- Volume 60 , Number 1
- Page: 61–70
Energy generation mechanisms in the in vitro -grown Mycobacterium lepraemurium
ABSTRACT
Mycobacterium lepraemurium was cultivated on Ogawa egg-yolk medium and its energy coupling mechanisms were investigated. Cell-free extracts prepared f rom in vitro-grown cells catalyzed phosphorylation coupled to the oxidation of generated NADH, added NADH, and succinateyielding ratios of phosphorus moles incorporated into high-energy bonds to oxygen atoms utilized (P/O ratios) of 0.75, 0.52, and 0.36, respectively. Ascorbate oxidation alone or in the presence of tetramethyl-pphenyline-diamine (TMPD) did not yield any adenosine triphosphate (ATP). However, ascorbate in the presence of added cytochrome c was coupled to ATP synthesis and yielded a P/O ratio of 0.12. The oxidative phosphorylation was uncoupled by all of the uncouplers used without any inhibition of oxygen consumption. ATP generation coupled to NADH oxidation was completely inhibited by the flavoprotcin inhibitors, such as rotcnone and amytal; these inhibitors had no effect, however, on ATP synthesis associated with succinate oxidation. Antimycin A or 2-n-hcptyl-4-hydroxy-quinoline-N-oxide (HQNO) and cyanide inhibited markedly the oxidations of NADH and succinate as well as the coupled ATP generation. The phosphorylation coupled to ascorbate plus cytochrome c was not affected by either of the flavoprotcin inhibitors or by antimycin A or HQNO, but was completely inhibited by cyanide. The thiolbearing agents p-chloromercuribenzoate (PCMB) and N-ethylmaleimide were the potent inhibitors of the phosphorylation associated with the oxidation of NADH and succinate. The results indicate that the three energy-coupling sites are functional in the respiratory chain of in wire-grown M. lepraemurium.RÉSUMÉ
Mycobactcrium lepraemurium a été cultivé sur milieu de jaune d'oeuf d'Ogawa et ses mécanismes de couplage d'énergie ont été étudiés. Des extraits acellulaircs préparés à partir de cellules cultivées in vitro ont catalysé la phosphorilation couplée à l'oxydation du NADH endogène, NADH exogène, et des rapports de production de succinate des moles de phosphore incorporés dans des liens à haute énergia à des atomes d'oxygène utilisés (rapport P/O) de respectivement 0, 75, 0, 52, et 0, 36. L'oxydation de l'ascorbate seul ou en présence de diaminc de tétraméthyl-p-phénylinc (TMPD) n'a pas d'adénosinc triphosphate (ATP). Cependant, de l'ascorbate en présence de cytochrome c additionnel était couplée à la synthèse d'ATP et produisait un rapport P/O de 0, 12. La phosphorilation oxydative était découplée par tous les découpleurs utilisés sans inhibition de la consommation d'oxygène. La production d'ATP couplée à l'oxydation du NADH était complètement inhibée par les inhibiteurs de la flavoprotéinc, telle que la rotenone et l'amytal; ces inhibiteurs n'avaient pas d'effet, cependant, sur la synthèse d'ATP associée à l'oxydation du succinate. L'antimycine A ou le 2-n-heptyle-4-hydroxiquinolincn-oxyde (HQNO) et la cyanide inhibaient de façon marquée les oxydations du NADH et du succinate ainsi que le couplage de la production d'ATP couplé. La phosphorilation couplée à l'ascorbate plus cytochrome c n'était affectée par aucun des inhibiteurs de la flavoprotéine ni par l'antimycine A ou le HQNO, mais était complètement inhibée par la cyanide. Les agents p-chloromercuribenzoate (PCMB) et N-ethylmaleimide et porteurs de thiol étaient les inhibiteurs puissants de la phosphorilation associée à l'oxydation du NADH et du succinate. Les résultats indiquent que les trois sites de couplage d'énergie sont fonctionnels dans la chainc respiratoire de M. lepraemurium cultivé in-vitro.RESUMEN
Se estudiaron los mecanismos acopladores de energía del Mycobacterium lepraemurium crecido en el medio de Ogawa-yema de huevo. Los ext ractos libres de células de las micobacterias cultivadas in vitro catalizaron la fosforilación acoplada a la oxidación del NADH generado y del NADH y succinato adicionados, exhibiendo índices de incorporación de fósforo (en moles) en enlaces de alta energía/átomos de oxígeno utilizados (índices P/O) de 0.75, 0.52, y 0.36, respectivamente. La oxidación del ascorbato sólo o en presencia de tetrametil-p-fenilen diamina (TMFD) no produjo nada de trifosfato de adenosina (ATP). Sin embargo, en presencia de citocromo c exógeno, el ascorbato fue acoplado a la síntesis de ATP y produjo un índice P/O de 0.12. La fosforilación oxidativa fue desacoplada por todos los desacopladores usados sin que ocurriera ninguna