- Volume 60 , Number 1
- Page: 54–60
Effect of skin test with M. leprae soluble antigen on reaction to a subsequent test with the same antigen
ABSTRACT
Soluble skin-test antigens (STA), produced f rom armadillo-derived Mycobacterium leprae by Drs. Rees and Convit, were expected to meet the long-felt need of a test for leprosy infection and also to serve as tests for measuring postvaccination sensitization induced by vaccine preparations against leprosy. The present paper reports results f rom two studies examining the influence, if any, of skin testing with Rees' STA on reaction to a subsequent test with the same antigen. In the first study, 2168 persons f rom households of leprosy patients and f rom neighboring households were skin tested with Rees' STA twice at an interval of 6 months. In the second study, 1700 persons, free f rom leprosy, received cither Rees' STA or normal saline by random allocation. A random subset of 850 persons was tested with Rees' STA after 3 months. The remaining 850 persons were tested with Rees' STA after 6 months. In addition, 242 leprosy patients were given Rees' STA or normal saline by random allocation, and all of these patients were tested with Rees' STA after 6 months. The results of the two studies showed that among persons reacting with a small size of reaction to Rees' STA, the size of the reaction to the repeat test was significantly larger. However, f rom the results of the second study, which included a control group, it was clearly seen that the quantum of boosting or sensitizing effect of the first test as well as that of new sensitization was small over a period of 3-6 months. Thus, the significant increase in reaction size to the second test among those with a small-size reaction to the first test was mostly due to the design effect and was not attributable to the STA. In view of the finding that even without any intervention, reactions to a repeat test would be much larger among those with a small-size reaction to the first test, it becomes important while designing studies for measuring postvaccination sensitivity that provision be made for a control group to ensure a direct measure of the effect of a vaccine.RÉSUMÉ
On avait espéré que les tests cutanés basés sur les antigènes solubles produits à partir de Mycobacterium leprae obtenus de tatous par les Docteurs Rees et Convit, puissent satisfaire les besoins ressentis depuis longtemps pour un test d'infection lépreuse; on avait également espéré que ces tests puissent mesurer la sensibilisation post-vaccinale induite par des préparations de vaccins vis-à-vis de la lèpre. Le présent article rapporte les résultats de deux études examinant l'influence éventuelle des tests cutanés basés sur l'antigène soluble de Rees sur la réaction vis-à-vis d'un test ultérieur avec le même antigène. Dans la première étude, 2168 personnes vivant dans des maisons avec des patients présentant la lèpre ou dans des maisons voisines, ont eu à deux reprises, à un intervalle de 6 mois un test cutané avec l'antigène de Rees. Dans la seconde étude, 1700 personnes, indemnes de lèpre, ont reçu soit l'antigène soluble de Rees, soit une solution saline normale. L'allocation de ces personnes à l'un ou l'autre groupe, s'est déroulée au hasard. Un sous-groupe randomisé de 850 personnes fut testé avec l'antigène soluble de Rees après 3 mois. Les autres 850 personnes furent testées avec l'antigène de Rees après 6 mois. De plus, 242 patients lépreux ont reçu soit l'antigène soluble de Recs, soit la solution saline normale avec allocation au hasard, et tous ces patients ont été testés avec l'antigène de Recs après 6 mois. Les résultats des deux études ont montré que parmi les personnes qui avaient une réaction de petite taille vis-à-vis de l'antigène soluble de Rees, la taille de la réaction augmentait de manière significative lorsque le test était répété. Cependant, à partir des résultats de la deuxième étude, qui comprenait un groupe témoins, on a clairement vu que l'effet sensibilisateur du premier test aussi bien que celui d'une sensibilisation répétée était faible sur une période de 3 à 6 mois. En conséquence, l'augmentation significative de la taille de la réaction au second test parmi ceux qui avaient une réaction de petite taille était due principalement à un artefact et ne pouvait être attribuée à l'antigène soluble. Au vu de l'observation que, même en l'absence d'intervention, les réactions à un test répété seraient beaucoup plus importantes parmi ceux qui avaient présenté une réaction de petite taille pour le premier test, il devient important lors de l'établissement de protocoles d'étude pour mesurer la sensibilité post-vaccinale, de prévoir un groupe témoin afin d'assurer une mesure directe de l'effet du vaccin.RESUMEN
