either BCG sonicate or WML responded to p65 and p70.
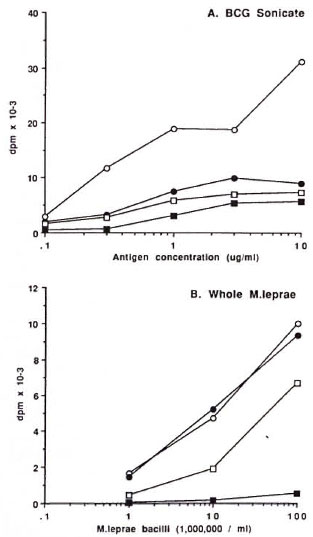
Fig. 1. Mean proliferative responses of groups ofleprosy patients and healthy controls to differing concentrations of BCG sonicate (A) and whole M. leprae (B). ○ = controls; ● = TT/BT patients; □ =BB/NL patients; ■ = LL patients.
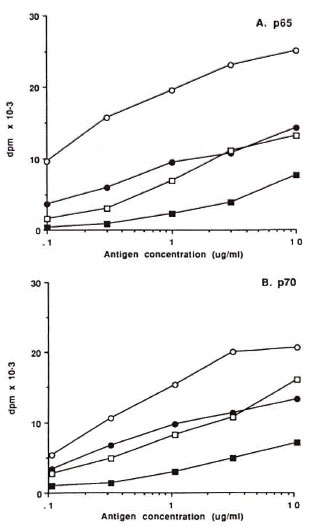
Fig. 2. Mean proliferative responses of groups ofleprosy patients and healthy controls to differing concentrations of p65 (A) and p70 (B). ○ = controls; ● =TT/BT patients; □ = BB/BL patients; ■ = LL patients.
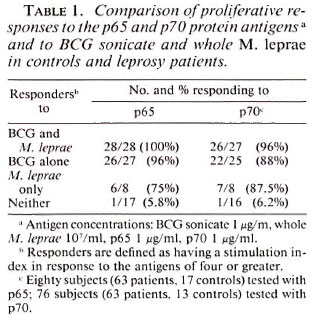
The proportion of responders to each antigen in controls and the three categories of leprosy patients were compared at the optimal antigen concentrations (Table 2). All LL patients and the majority of BB/BL patients failed to respond to WML (Table 2) or MLS (data not shown); whereas 39% of LL and 79% of BB/BL patients responded to BCG sonicate, p65, and p70. The magnitude of the proliferative responses to crude antigens (BCG sonicate and WML) and purified antigens (p65 and p70) was significantly diminished in the patients compared to controls (Table 2). This effect was also apparent for PPD and MLS (data not shown). When compared to TT/BT patients, the mean response of LL patients was significantly reduced for WML (p < 0.001), p65 (p < 0.05), and p70 (p < 0.01). Comparison of the mean dose response curves of patients and controls for the four antigens showed a similar trend. Lepromatous leprosy patients had significantly reduced but demonstrable responses to p65 and p70 (Fig. 2) as well as to crude BCG sonicate, while being anergic to WML (Fig. 1) and MLS. There was a slight reduction in the responses to concanavalin A in patients compared with controls. The control subjects had a mean 3H-thymidine incorporation of 45,807 dpm (S.E.M. ± 8019) compared with TT/BT mean 28,602 (± 3618)dpm; BB/BL mean 18,084 (± 2821) dpm; and LL mean 26,742 (± 3172) dpm.
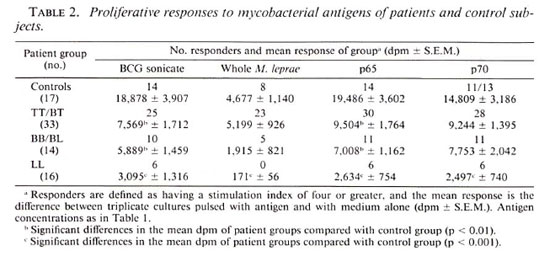
The effect of other factors on responsiveness to p65 and p70 was examined. There was no difference in the magnitude of responses between males and females in the same disease category. Although significantly more of the control subjects were younger than the patients, there were no differences in the p65 and p70 responses between younger (< 40 years) and older patients of the same disease category. More of the control subjects (82%) had evidence of a BCG vaccination scar than patients (23%, p < 0.05) but, again, there was no difference in the p65 and p70 responses between those patients of the same group with and without apparent vaccination scars. Treated and untreated leprosy patients in the same category had similar levels of responses to each antigen. The six patients tested at the onset of leprosy reaction displayed no significant differences in antigen responses when compared with patients in the same category who were not in reaction. In the subgroup of 37 patients lepromin tested, 22 were lepromin positive. The proportion of p65 and p70 responders was 95% and 86%, respectively, in lepromin-positive patients and 53% for both antigens in lepromin-negative patients. There was no significant difference in the mean proliferative response to p65 and p70 in lepromin-positive and -negative subjects.
