- Volume 60 , Number 1
- Page: 44–53
T-cell responses of leprosy patients and healthy contacts toward separated protein antigens of Mycobacterium leprae
ABSTRACT
Sonicated extracts of Mycobacterium leprae were separated by two-dimensional gel electrophoresis and electroeluted into 400 distinct soluble fractions. These fractions were probed with T lymphocytes f rom leprosy patients of different disease types, healthy contacts, and unexposed healthy individuals. Proliferative responses were visualized using three-dimensional stimulation profiles. T cells f rom many patients and contacts responded to a multitude of antigen fractions of different molecular masses and isoelectric points. T cells f rom unexposed individuals gave significant responses to lysates or whole organisms of M. leprae, but no or only marginal responses to separated antigen fractions. T cells of polar tuberculoid (TT) and the majority of polar lepromatous (LL) leprosy patients responded only to separated antigen fractions but not to lysates or whole organisms of M. leprae. The remaining LL patients were totally unresponsive and even failed to respond to separated M. leprae fractions. Thus, in some leprosy patients unresponsiveness to M. leprae seems to be caused by distinct components and can be broken by using separated antigen fractions; whereas in others, anergy remains. T cells of borderline tuberculoid (BT) patients, who were under chemotherapy, responded to separated antigen fractions as well as to lysates of M. leprae organisms. In contrast, BT patients who were untreated failed to react with any of the M. leprae preparations. Similarly, T cells of the majority of LL patients responding to separated fractions were under chemotherapy; whereas T cells f rom untreated LL patients gave no or only marginal responses to any of the M. leprae antigen preparations. These findings suggest some linkage between the degree of T-cell responsiveness and antileprosy drug treatment.RÉSUMÉ
Des extraits soniqués de Mycobacterium leprae ont été séparés par électrophorèse sur gel en deux dimensions et électrodilués dans 400 fractions solubles distinctes. Ces fractiones ont été testées avec des lymphocites T en provenance de patients lépreux, de dilïërents types de la maladie, des contacts en bonne santé et des individus en bonne santé non exposés à la lèpre. Des réponses prolifératives ont été observées en examinant des profils de stimulation en trois dimensions. Des cellules T de beaucoup de patients et contacts ont répondu à une multitude de fractions antigéniques de différentes masses moléculaires et points isoélectriques. Les cellules T d'individus non exposés ont donné des réponses significatives vis-à-vis des lysats ou d'organismes entiers de M. leprae, mais pas de réponse ou seulement des réponses marginales vis-àvis des fractions antigéniques séparées. Les cellules T de patients tuberculoïdes polaires (TT) et de la majorité des patients lépromateux polaires (LL) répondaient seulement aux fractions antigéniques séparées mais non aux lysats ou aux organismes entiers de M. leprae. Les autres patients LL ne répondaient absolument pas et ont même manqué de répondre aux fractions séparées de M. leprae. Chez certains patients lépreux, l'allergie vis-à-vis de M. leprae semble done être causée par différentes composantes et peut-être contournée en utilisant des fractions antigéniques séparées; tandis que chez d'autres, l'allergie reste stable. Les cellules T des patients borderline tuberculoïdes (BT) qui étaient sous chimiothérapie répondaient aux fractions antigéniques séparées aussi bien qu'au lysats ou aux organismes entiers de M. leprae. Par contraste, les patients BT qui n'étaient pas traités ne réagissaient à aucune des préparations de M. leprae. De même, les cellules T de la majorité des patients LL répondant aux fractions séparées étaient sous chimiothérapie; tandis que les cellules T des patients LL non traités ne donnaient pas de réponse ou seulement une réponse marginale à l'ensemble des préparations antigéniques de M. leprae. Ces observations suggèrent qu'il existe un lien entre le degré de réponse des cellules T et le traitement chimiothérapeutique de la lèpreRESUMEN