Se esperaba que los antígenos solubles para pruebas en piel (STA), producidos a partir del Mycobacterium leprae cultivado en armadillos por los Drs. Rees y Convit, sirvieran para demostrar la infección leprosa y también para medir la sensibilización postvacunal inducida por las diferentes vacunas contra la lepra. El presente trabajo reporta los resultados de dos estudios en donde se examinó la influencia de las pruebas con los STA de Rees, sobre la reacción a una prueba de repetición con el mismo antígeno. En el primer estudio, 2168 personas (convivientes y no convivientes con pacientes con lepra), se probaron con los STA en dos ocasiones con un intervalo de 6 meses. En el segundo estudio, 1700 personas libres de lepra, recibieron los STA o solución salina, al azar. Una muestra de 850 personas seleccionada también al azar se probó con los STA después de 3 meses. Las restantes 850 personas se probaron con los STA, 6 meses después. Además, 242 pacientes con lepra se inyectaron, al azar, con los STA o con solución salina, y lodos se probaron con los STA de Rees, 6 meses después. Los resultados de los 2 estudios mostraron que las personas que dieron una pequeña reacción a los STA, exhibieron una reacción significativamente mayor cuando se probaron una segunda vez con los STA. Sin embargo, los resultados del segundo estudio, el cual incluyó un grupo control, indicaron que la magnitud del efecto reestimulante o sensibilizante de la primer prueba en piel, así como la magnitud del efecto de una nueva sensibilización, fueron pequeñas durante un periodo de 3 a 6 meses. Así, el significante incremento en el tamaño de la reacción a la segunda prueba dérmica, entre aquellos individuos con una reacción de pequeño tamaño a la primer prueba en piel, no fue atribuible a los STA, sino al diseño experimental. En vista del hallazgo de que aún sin ninguna intervención, las reacciones a una prueba de repetición son mucho mayores en los individuos con una reacción de pequeño tamaño a la primer prueba intradérmica, se hace evidente la importancia de incluir siempre un grupo control en los estudios diseñados para medir la sensibilidad resultante de cualquier vacunación contra la lepra.Soluble skin-test antigens have been produced from armadillo-derived Mycobacterium leprae by Drs. Rees and Convit, referred to as Rees and Convit skin-test antigens (STA). Rees' STA is also referred to as leprosin or MLSA, and Convit's STA as SPA or SA. It was hoped that these STAs would meet the long-felt need for a test for leprosy infection to use in epidemiological studies. They were also expected to serve as tests for measuring postvaccination sensitization induced by vaccine preparations against leprosy. The Central JALMA Institute for Leprosy Field Unit has been carrying out a number of studies using these antigens, and these data were initially utilized to examine the influence, if any, of skin testing with Rees' STA on reaction to a subsequent test with the same antigen (Study 1).However, this study lacked a proper control group and, therefore, we undertook a controlled study to see whether testing with Rees' STA has any specific sensitizing or boosting effect on reactions to a subsequent test with the same antigen (Study 2). The findings of these two studies are presented in this paper.
MATERIAL AND METHODS
Study 1. The population for this study consisted of members of households of all the leprosy patients detected in a prevalence survey together with members of neighboring households in three villages in Sriperumbudhur Taluk of the Chingleput District, Tamil Nadu, India. In this population, two surveys, at an interval of 6 months, were conducted; the first from August-December 1987, the second from February-June 1988. In addition to clinical examination for leprosy, this population was skin tested with Rees' STA on both occasions.
A total of 2168 persons were skin tested and the results were read at both the first (first test) and second (second test) surveys. Those studied were of different ages and gender, and also differed with respect to leprosy disease status, i.e., noncascs, suspected cases, and cases of leprosy on the basis of clinical examination.
Study 2. This study was undertaken in 1700 healthy persons in another village of Sriperumbudhur Taluk during May-December 1989. Each person received 0.1 ml of either Rees' STA or normal saline by random allocation. A random subset of 850 persons was tested with STA after 3 months. The remaining 850 persons were tested with STA after 6 months.
In addition, 242 leprosy patients diagnosed in a prevalence survey in 12 villages were included in the study. The patient population was stratified according to type of leprosy, age, and sex. STA and normal saline were randomly allocated to the patients in each stratum. All of the patients were tested with STA after 6 months.
In each of the two studies, the same batch of Rees' STA (Lot WEL 3 CD-73) was used at both time points. Each person received 0.1 ml of the antigen containing 1 jug of protein intradermally on each of the two occasions but at different sites of the forearm (midvolar of right and left forearms, respectively, in Study 1 and upper dorsum of right and midvolar of the left forearms, respectively, in Study 2). The skin tests were performed by standardized testers, and 48 hr later the transverse diameters of indurations, in millimeters, were read by standardized readers, following standard procedures (4).