inhibición del consumo de oxígeno. La generación de ATP acoplada a la oxidación del NADH fue completamente inhibida por los inhibidores de flavoproteínas, retenona y amital; sin embargo, estos inhibidores no tuvieron efecto sobre la síntesis del ATP asociada a la oxidación del succinato. La antimicina A o óxido de la 2-n-heptil-4-hidroxiquinolina (HQNO) y el cianuro, inhibieron marcadamente las oxidaciones del NADH y del succinato, así como la generación acoplada del ATP. La fosforilación acoplada al ascorbato en presencia de citocromo c, no fue afectada ni por los inhibidores de flavoproteínas ni por la antimicina A o HQNO, pero fue completamente inhibida por cianuro. Los agentes tiolados p-cloromercuribenzoato (PCMB) y N-etilmaleimida, fueron potentes inhibidores de la fosforilación oxidativa del NADH y succinato. Los resultados indican que los 3 sitios acopladores de energía son funcionales en la cadena sitios acopladores de energía son funcionales en la cadena respiratoria del M. lepraemurium cultivado in vitro.Mycobacterium lepraemurium. the etiologic agent of murine leprosy, was first discovered by Stefansky in 1903 (23). Despite numerous attempts for its in vitro cultivation, the mycobacterium remained an obligate intracellular parasite until 1970 when Ogawa and Motomura(20) reported the growth of M. lepraemurium on Ogawa eggyolk medium. These findings have subsequently been confirmed (7,18,21).
It has long been established that adenosine triphosphate (ATP) is the most important mctabolically available form of energy in living cells. Energy coupling reactions that accompany biological oxidation of substrates have been studied extensively in many bacterial systems (3,6) We have reported (10) such mechanisms in in vivo -grown M. lepraemurium. However, when in vivo- grown cells are used for metabolic studies, it is always argued whether the results reported are not due to the presence of host tissues, although in very small amounts, in the purified preparations used during such studies. Although M. lepraemurium nearly 70 years after its discovery has been cultivated in vitro, energy conservation mechanisms using in vitro -grown cells have not yet been studied. Therefore, we investigated the mechanisms of electron transport and energy generation in in vitro -grown M. lepraemurium. The experimental data show that this mycobacterium is capable of conserving oxidative energy by means of three energy coupling sites observed to be present and operative in the electron transport chain.
MATERIALS AND METHODS
Microorganism. The Hawaiian strain of M. lepraemurium used in these studies was originally obtained from Dr. P. Greenberg (La Roche Research Laboratory, Nutley, New Jersey, U.S.A.). It was maintained in Spraguc Dawley rats or C3H mice by subcutaneous serial passages every 4-5 months.
In vitro cultivation of M. lepraemurium. Primary as well as successive subcultures of M. lepraemurium were obtained on Ogawa egg-yolk medium as described earlier (7). Since malachite green interferes with the spectral studies, it was added in the egg-yolk medium only to obtain primary cultures and was omitted from the medium used to obtain subcultures.
Preparation of cell suspensions. The growth of the fourth subcultures from the surface of Ogawa egg-yolk medium was removed and suspended in 50 mM sodiumpotassium phosphate buffer, pH 6.5. The cell suspension was centrifuged at 10,000 x g x 10 min at 4ºC in a Bcckman J-21B centrifuge. The pellet was washed twice more, and cell-free extracts were prepared as described below.
Preparation of cell-free extracts. To prepare cell-free extracts, the cells were suspended 1:4 w/v (wet weight) in a sonication medium containing 50 mM Tris (hydroxymethyl) aminomethane buffer, pH 6.5, 300 mM sucrose, 5 mM ethylenediamine tetraaceticacid (EDTA-Na2)and 50 mM MgCL2. 6H2O. The cell suspensions were passed twice through a chilled Aminco-French Pressure Cell at 18,000 psi (1 psi = 703.07 kg/m2). The homogenate was centrifuged at 20,000 xg x 30 min in a Beckman J-21B refrigerated centrifuge. The resulting supernatant was used as the cell-free extract. To obtain particulate and supernatant fractions, 20,000 x g cell-free extracts were further centrifuged at 150,000 x g x 120 min in a Beckman model L-65 ultracentrifuge using the rotor type 50 Ti at 2ºC. The resulting supernatant was used as the 150,000 x g supernatant fraction, and the pellet after washing once was suspended in an appropriate volume of sonication medium and used as 150,000 x g particulate fraction.