Se separaron extractos sonicados de Mycobacterium leprae por electroforésis bidimensional en gcl, y se electroeluyeron en 400 distintas fracciones solubles. Estas fracciones se probaron con linfocitos T de pacientes con diferentes tipos de lepra, de contactos sanos, y de individuos sanos no expuestos a la lepra. Las respuestas proliferativas se visualizaron usando perfiles de estimulación tridimensional. Las células T de muchos pacientes y contactos respondieron a una multitud de fracciones antigénicas de diferentes masas moleculares y puntos isoeléctricos. Las células T de los individuos no expuestos dieron respuestas significativas contra los microorganismos completos o sus lisados pero no respondieron, o lo hicieron marginalmente, en contra de las fracciones antigénicas separadas. Las células T de los pacientes con lepra TT y de la mayoría de los pacientes con lepra LL, respondieron sólo a las fracciones antigénicas separadas pero no a los lisados o a los microorganismos completos. El resto de los pacientes LL fueron totalmente no-respondedores. Así, en algunos pacientes con lepra, la falta de respuesta al M. leprae parece estar causada por distintos componentes y puede revertirse usando fracciones antigénicas separadas; en otros, la anergia permanece. Las células T de los pacientes con lepra tubcrculoide subpolar (BT) en tratamiento, respondieron tanto a las fracciones antigénicas separadas como a los lisados o a los organismos completos. En contraste, los pacientes BT no tratados fallaron a responder con cualquiera de las preparaciones de M. leprae. De manera similar, la mayoría de los pacientes LL, cuyas células T respondieron a las fracciones separadas, estaban en tratamiento, mientras que no respondieron las células de los pacientes no tratados. Estos resultados sugieren cierta relación entre el grado de respuesta de las células T y el tratamiento con drogas anti-leprosas.Leprosy is a spectral disease caused by the acid-fast bacillus Mycobacterium leprae (13,25,26) . Although the disease can be treated chemotherapeutically, effective control will ultimately depend on active vaccination (2). At present, no antileprosy vaccine is available although clinical trials using BCG, BCG plus killed M. leprae, and M. leprae- related organisms are currently under way (14). Current evidence suggests that in leprosy not only protection but also pathogenesis and anergy depend on specific immunity (13, 14, 26). Therefore, a subunit vaccine composed of a few well-defined protective antigens in a potent delivery system would be highly desirable. Protective immunity against leprosy is mediated by specific T lymphocytes which recognize antigenic peptides in the context of gene products of the major histocompatibility complex on appropriate presenter cells (13, 14, 26). Thus, identification of protective antigens with vaccine potential crucially depends on the application of viable T lymphocytes.
Recently, a recombinant expression library of M. leprae has been established and several recombinant proteins (r-proteins) have been identified with monoclonal antibodies (29). Many of these r-proteins have been shown to stimulate T lymphocytes, as well (14). However, in light of the high number of different proteins M. leprae is composed of, the number of r-protein antigens identified thus far appears to be extremely small. Furthermore, it is known that the antigen repertoire for B cells and T cells can differ markedly. Hence, procedures depending on preselection with antibodies may miss important T-cell antigens, and alternative strategies must be considered.
We have developed a method which allows rapid and direct screening with T lymphocytes of hundreds of distinct fractions of bacteria (10). Here we use this method for comparing the repertoire toward M. leprae antigens of T lymphocytes from the peripheral blood of tuberculoid (TT), borderline tuberculoid (BT), and lepromatous (LL) leprosy patients with those from healthy household contacts and normal donors from nonendemic countries without known exposure to M. leprae. It is hoped that such studies will ultimately facilitate the identification of antigens of protective and prognostic value.
MATERIALS AND METHODS
Bacterial antigens. Killed M. leprae organisms were kindly provided by Dr. P. Brennan (Colorado State University, Fort Collins, Colorado, U.S.A.). Bacteria were disrupted with a sonicator (Branson Instruments Co., Stamford, Connecticut, U.S.A.) and treated with 2 µ g/ml Dnase I and 2 ml Rnase (Sigma Chemical Co., St. Louis, Missouri, U.S.A.). Afterward proteases were inhibited with PMSF (1 mM; Serva Biochemicals, Montvale, New Jersey, U.S.A.) and EDTA (5 mM; Sigma). Insoluble membrane components were removed by centrifugation (15,000 x g x 1 hr), and the supernatants were stored at - 70ºC.
Two-dimensional polyacrylamide gel electrophoresis (PAGE) and protein transfer. Proteins were separated and isolated from two-dimensional gels as described earlier (10). In short, 150 µ g/gel M. leprae antigens were separated according to their isoelectric point (first dimension) and according to their size (second dimension) on native Polyacrylamide gradient gels (10%-12.5%). Proteins were transferred into 400 holes each containing 100 µ l of 5 mM Tris buffer by electroelution. Duplicates of each fraction (ca. 7 µ l) were transferred to 96-well microliter plates, sterilized with ultraviolet light, and directly tested with T cells. These antigen fractions as well as the control antigens are not toxic as assessed by pilot experiments using peripheral blood leukocytes from healthy individuals.