Statistical analysis. The paired t -test was used to test for significance of the mean increase in reaction size. The chi-squared test was employed for comparing proportions. Since most of the distributions were not uni- modal, the Mann-Whitney U test was employed to compare two means. For comparing more than two means, the F-test (if permissible) or the Kruskal-Wallis test was used.
RESULTS
Study 1. Considering reactions to the two tests with STA, performed at an interval of 6 months, the size of reaction to the second test, in general, was larger than that to the first test. The mean sizes of reaction to the first and second tests were 14.1 mm and 16.9 mm, respectively, the mean increase in reaction size being 2.8 mm (p < 0.01). Of the 558 persons reacting with 0-9 mm to the first test, as many as 440 (79%) had reactions of 10 mm or more to the second test. Even among children aged 1-4 years this proportion was as high as 69%. Considering reactions of 10 mm or more to be "positive," the positivity rate went up from 74% at the first test to 93% at the second test (p < 0.01), showing that a vast majority of persons who were "negative" to the STA became "positive" within 6 months' time. These results were very similar in the patient population also, irrespective of the clinical classification of the leprosy.
The difference in mean reaction size to the two tests according to age and size of reaction to the first test is shown in Table 1. An analysis of variance showed that the mean difference in reaction size to the two tests was independent of age (p > 0.2) but was highly dependent on the size of reaction to the first test (p < 0.001).
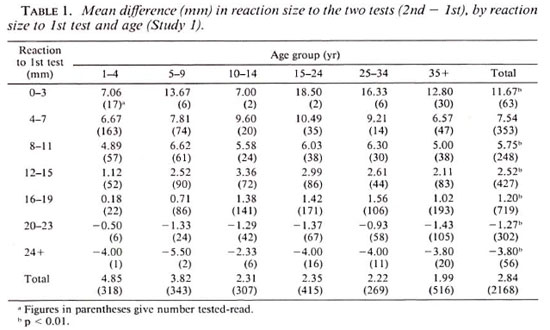
Study 2. The pattern of results among those tested with STA at 0 and 3 months and at 0 and 6 months was very similar to that observed in Study 1. These results showed that the difference in mean reaction size to the two tests was larger at 6 months compared to that at 3 months (p < 0.05). The overall increase in mean reaction size was 0.44 mm and 1.67 mm at 3 and 6 months, respectively.
Table 2 gives data on the mean sizes of reactions to STA at 3 and 6 months for the tested and control groups at 0 months according to age and sex. With the design adopted, the increase in mean reaction size, if any, in the tested group in comparison with that in the control group can be attributed to the boosting or sensitizing effect of the first test at 0 months. The overall increase was only marginal; 1.1 mm (p < 0.05) at 3 months and 0.7 mm (p > 0.1) at 6 months. This increase was mostly confined to males.
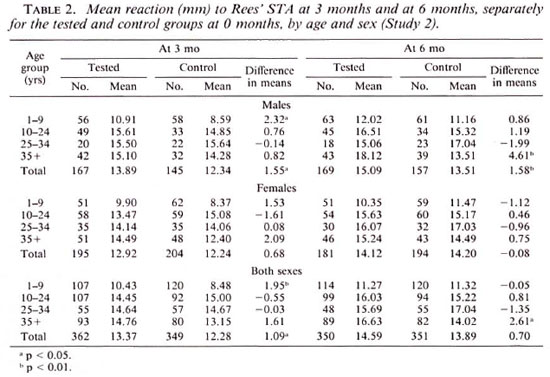
In the patient population, the comparisons are based on 182 patients who were test-read on both occasions. Considering all patients, the mean reaction size to STA at 6 months in those initially tested was similar to that in the control group; 15.7 mm and 16.4 mm in the two groups, respectively (p > 0.4). Among the 150 patients belonging to the tuberculoid leprosy or borderline tuberculoid leprosy group, mean sizes of reactions were 16.7 mm and 16.9 mm (p > 0.8). Among the 22 patients belonging to the borderline lepromatous leprosy or lepromatous leprosy group, mean reaction sizes were 10.1 mm and 11.9 mm (p > 0.6) in the initially tested and control groups, respectively.
Although it was not the purpose of Study 2 to obtain estimates of the proportion "positive" to STA after different intervals of time, the available material was used to study the mean size of reaction and proportion of persons "positive" (10 mm or more) to STA at 0, 3, and 6 months among persons tested with STA for the first time. Both the mean size of reaction and proportion positive to STA at 3 months did not differ significantly from those at 0 months; whereas they were slightly higher at 6 months, particularly in the youngest age group. Considering the age group 1-9 years, among 221 and 120 children tested for the first time at 0 and 6 months, the proportions positive to STA were 51.1% and 55.8% (p > 0.1) and the mean sizes of reactions were 9.2 mm and 11.3 mm (p < 0.01), respectively. Since the two groups ofchildren were random samples from the same population, this increase can be attributed to new sensitization.