Oxidation and coupled phosphorylation. Oxygen consumption by various substrates was measured at 32ºC using a Y.S.I, oxygen polarograph (Yellow Springs Instrument Co. Inc., Yellow Springs, Ohio, U.S.A.) in a reaction mixture containing 50 mM sucrose, 5 mM MgCl2-6H2O, 5 mM KF, 2 mM adenosine diphosphate (ADP), 4 mM K2HPO4 and 50 mM Tris-HCl buffer, pH 6.5. The reaction mixture containing the cell free extracts, particulate, or supernatant fraction (3-4.5 mg protein) was incubated for 3 min and then the reaction, in a total volume of 3 ml, was started either by generating NADH or by adding 3.3 mM NADH, 10 mM succinate, ascorbate, glycerol, 3 mM dithiothreitol or DL penicillamine. NADH generating system contained 12 units of alcohol dehydrogenase, 10 mM semicarbazide, 40 mM ethanol, and 1 mM NAD + . The reaction was carried out for at least 10 min.
For adenosine triphosphate (ATP) determination, the reaction in a total volume of 2 ml was carried out in the same reaction mixture used for oxygen consumption at 32ºC for 10 min. The reaction was terminated by adding 0.1 ml of 0.1 N HC1. The ATP formed was extracted with 15% perchloric acid, neutralized according to the procedure of Gibson and Morita (5), and the amount of ATP in the neutralized supernatant was determined by the luciferin-luciferase method as described by Strehler (24). All possible controls, such as reaction mixtures with or without substrate as well as reaction mixtures containing bacterial fractions boiled for 5 min to exclude nonenzymatic oxidation, were included. The phosphorus moles incorporated into highenergy bonds to oxygen atoms utilized (P/O) ratios were corrected by subtracting the endogenous values of O2 consumed and the amount of ATP exhibited by the cell-free extracts.
Uncouplers and inhibitors. The following uncouplers and inhibitors were used during this study: 2,4-dinitrophenol (DNP), 2,6-dibromophenol (DBP), pentachlorophenol (PCP), m,chlorocarbonylcyanide phenylhydrazone (CCCP), dicoumarol, gramicidin, rotenone, amytal, thenoyltrifluoroacetone (TTFA), antimycin A, 2-nheptyl-4-hydroxyquinoline-N-oxide (HQ-NO), cyanide, p-chloromercuribenzoatc (PCM 13), and N-ethylmaleimide.
Cyanide was dissolved in water. PCMB was dissolved in 0.1 M glycyl glycine buffer (pH 7.5). All other uncouplers and inhibitors were dissolved in ethanol. The volume of ethanol used did not affect oxidative or phosphorylative activity of the cell-free preparation.
Difference spectra. The reduced minus oxidized difference spectra were obtained by means of a Cary model 118C dual-beam recording spectrophotometer equipped with an intense light source. To study the effect of inhibitors on the difference spectra, an appropriate concentration of inhibitors was added in both cuvettes, incubated for about 5 min, and then the substrate was added in the sample cuvette and the spectra were taken.
Protein determination. Protein concentration in the cell-free preparations was determined using the Folin-Ciocalteau reagent by the method of Lowry, et al. (17). Crystalline bovine serum albumin was used as a standard.
Chemicals. ATP, ADP, alcohol dehydrogenase, NAD + , and NADH, as well as all the inhibitors and uncouplers were purchased from the Sigma Chemical Co., St. Louis, Missouri, U.S.A. All other chemicals were of the highest grade available.