T-cell proliferation assay. Peripheral mononuclear blood cells were isolated by centrifugation (Ficoll-Hypaque, 1.077 kg/1) approximately 12 hr after blood donation. T cells were enriched over nylon wool columns (9). Although these preparations still contain a significant number of other leukocytes (primarily B cells), proliferative responses are almost exclusively caused by T cells. B cells and macrophages will not proliferate in such cultures. We, therefore, operationally define the nylon-wool-enriched cell population as T lymphocytes. Autologous peripheral blood mononuclear cells were irradiated with 3000 rad and used as accessory cells. T cells and accessory cells were added to the antigen fractions and cultured in RPMI 1640 (200 Ml/well; Gibco Laboratories, Grand Island, New York, U.S.A.), supplemented with 10% heat-inactivated AB+ human serum, 2 mM glutamine, and antibiotics at 37ºC, 5% CO2 . For controls, whole M. leprae organisms and M. leprae lysates were used (2.5 µ g/ml, unless otherwise stated). The cells were pulsed with 2.5 U/well recombinant human interleukin 2 (IL-2) (a kind gift of Hoffmann- LaRoche) on day 6. On day 8, 0.5 /uCi/well 3H-thymidine (Amersham) was added, and the incorporated radioactivity was measured after 18 hr by liquid scintillation counting. Stimulation indices (SI) were calculated from the mean value of duplicate stimulations divided by the background value. SI  3 were considered positive proliferative responses. Since the supply of blood cells from patients was limited, final numbers of T cells and accessory cells had to be adjusted appropriately. Usually, they ranged in the order of 3 x 104 T cells and accessory cells, respectively (for further details see tables). In pilot experiments it was found that positive responses could be obtained under our assay conditions with as few as 3 x 103 T cells and accessory cells each. Such low cell numbers could be used because antigen-induced T-cell proliferation was further amplified by the addition of exogenous IL-2. At these low cell numbers, we consider it unlikely that the uptake 3H-thymidine is significantly affected by release of "cold" thymidine from accessory cells.
3 were considered positive proliferative responses. Since the supply of blood cells from patients was limited, final numbers of T cells and accessory cells had to be adjusted appropriately. Usually, they ranged in the order of 3 x 104 T cells and accessory cells, respectively (for further details see tables). In pilot experiments it was found that positive responses could be obtained under our assay conditions with as few as 3 x 103 T cells and accessory cells each. Such low cell numbers could be used because antigen-induced T-cell proliferation was further amplified by the addition of exogenous IL-2. At these low cell numbers, we consider it unlikely that the uptake 3H-thymidine is significantly affected by release of "cold" thymidine from accessory cells.
Cell donors. Studies with T cells from leprosy patients and healthy contacts were performed at the Cancer Research Institute, Tata Memorial Hospital, Bombay, India. This study comprised 2 TT, 9 BT, and 10 LL patients as well as 10 healthy contacts (family members and hospital employees). In addition, six individuals who never had been in a leprosy-endemic country were included in this study. Classification of leprosy patients was done according to Ridley and Jopling (25).
RESULTS
T-cell responses of TT and BT patients toward separated antigens of M. leprae. T cells of the two TT patients tested responded to a restricted number of antigens with a focus on fractions > 50 kDa (Fig. 1 corresponding to Donor A from Table 1). In contrast, T cells of these patients failed to respond to killed M. leprae organisms or to bacterial lysates (Table 1). T cells of 2 of 9 BT patients reacted strongly toward whole M. leprae and bacterial lysates as well as toward separated antigens (Table 1). One of these patients (Donor A, Table 1) responded to numerous protein fractions of > 20 kDa of varying pH (pH 6.6 to 3.8); whereas the other one (Donor B, Table 1) primarily reacted with few fractions > 50 kDa, pH 6.3 to 5.4. Undetectable or only weak responses to any antigen preparation or fraction were observed with T cells from the other seven BT patients. These individuals had not been treated with drugs while the two T-cell respon ders were under chemotherapy at the time of blood sampling.