The responses of individual subjects to crude mycobacterial antigens and p65 and p70 were compared. There was strong correlation between the responses to BCG sonicate and p65 (r = 0.816, p < 0.001) and to BCG sonicate and p70 (r = 0.744, p < 0.001) both for controls and leprosy patients (Fig. 3). A similar correlation was observed between responses to WML and p65 (r = 0.661, p < 0.001) and p70 (r = 0.625, p < 0.001) (Fig. 3). The same pattern was observed for the correlation between PPD and p65 (r = 0.504, p < 0.001) and p70 (r = 0.463, p < 0.001) and between MLS and p65 (r = 0.696, p < 0.001) and p70 (r = 0.627, p < 0.001). The proliferative responses to p65 and p70 were also strongly correlated in the whole group (r = 0.918, p < 0.001), and there were no significant differences in this correlation in patients in the different leprosy categories.
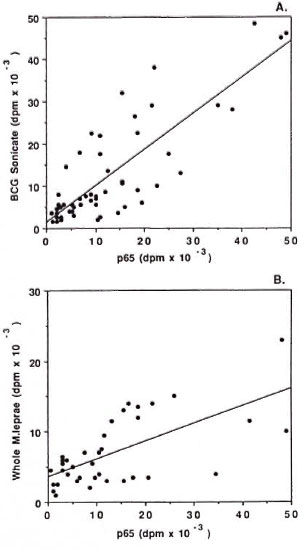
Fig. 3. Correlation between proliferative responsesof individual patients and controls to p65 (1 µ g/ml)and BCG sonicate (1 µ g/ml) (A: r 0.816) and p65 (1 µ g/ml) and whole M. leprae (107 (B: r = 0.661).
DISCUSSION
This study demonstrates that T-cell responsiveness to the mycobacterial stress proteins p65 and p70 is related to responsiveness to BCG or to M. leprae in both leprosy patients and healthy control subjects from a leprosy-endemic area. We have previously demonstrated that control subjects who were neither exposed to tuberculosis or leprosy nor BCG vaccinated do not respond to purified p65 or p70 (2,4). It is now apparent that endemic-control subjects and patients who are unresponsive to crude mycobacterial antigens have not been primed to respond to mycobacterial antigens per se or to the purified heat-shock proteins by exposure cither to other parasitic or bacterial infections common in an endemic country (Table l).This is a significant finding since the "common bacterial antigen" is a homolog of the p65 antigen (19), while variants of heat-shock protein p70 (hsp70) occur in both bacteria, such as E. coli, and parasites including malaria, filaria and leishmania (20,21). The results complement a study of Melanesian family contacts of leprosy patients in which a similar correlation was observed between responsiveness to p65 and p70 and BCG and M. leprae sonicates (1). Limiting dilution analysis in those contacts revealed that the precursor cell frequency for lymphocytes responding to either p65 or p70 mirrored the bulk proliferative response of PBMC to the same antigen.
The immunological hallmark of polar lepromatous leprosy is specific anergy to M. leprae (11). This anergy does not extend completely to the shared mycobacterial antigens p65 and p70 of M. bovis BCG since both stimulated a T-cell response, albeit reduced, in the BCG-responsive LL patients (Fig. 2). Both of these antigens are present in M. leprae sonicate (3,4), and both are capable of priming for anti-p65 and anti-p70 responses since subjects reactive to M. leprae alone, but not to BCG or PPD, can recognize BCG-derived p65 and p70 (Table 1). There are a number of possible explanations for these observations.