DISCUSSION
The objective of the studies reported here was to see whether skin testing with M. leprae soluble antigen had any effect on reactions to a retest with the same antigen. Data from Study 1 demonstrated that there was a significant increase in the size of reaction to Rees' STA, even within a short span of 6 months, both among leprosy patients and others. The increase was very much pronounced in persons with small sizes of reactions to the first test, irrespective of age. However, this increase could be due to several factors, such as design effect (i.e., the effect of regression to the mean), new sensitization (specific and nonspecific), and a boosting and sensitizing effect of the first test. Since this study did not include a control group, i.e., a group which did not receive STA on the first occasion, it was not possible to know exactly the magnitude of the boosting/sensitizing effect of STA, if any, on reactions to a subsequent test. In order to understand these results better, a properly controlled study was therefore undertaken.
The phenomenon of boosting of a reaction in a subsequent test due to the initial test has been well documented in respect to the tuberculin test (1,5,9,11). Narain, et al. reported that a test with 1 TU of PPD RT 23 with TWeen 80 was responsible for a significantly larger reaction to a subsequent test given 2 months later, and that this effect increased with age (11). There was an increase in the mean reaction size of 1.7 mm in the group tested twice as compared to that in the control group tested only once at 2 months. This boosting effect, observed 2 months after previous testing, was not seen after a period of 18 months (10). It has also been reported that this enhancing effect may be more marked in communities with a high prevalence of nonspecific sensitivity(9,11). Comstock reported that although there was no evidence that subjects could be sensitized by doses of tuberculin used in routine testing, those doses were capable of boosting sensitivity that had waned below the level of detection back toward its original degree of intensity (1). Ferebec and Mount observed that, on the second test, the percentage of reactors was higher in the subgroups which had been tested with tuberculin originally than in those tested first with diluent (5).
However, no studies seem to have been reported in the literature on the boosting or sensitizing effect of skin testing with M. leprae soluble antigens. Ponnighaus and Fine (9) in their studies based on 91 indiv iduals have observed skin-test "conversions" following Rees' STA (batch CD-19) in 39% of individuals. They also noted "natural reversion" following STA in 30% of the individuals. They could not hypothesize "to what extent this may reflect temporal variations in skin sensitivity to this antigen among members of this population or boosting of the skin test response or actual sensitization by the initial skin test." They attempted to make use of this 39% conversion figure to work out the "corrected" conversion rates to judge vaccine effects in their studies. Convit, et al. (2) have reported postvaccination sensitization results using Convit's STA. However, their studies did not include a control group receiving only the STA twice. Gill, et al. (6) also reported results of postvaccination sensitization using Rees' STA (batch CD-19) in a nonendemic country for leprosy. Although no details on individuals who received only the Rees' STA were given, no boosting effect was apparently perceptible in their study. In none of the studies using M. leprae soluble antigens reported above was there a control group, i.e., a group that did not receive STA on the first occasion, which would have helped to obtain a direct measure of the boosting or sensitizing effect of the STA.
The results of the second study reported here, which included a control group, clearly showed that the quantum of the boosting or sensitizing effect with Rees' STA over a period of 3-6 months was small, a finding similar to that reported with a tuberculin test. No boosting effect was seen in leprosy patients.
In the two studies reported here, it was observed that among persons with small reaction sizes to STA, the size of reaction to the repeat test was significantly higher than that to the first test, giving the impression that the first test had a strong enhancing effect on reactions to the second test. How ever, from the results of the second study, which included a control group, it was clearly seen that the quantum of the boosting or sensitizing effect of the first test as well as that of new sensitization was small over a period of 3-6 months. Thus, the significant increase in reaction size to the second test among those with small reaction sizes to the first test was mostly due to the design of the study and cannot be attributed to the boosting or sensitizing effect of the first test.
It was also seen in the first study that the increase in reaction size to the repeat test was the highest in those with very small reactions to the first test and gradually decreased as the size of reaction to the first test increased. In fact, for persons with very high initial reactions, there was a decrease in reaction size to the second test (Table 1). In a group of persons with very small reactions to the first test, readings to the second test, on the average, would show an increase. Similarly, in a group of persons with readings of very large reactions to the first test, readings to the second test would tend to show a decrease on the average. This interesting phenomenon of the effect of regression to the mean has been reported in the literature (3). These results clearly demonstrate that to carry out such studies with skin-test antigens without proper controls may lead to erroneous conclusions.