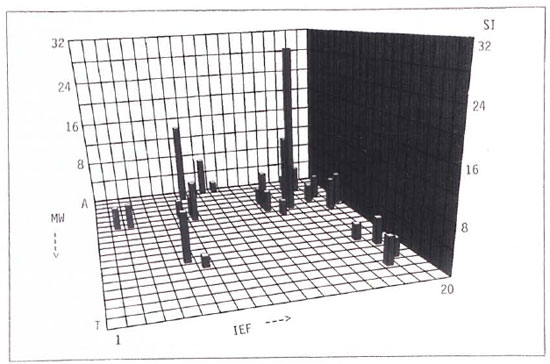
Fig. 1. Lysates of (150 pg/gel) were separated by isoelectic focussing (first dimension) and by polyacrylamide gel electrophoresis (second dimension). First dimension (pI), 1-20; second dimension (size), A-T. Each square represents one of 400 antigen fractions. Separated antigens where clectroeluted and prepared as described (10). Duplicates of each antigen fraction were cultured with 11,500 T cells and 33,000 accessory cells/well. T cells were stimulated with 2.5U rIL-2/well on day 6 and pulsed with 0.5 µ Ci/well 3H-thymidine on day 8. Background value = 122 cpm.
T-cell responses of LL patients toward separated antigens of M. leprae. As shown in Table 2, T cells of 7 of 10 LL patients responded to two-dimensional-separated protein fractions, although none ofthese donors reacted with killed M. leprae organisms and one of them showed minor responses to bacterial lysatcs (Table 2). T cells of the other three LL patients failed to respond to any of the antigen preparations and fractions tested. The majority (6 of 7) of LL patients under chemotherapy but only 1 of 3 untreated patients strongly responded to two-dimensional-separated protein fractions, suggesting a link between T-cell responsiveness and chemotherapy. Examples of two donors showing different response patterns are depicted in Figure 2. T cells of one LL patient (Donor E) primarily responded to a fraction of 90-100 kDa, pH 4.25, and weakly to numerous antigen fractions of < 40 kDa (Fig. 2A). T lymphocytes of another LL patient (Donor F) displayed marked reactivity against separated antigen fractions of > 160 kDa and of < 20 kDa of varying pH values (Fig. 2B). No coherent response pattern of the seven LL patients to separated antigen fractions was found.
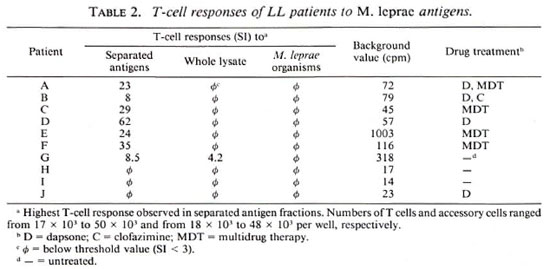
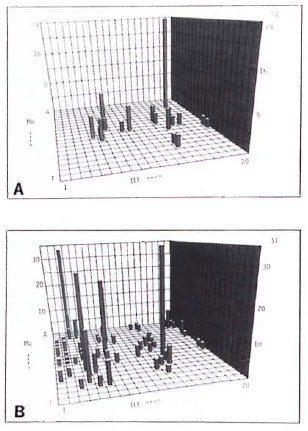
Fig 2. Representative T-cell responses of two LL patients against two-dimensional-separated antigens of M. leprae . M. leprae antigens were separated and tested with T cells as described in Fig. 1. Cell numbers = 35,000 T cells and 50,000 accessory cells/well (A); 27,500 T cells and 20,000 accessory cells/well (B); background values = 1003 cpm (A) and 116 cpm (B).
T-cell responses of healthy leprosy contacts toward separated antigens of M. leprae. T-cell responses of the 10 healthy contacts ranged from strong reactions toward only a few two-dimensional-separated antigens to broadly distributed reactivity patterns against numerous fractions (Table 3; Fig. 3, where Fig. 3A corresponds to Donor A and Fig. 3B to Donor B). T cells of five contacts were stimulated by separated antigen fractions as well as by killed- M. leprae organisms and bacterial lysates (Donors A to E, Table 3); those of one contact were only stimulated by M. leprae organisms and lysates (Donor F). In another donor, T cells were stimulated by killed- M. leprae organisms only (Donor G); in Donor H, weak T-cell responses to separated antigens but no reactivity against killed M. leprae and bacterial lysates were observed. T cells from two contacts failed to respond to any of the preparations tested (Donors I and J). Thus, the T-cell reactivity pattern of leprosy contacts was remarkably heterogeneous, and ranged from nonresponders to individuals displaying reactivity to a wide variety of M. leprae antigens.