First, LL patients may be responding to regions of the M. bovis p65 and p70 not present in the M. leprae homologs. This may be the case for p70 since there is 30% divergence in the deduced amino acid sequences for M. leprae and M. tuberculosis p70 in the C-terminal half of the molecule (McKenzie, et al., 1991, in press). This is less likely for the p65 antigen since there is 95% identity between M. bovis (BCG) and M. leprae p65 across the whole molecule(19). The second possibility is that unresponsiveness to heat-shock proteins is not included in the M.leprae -specific ancrgy characteristic of lepromatous leprosy. Thirdly, the responses to p65 and p70 present in the complex M. leprae preparations could be inhibited by antigen-specific suppressor cells, which have been demonstrated in one study for p65 (16), or by nonspecific suppressor effects such as those induced by mycobacterial glycolipid antigens present in the intact bacilli (19). Interestingly, when lymphocytes from contacts of Melanesian leprosy patients who were low responders to p65 and p70 were examined by limiting dilution analysis, no evidence for suppression, either specific or nonspecific, was obtained (1). Thus, the precise explanation for the responses of lepromatous patients to BCG p65 and p70 remains uncertain, and will require comparative studies in leprosy patients with M. leprae and BCG heat-shock proteins and defined T-cell epitopes of both proteins.
There was a moderately strong correlation between responses to the crude mycobacterial antigens and p65 and p70. This was also observed in a larger group of family contacts (1), and confirms that both proteins are dominant T-cell immunogens. In addition, a strong correlation was demonstrated between responses to the p65 and p70 in individual subjects. Activation of cellular responses to the two proteins may be due to their increased expression in M. leprae secondary to metabolic or oxidative stress within the host macrophage and subsequent presentation to host T cells. Since both p65 and p70 have partial identity with human heat-shock proteins p60 and p70 (10,20) activation of strong cellular-immune responses may be accompanied by the emergence of autoreactive lymphocytes in genetically susceptible individuals (13,21).
Acknowledgment. The Mycobacterial Research Laboratory is fully supported by The Leprosy Mission International. We thank Dr. R. J. W. Rees for the provision of M. leprae through the IMMLEP component of the WHO/UNDP/World Hank Tropical Diseases Research Programme. This study was only possible through the excellent cooperation of the doctors and stall'at Ananbadan Leprosy Hospital. We would also like to thank Dr. R. B. Adiga, Chief of the Public Health Division, Ministry of Health. His Majesty's Government of Nepal, for this support of this research. We thank Dr. L. Hellqvist for purifying the p65 and p70 antigens and Lynne White for assistance in preparing the manuscript.
REFERENCES
1. ADAMS, E., GARSIA, R, J., HELLQVIST, L., HOLT, P. and BASTEN, A. T cell reactivity to the purified mycobacterial antigens p65 and p70 in leprosy patients and their household contacts. Clin. Exp. Immunol. 80(1990)206-212.
2. BRITTON, W. J., HELLQVIST, L., BASTEN, A. and INGLIS, S. Immunoreactivity of a 70kD protein purified from Mycobacterium bovis (BCG) by monoclonal antibody affinity chromatography. J. Exp. Med. 164(1986)695-708.
3. BRITTON, W. J., HELLQVIST, L., BASTEN, A. and RAISON, R. L. Mycobacterium leprae antigens involved in human immune responses. I. Identification of four antigens by monoclonal antibodies. J. Immunol. 135(1985)4171-4177.
4. BRITTON, W. J., HELLQVIST, L., GARSIA, R. J. and BASTEN, A. Dominant cell wall proteins of Mycobacterium leprae recognized by monoclonal antibodies. Clin. Exp. Immunol. 67(1987)31-42.
5. BUCHANAN, T. M., NOMAGUCHI, H., ANDERSON, D. C, YOUNG, R. A., GILLIS, T. P., BRITTON, W. J., IVANYI, J., KOLK, A. H., CLOSS, O., BLOOM, B. R. and MEHRA, V. Characterization of antibodyreactive epitopes on the 65-kilodalton protein of Mycobacterium leprae. Infect. Immun. 55(1987)1000-1003.
6. CLOSS, O., REITAN, L. J., NEGASSI, K., HARBOE, M. and BELEHU, A. In vitro stimulation of lymphocytes of leprosy patients, healthy contacts of leprosy patients and subjects not exposed to leprosy. Comparison of an antigen fraction prepared from Mycobacterium leprae and tuberculin purified protein. Scand. J. Immunol. 16(1982)103-115.
7. EMMRICH, R., THOLE, J. E. R., VAN EMBDEN, J. D. A. and KAUFMANN, S. H. E. A recombinant 64 kDa protein of Mycobacterium bovis BCG specifically stimulates human T cell clones reactive to mycobacterial antigens. J. Exp. Med. 163(1985)1024-1026.
8. ENGERS, H. D., ABE, M., BLOOM, B. R., MEHRA,V., BRITTON, W., BUCHANAN, T. M., KHANOLKAR, S. K., YOUNG, D. B., CLOSS, O., GILLIS, T., HARBOE, M., IVANYI, J., KOLK, A. H. J. and SHEPARD, C. C. Results of a World Health Organization (WHO) sponsored workshop on monoclonal antibodies to Mycobacterium leprae (Letter). Infect. Immun. 48(1985)603-605.