M. leprae soluble antigens have not been found useful in detecting leprosy infection in an endemic area for leprosy (8). The "soft" nature of reactions to these antigens and the difficulties in the reproducibility of the readings have also been reported (7). However, these antigens as well as lepromin are often used in measuring postvaccination sensitivity toantileprosy vaccines (2,6,12,13). In view of the results reported in this paper, that even without any intervention reactions to a repeat test would be much larger particularly among those with small reaction sizes to the first test, it becomes important that while designing studies for measuring postvaccination sensitivity, provision for a control group should be made. This would help in obtaining a direct measure of the effect of a vaccine which does not include effects due to the design, boosting or sensitizing effect of the first test, and new sensitization.
In conclusion, the results of the studies reported here have shown that: a) there was a very slight boosting effect with M. leprae soluble antigen, similar to that reported with tuberculin, and b) it would be dangerously misleading to carry out similar studies without proper controls.
REFERENCES
1. COMSTOCK, G. W. Tuberculin conversions: true or false? (Editorial) Am. Rev. Respir. Dis. 118 (1978) 215-219.
2. CONVIT, J., ARANZAZU, N., ZUNIGA, M., ULRICH, M., PINARDI, M. E., CASTELLAZZI, Z. and ALVARARDO, J. Immunotherapy and immunoprophylaxis of leprosy. Lepr. Rev. Special Issue (1989)47S-60S.
3. DAVIS, C. E. The effect of regression to mean in epidemiological and clinical studies. Am. J. Epidemiol. 104(1976)493-498.
4. EDWARDS, L. B., PALMER, C. E. and MAGNUS, K. BCG vaccination. Geneva: World Health Organization, 1953, pp. 32-33. Monograph Ser. No. 12.
5. FEREBEE, S. H. and MOUNT, F. W. Evidence of booster effect in serial tuberculin testing. Am. Rev. Respir. Dis. 88(1963)118-119.
6. GILL, H. K., MUSTAFA, A. S. and GODAL, T. Induction of delayed type hypersensitivity in human volunteers immunized with a candidate leprosy vaccine consisting of killed Mycobacterium leprae. Bull. WHO 64(1986)121-126.
7. GUPTE, M. D. and ANANTHARAMAN, D. S. Use of soluble antigens in leprosy epidemiology. Lepr. Rev. 59(1988)329-335.
8. GUPTE, M. D., ANANTHARAMAN, D. S., NAGARAJU, B., KANNAN, S. and VALLISHAYEE, R. S. Experiences with Mycobacterium leprae soluble antigens in a leprosy endemic population. Lepr. Rev. 61(1990)132-144.
9. KNIGHT, R. A., KABORKJIAN, M. E. and HARRIS, H. W. An investigation of the influence of PPD-B hypersensitivity on the booster effect associated with multiple tuberculin test with PPD-S. (Abstract) Am. Rev. Respir. Dis. 88(1963)119.
10. NARAIN, R., GOTHI, G. D., GANAPATHY K. T. and SHYAMA SUNDER, C. V. Effect on tuberculin allergy of tuberculin tests given 18 months earlier. Indian J. Med. Res. 69(1979)886-892.
11. NARAIN, R., NAIR, S. S., RAMANATIIA RAO, G. CHANDRASEKAR, P. and LAL. P. Enhancing of tuberculin allergy by previous tuberculin testing. Bull. WHO 34(1966)623-638.
12. PONNIGHAUS, J. M. and FINE, P. E. M. Sensitization studies with potential leprosy vaccine preparations in northern Malawi. Int. J. Lepr. 54(1986)25-37.
13. SAMUEL, N. M., NEWPANI, K., SAMUEL, S. and ADIGA, R. B. Vaccination of borderline tuberculoid patients with BCG plus killed Mycobacterium leprae. Jpn. J. Lepr. 55(1986)29-34.
1. M.D., CJIL Field Unit (Indian Council of Medical Research), 271 Nehru Bazaar, Avadi, Madras 600054, India.
2. M.Sc., CJIL Field Unit (Indian Council of Medical Research), 271 Nehru Bazaar, Avadi, Madras 600054, India.
3. M.A., CJIL Field Unit (Indian Council of Medical Research), 271 Nehru Bazaar, Avadi, Madras 600054, India.
4. M.Sc., CJIL Field Unit (Indian Council of Medical Research), 271 Nehru Bazaar, Avadi, Madras 600054, India.
CJIL Field Unit (Indian Council of Medical Research), 271 Nehru Bazaar, Avadi, Madras 600054, India.
Received for publication on 10 January 1989.
Accepted for publication in revised form on 7 January 1992.