Fig 3. Representative T-cell responses of two healthy leprosy contacts against two-dimensional-separated antigens of M. leprae . M. leprae antigens wereseparated and tested with T cells as described in Fig.1. Cell numbers = 25,500 T cells and 23,000 accessory cells/well (A); 21,500 T cells and 12,000 accessory cells/well (B); background values = 1967 cpm (A) and 113cpm (B); control SI to whole M. leprae lysate = 13 (A);4,4 (B); control SI to killed- M. leprae organisms = 7(A); 40 (B).
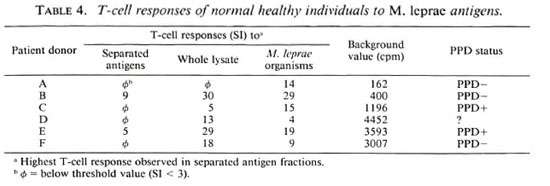
T-cell responses of unexposed donors toward separated antigens of M. leprae. Table 4 summarizes the results of this part of the study which comprises 2 PPD-positive, 3 PPD-negative individuals, and 1 of unknown PPD status. T cells from all donors tested responded to killed- M. leprae organisms and T cells from 5 of 6 donors (Donors B to F) were stimulated by lysates as well. Nevertheless, T cells of 2 of 6 donors responded to two-dimensional-separated antigens only, and even then only weakly (Donors B and E).
DISCUSSION
T lymphocytes are major mediators of both protection against and the pathogenesis of leprosy (13,14,16)-The spectral form of this disease is directly correlated with the quality and quantity of the specific T-cell response. Its more benign pole, TT, is characterized by strong T-cell responses to M. leprae antigens; whereas at its more malign pole, LL, T-cell responses are suppressed or even anergic (25). It has been proposed that distinct antigens of M. leprae are correlated with or even responsible for the different disease stages (3,8). Hence, subunit vaccines composed of protective antigens only would be preferable to vaccines based on whole M. leprae organisms. We therefore attempted to characterize the repertoire toward M. leprae antigens of T lymphocytes from patients of defined disease types, healthy contacts, and individuals from nonendemic countries.
Recently, recombinant protein antigens were selected from the λgt11 library using monoclonal antibodies (29). Although several of these antigens have been found to stimulate T cells, none of them has proven to be protective, as yet (14). Furthermore, the number of T-cell antigens identified in this way remains small (< 10) as compared to the large number of proteins (> 1000) M. leprae is composed of. This discrepancy significantly hampers direct identification of distinct T-cell antigens with vaccine potential. T-cell testing of proteins separated by one-dimensional SDS-PAGE has allowed association of T-cell responses with molecular mass regions (1,4,6,16-19,23).The exact characterization of stimulatory protein antigens, however, is not possible due to the limited resolution power of this technique. Hence, separation methods with higher resolution capacities are urgently required.
We have developed a system which allows separation of M. leprae lysates by twodimensional PAGE and direct transfer into soluble phase ofseparated fractions (10). This technology allowed us to test several hundreds of distinct fractions of M. leprae lysates with T lymphocytes. We are aware that this approach also has certain drawbacks. It is practically impossible to determine the protein concentrations of the 400 single fractions, but since this concentration reflects the relative proportion of a given antigen in the whole M. leprae preparation, we consider this approach feasible. The system was standardized using for each two-dimensional PAGE the same amount of protein from one single batch of M. leprae lysate. Furthermore, due to the limited availability of patients' blood cells and due to the high numbers of antigen fractions tested, we had to use low and varying con centrations of T cells and accessory cells. Therefore, antigen-induced T-cell re sponses were further amplified by adding exogenous IL-2 after 6 days of culture. After this time of culture, only antigen-stimulated T cells respond to IL-2; whereas unrelated T cells or B cells do not. This system, there fore, allows significant amplification of antigen-specific T-cell responses and significantly increases the sensitivity of the assay. We are, therefore, confident that our test system determines even weak T-cell responses which would remain undetectable under normal culture conditions performed without IL-2.