9. FOURNIE, J. J., ADAMS, E., MULLINS, R. J. and BASTEN, A. Inhibition of human lymphoproliferative responses by mycobacterial phenolic glycolipids. Infect. Immun. 57(1989)3653-3659.
10. GARSIA, R. J., HELLQVIST, L., BOOTH, R. J., RADFORD, A., BRITTON, W. J., ASTBURY, L.,TRENT, R. J. and BASTEN. A. Homology of the 70-kilodalton antigens from Mycobacterium leprae, M. bovis, and M. tuberculosis with the conserved heat shock protein 70 of eucaryotes. Infect. Immun. 57(1989)204-212.
11. HARBOE, M. The immunology of leprosy. In: Leprosy. Hastings, R. C, ed. Edinburgh: Churchill Livingstone, 1985, p. 53.
12. KAUFMANN, S. H. E., VATH, U., THOLE, J. E. R., VAN EMBDEN, J. D. A. and EMMRICH, F. Enumeration of T cells reactive with Mycobacterium tuberculosis organism and specific for the recombinant 64 kDa protein. Eur. J. Immunol. 17(1987)351-357.
13. LAMB, J., BAL, V., MENDEZ SAUPERINO, P., MEHLERT, A., SO, A., ROTHBARD, J., FIRDAL, S., YOUNG, R. A. and YOUNG, D. B. Stress proteins may provide a link between the immune response to infection and autoimmunity. Int. Immunol. 1(1989)191-195.
14. MUNK, M. E., SHOEL, B. and KAUFMANN, S. H. E. T cell responses of normal individuals tward recombinant protein antigens of Mycobacterium tuberculosis. Eur. J. Immunol. 18(1988)1835-1838.
15. MYRVANG, B., GODAL, T., RIDLEY, D. S., FROLAND, S. S. and SONG, Y. K. Immune responsiveness to Mycobacterium leprae and other mycobacterial antigens throughout the clinical and histopathological spectrum of leprosy. Clin. Exp. Immunol. 14(1973)541-553.
16. OTTENHOFF, T. H. M., ELFERINK, D. G.,KLATSER, P. R. and DE VRIES, R. R. P. Cloned suppressor T cells from a lepromatous leprosy patient suppress Mycobacterium leprae reactive helper T cells. Nature 332(1986)462-464.
17. RIDLEY, D. S. and JOPLING, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
18. SHINNICK, T. M., SWEETSER, D., THOLE, J., VA N EMBDEN, J. and YOUNG, R. A. The etiologic agents of leprosy and tuberculosis share in immunoreactive protein antigen with the vaccine strain Mycobacterium bovis BCG. Infect. Immun. 55(1987)1932-1935.
19. SHINNICK, T. M., VODKIN, M. H. and WILLIAMS, J. C. The Mycobacterium tuberculosis 65-kilodalton antigen is a heat shock protein which corresponds to common antigen and to E. coli GroEL protein. Infect. Immun. 56(1988)446-449.
20. YOUNG, D. B., LATHIGRA, R., HENDRIX, R.,SWEETSER, D. and YOUNG, R. A. Stress proteins are immune targets in leprosy and tuberculosis.Proc. Natl. Acad. Sci. U.S.A. 85(1988)4267-4270.
21.YOUNG, D. B., LATHIGRA, R. and MEHLERT, A.Stress induced proteins as antigens in infectious diseases. In: Stress induced Proteins. Pardue, M. L., Feramisco, J. R. and Lindguist, S., eds. NewYork: Alan R. Liss Inc., 1989.
22. YOUNG, R. A., MEHRA, V., SWEETSER, D.,BUCHANAN, T. M., CLARK-CURTISS, J., DAVIS, R.W. and BLOOM, B. R. Genes for the major proteinantigens of the leprosy parasite Mycobacterium leprae. Nature 316(1985)450-452.
1. B.Appl.Sc.; Mycobacterial Research Laboratory, Anandaban Leprosy Hospital, Kathmandu, Nepal.
2. M.D., Mycobacterial Research Laboratory, Anandaban Leprosy Hospital, Kathmandu, Nepal.
3. Ph.D., Centenary Institute of Cancer Medicine and Cell Biology. Sydney, New South Wales 2006. Australia.
Reprint requests to Dr. Britton.
Received for publication on 12 August 1991.
Accepted for publication on 29 October 1991.