Our studies revealed a marked variability in the T-cell repertoire to M. leprae antigens among healthy leprosy contacts. This find ing is consistent with and extends studies by others using one-dimensional separation(6,18,19). Although we failed to identify dominant regions as defined by molecular mass and isoelectric point (pI), the responses of 4 of 6 contacts were directed to protein fractions of molecular mass 50-80 kDa and pi 5.2 to 5.5. This region includes the stress protein hsp60 known to represent an immunodominant antigen (5,14,15,27,28) . Indeed, we found that hsp60 of M. bovis, which is almost identical to its cognate in M. leprae, has a pI of 5.1 as assessed in our two-dimensional system (data not shown).
In the majority of cases, T cells of leprosy contacts (5 of 6) and BT patients (3 of 3) who responded to separated antigens were also stimulated by lysates or by whole organisms of M. leprae. In contrast and consistent with earlier findings by others, T cells from LL patients reacted with separated antigens only and not with whole M. leprae organisms or lysates (19,23). These findings strongly suggest the existence of suppressor entities in M. leprae. Removing these entities could allow T-cell stimulation by different M. leprae antigens (3,8). Interestingly, in our study such type of reactivity was also observed with TT patients and even one household contact. Others have also reported unresponsiveness in contacts (19); whereas our observation with T cells from TT patients stands in contrast to previous studies (18,19,23). We cannot explain this discrepancy.
T cells from 2 of 2 BT patients and 6 of 7 LL patients under drug treatment responded to M. leprae antigens; whereas T cells of 6 of 7 BT patients and 2 of 3 LL patients who were untreated failed to show any T-cell reactivity toward M. leprae. The remaining one BT and LL patient, each, gave only marginal responses. Consistent with this observation, it has been found previously that T-cell clones from LL patients undergoing long-term treatment with dapsone respond much more strongly to M. leprae and BCG than do T cells from untreated patients (7). These findings suggest reactivation of M. leprae- specific T-cell responses by antileprosy chemotherapy.
In our study, 6 of 9 BT patients and 3 of 10 LL patients failed to respond to any M. leprae antigen preparation including separated protein fractions, suggesting complete anergy (11). Only one of these anergic patients was under drug treatment. Interestingly, 2 of 10 healthy contacts also failed to show any response. Thus, unresponsiveness to M. leprae antigens was observed in different groups of donors in our study.
T cells from normal healthy donors from nonendemic countries responded weakly to two-dimensional-separated protein fractions, although strong reactivities were observed against lysates and whole M. leprae organisms. Similar results have been obtained in a study using one-dimensional separated M. leprae antigens (6). We have previously shown that gamma/delta T cells are stimulated by unseparated lysates of M. tuberculosis and M. leprae (20). To a great extent this response is nonspecific and is caused by low molecular weight (< 3 kDa) and protease-resistant material which escaped our gels (24). Perhaps the responses of donors without previous exposure to M. leprae by whole M. leprae organisms were primarily due to the oligoclonal activation of gamma/delta T cells. The rather weak response of two donors toward two-dimensional-separated fractions would then be caused by T cells with specificity for crossreactive antigens from other microbes.
Our two-dimensional-separation technique revealed a broad T-cell repertoire toward M. leprae antigens in many patients and healthy contacts and, hence, further underlines the necessity of refined antigen separation methods. Only a few antigens of M. leprae have been defined by screening with monoclonal antibodies (for review see 14). This number is far too small to account for the broad reactivity patterns observed here. Despite the high resolution power of the two-dimensional gel electrophoresis applied in our study, this approach requires further improvements. Furthermore, we shall attempt to determine the functions of T cells responding to separated M. leprae fractions, such as interleukin secretion (particularly interferon-7), cytolysis and suppression(3,12,33) While the former two activities can be measured directly using well-established assay systems, the determination of suppression can only be done indirectly. However, co-stimulation assays with mitogens or unrelated antigens in the presence of separated fractions of M. leprae might allow identification of distinct antigens related to suppression (3). Once such improved procedures have been established, it might become possible to identify antigens characteristic for protection, pathogenesis, or different disease types, respectively. Such strategies may facilitate the identification of the antigens to be used in an antileprosy subunit vaccine as well as in the early diagnosis of leprosy.
Acknowledgment. This work received financial support from the UNDP/World Bank/WHO Special Program for Research and Training in Tropical Diseases, the World Health Organization as part of its program for Vaccine Development, the German Leprosy Relief Association, and the EC-India Science and Technology Cooperation Program. H. Gulle was supported by the Boehringer Ingelheim Fund. We are grateful to Mrs. R. Mahmoudi for her secretarial assistance. We thank Dr. P. Brennan, Fort Collins, Colorado, U.S.A., for providing killed M. leprae organisms and Hoffmann - LaRoche (Basel) for recombinant IL-2. Grateful acknowledgment is made of the superintendent. Richardson Leprosy Hospital, Miraj, India, for providing the opportunity to conduct the clinical work.
REFERENCES
1. ABOU-ZEID, C, FILLEY, E., STEELE, J. and ROOK, G. A. W. A simple new method for using antigens separated by Polyacrylamide gel electrophoresis to stimulate lymphocytes in vitro after converting bands cut from Western blots into antigen-bearing particles. J. Immunol. methods 98(1987)5-10.
2. BLOOM, B. R. and GODAL, T. Selective primary health care: strategics for control of disease in the developing world. V. Leprosy. Rev. Infect. Dis. 5(1983)765-780.
3. BLOOM, B. and MEHRA, V. Immunological unresponsiveness in leprosy. Immunol. Rev.80(1984)5-86(60 ref.).
4. CONVERSE, P. J., OTTENHOFF, T. H. M., GEBRE, N., EHRENBERG, J. P. and KIESSLING, R. Cellular, humoral, and gamma interferon responses to Mycobacterium leprae and BCG antigens in healthy individuals exposed to leprosy. Scand. J. Immunol. 27(1988)515-525.
5. EMMRICH, F., THOLE, J., VAN EMBDEN,,J. and KAUFMANN, S. H. E. A recombinant 64 kilodalton protein of Mycobacterium bovis bacillus Calmette-Guerin specifically stimulated human T4 clones reactive to mycobacterial antigens. J. Exp. Med. 163(1986)1024-1029.
6. FILLEY, E., ABOU-ZEID, C, WATERS, M. and ROOK, G. The use of antigen bearing nitrocellulose particles derived from Western blots to study proliferative responses to 27 antigenic fractions from Mycobacterium leprae in patients and controls. Immunology 67(1989)75-80.
7. GILL, H. K., RIDLEY, D. S., GANESAN, J., MUSTAFA, A. S., REES, R. J. W. and GODAL, T. Mycobacterium leprae reactive T cell clones from lepromatous leprosy patients after prolonged dapsone chemotherapy. Lepr. Rev. 61(1990)25-31.
8. GODAL, T., MYRVANG, B., FROLAND, S. S., SHAO, J. and MELAKU, G. Evidence that the mechanism of immunological tolerance ('central failure') is operative in the lack of host resistance in lepromatous leprosy. Scand. J. Immunol. 1(1972)311-321.
9. GREAVES, M. F. and BROWN, G. Purification of human T and B lymphocytes. J. Immunol. 112(1974)420-423.
10. GULLE, H., SCHOEL, B. and KAUFMANN, S. H. E. Direct blotting with viable cells of protein mixtures separated by two-dimensional gel electrophoresis. J. Immunol. methods 133(1990)253-261.
11. KAPLAN, G. and COHN, Z. A. Leprosy and cellmediated immunity. Curr. Opin. Immunol. 3(1991)91-96.
12. KAUFMANN, S. H. E. CD8' T lymphocytes in intracellular microbial infections. Immunol. Today 8(1988)168-174.
13. KAUFMANN, S. H. E. Leprosy and tuberculosis vaccine design. Trop. Med. Parasitol. 40(1989)251-257.
14. KAUFMANN, S. H. E. and YOUNG, D. B. Vaccination against tuberculosis and leprosy. Immunobiology (1991) in press.
15. LAMB, J. R., IVANYI. J., REES, A. D. M., ROTHBARD, J. B., HOWLAND, K., YOUNG, R. A. and YOUNG, D. B. Mapping of T cell epitopes using recombinant antigens and synthetic peptides. EMBO J. 6(1987)1245-1249.
16. LAMB, J. R., O'HEHIR, R. E. and YOUNG, D. B. The use of nitrocellulose immunoblots for the analysis of antigen recognition by T lymphocytes. J. Immunol. methods 110(1988)1-10.
17. LAMB, J. R. and YOUNG, D. B. A novel approach to the identification of T cell epitopes in Mycobacterium tuberculosis using human T lymphocyte clones. Immunology 60(1987)1-5.
18. LEE, S. P., STOKER, N. G., GRANT, K. A., HANDZEL, Z. T., HUSSAIN, R., MCADAM, K. P. W. J. and DOCKRELL, H. M. Cellular immune response of leprosy contacts to fractionated Mycobacterium leprae antigens. Infect. Immun. 57(1989)2475- 2480.
19. MENDEZ-SAMPERIO, P., LAMB, J., BOTHAMLEY, G., STANLEY, P., ELLIS, C. and IVANYI, J. Molecular study of the T cell repertoire in family contacts and patients with leprosy. J. Immunol. 142(1989)3599-3604.
20. MUNK, M. E., GATRILL, A. and KAUFMANN, S. H. E. Target cell lysis and IL-2 secretion by γ/δ lymphocytes after activation with bacteria. J. Immunol. 145(1990)2434-2439.
21. MYRVANG, B., GODAL, T., RIDLEY, D. S., FROLAND, S. S. and SONG, Y. K. Immune responsiveness to Mycobacterium leprae and other mycobacterial antigens throughout the clinical and histopathological spectrum of leprosy. Clin. Exp. Immunol. 14(1973)541-553.
22. NATHAN, C. F., KAPLAN, G., LEVIS, W. R., NUSRAT, A., WITMER, M. D., SHERWIN, S. A., JOB, C. K., HOROWITZ, C. R., STEINMAN, R. M. and COHN, Z. A. Local and systemic effects of intradermal recombinant interferon-γ in patients with lepromatous leprosy. N. Engl. J. Med. 315(1986)6-15.
23. OTTENHOFF, T. H. M., CONVERSE, P. J., GEBRE, N., WONDIMU, A., EHRENBERG, J. P . and KIESS- LING, R. T cell responses to fractionated Mycobacterium leprae antigens in leprosy. The lepromatous nonresponder defect can be overcome in vitro by stimulation with fractionated M. leprae components. Eur. J. Immunol. 19(1989)707-713.
24. PFEFFER, K., SCHOEL, B., GULLE, H., KAUFMANN, S. H. E. and WAGNER, H. Primary responses of human T cells to mycobacteria: a frequent set of γ/δT cells are stimulated by protease resistant ligands. Eur. J. Immunol. 20(1990)1175-1179.
25. RIDLEY, D. S. and JOPLING, W . H. Classification of leprosy according to immunity: a five-group system. Int. J. Lepr. 34(1966)255-273.
26. SANSONETTI, P. and LAGRANGE, P. H. The immunology of leprosy: speculations on the leprosy spectrum. Rev. Infect. Dis. 3(1981)422-469.
27. Shinnick, T. M., Sweetser, D., Thole, J., van Embden, J. and young, R. A. The etiologic agents of leprosy and tuberculosis share an immunoreactive protein antigen with the vaccine strain Mycobacterium bovis BCG. Infect. Immun. 55(1987)1932-1935.
28. Van Schooten, W. C. A., Ottenhoff, T . H. M., Klatser, P. R., Thole, J., de Vries, R. R. P. and KOLK, A. H. J. T cell epitopes on the 36K and 65K Mycobacterium leprae antigens defined by human T cell clones. Eur. J. Immunol. 18(1988)849-854.
29. YOUNG , R. A., MEHRA , V., SWEETSER, D., BUCHANAN, T., CLARK-CURTISS, J., DAVIS, R. W. and BLOOM, B. R. Genes for the major protein antigens of the leprosy parasite Mycobacterium leprae. Nature 316(1985)450-452.
1. Ph.D., Professor, Department of Immunology, University of Ulm, Albert-Einstein-Allee 11,7900 Ulm, Germany.
2. Ph.D., Professor, Department of Immunology, University of Ulm, Albert-Einstein-Allee 11,7900 Ulm, Germany.
3. Ph.D., Director, Cancer Research Institute, Parel, Bombay 400012, India.
4. Ph.D., Director, Cancer Research Institute, Parel, Bombay 400012, India.
5. M.D., Director, Cancer Research Institute, Parel, Bombay 400012, India.
6. Ph.D., Professor, Department of Immunology, University of Ulm, Albert-Einstein-Allee 11,7900 Ulm, Germany.
Reprint requests to Prof. Dr. S. H. E. Kaufmann.
Received for publication on 15 July 1991.
Accepted for publication in revised form on 9 September 1991.