RESULTS
Phosphorylation coupled to the oxidation of various substrates. Reaction mixtures without substrate and heat-inactivated bacterial fractions did not show any oxidation. The data in Table 1 represent the rates of oxidation of various substrates and the associated ATP generation by the cell-free extracts (20,000 x g) prepared from in vitro- grown M. lepraemurium. The rates of oxidation of generated NADH or exogenously added NADH were the same, but the amount of ATP formed with generated NADH was always higher than that obtained with added NADH in the same experiment. The generated system was, therefore, used in all subsequent experiments when NADH was the substrate. When succinate was used as the substrate, P/O ratios of 0.36 were obtained. Although the rate of oxidation of ascorbate was higher than NADH and succinate, its oxidation did not yield any ATP. Ascorbate was very rapidly oxidized in the presence of added tetramethyl-p-phenyline-diamine (TMPD) (0.01 mM) but the oxidation was not coupled to phosphorylation. However, in the presence of 0.01 mM cytochrome c, ascorbate was also oxidized readily and yielded a P/O ratio of 0.12. Cytochrome c in the in vitro -grown M. lepraemurium (9) is present in very small amounts. Added cytochrome c seems to couple with the endogenous cytochrome c, since the rate of ascorbate oxidation is enhanced in the presence of added cytochrome c. The rate of oxidation of glycerol was the same as obtained with NADH but no phosphate esterification occurred during glycerol oxidation. Sulfhydryl compounds, dithiothreitol and DL penicillamine were actively oxidized but without any associated ATP production. These results suggest that all of the phosphorylation sites were operative in the respiratory chain of in vitro -grown M. lepraemurium (10).
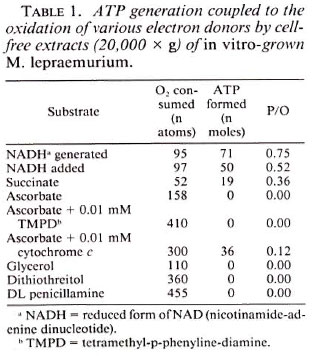
Requirements for ATP production. The data in Table 2 show that with generated NADH as the substrate, although the rates of oxygen uptake were not affected, the P/O ratios decreased considerably when either ADP, Pi, or MgCL2 were omitted from the reaction mixture. The results suggest that AMP could replace ADP as a phosphate acceptor possibly due to the presence of enzyme pyrophosphatase in the cell-free extract. The omission of KF from the reaction mixture caused a slight stimulation of NADH oxidation but it caused 79% inhibition of the coupled phosphorylation. The addition of NAD + or ethylene glycol tetraacetic acid (EGTA) to the complete reaction mixture did not enhance the phosphorylation efficiency, and the omission of sucrose caused no appreciable decrease in NADH oxidation as well as the associated ATP production. In order to obtain good P/O ratios, it was necessary to use freshly harvested cells to prepare cell-free extracts. Although the cell-free extracts prepared from frozen cells did not lose any oxidative activity, their phosphorylative activity was completely lost. Similar results were obtained with in vivo -grown M. lepraemurium (10).
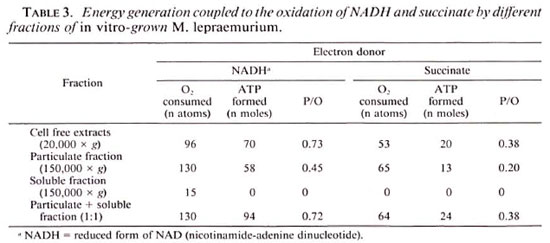
ATP production by various cell-free fractionated systems. The relative rates of oxidation and coupled phosphorylation by cell-free extracts, particulate and soluble fractions of in vitro- grown M. lepraemurium are shown in Table 3. Although the rates of oxidation of NADH and succinate by the particulate fraction were higher than those obtained with cell-free extracts, the amounts of ATP formed were comparatively lower, resulting in lower P/O ratios. NADH was oxidized at a very slow rate by the soluble fracti on without any ATP formation. The soluble fraction failed to oxidize succinate. When particulate and soluble fractions were combined 1:1 based on protein, the rates of oxidation of NADH and succinate were similar to those obtained by the particulate fraction alone, but the amounts of coupled ATP produced were higher and thus the P/O ratios with both substrates were the same as obtained with cell-free extracts. These results show that both particulate and soluble fractions are necessary for efficient ATP production by in vitro- grown M. lepraemurium. In all subsequent experiments, crude cell-free extracts (20,000 x g) were used during this study.
Effect of uncouplers on ATP production.
In almost all cases low concentrations of various uncouplers of oxidative phosphorylation, i.e., DNP. DBP, PCP, CCCP, and dicoumarol, abolished ADP phosphorylation without causing any inhibition of the oxidation of either NADH or succinate (Table 4). In addition, dicoumarol and gramicidin (12 µ g/mg protein) effectively inhibited ATP synthesis linked with oxidative phosphorylation. The energy is thus generated through the process of oxidative phosphorylation by the in vitro -grown M. lepraemurium.
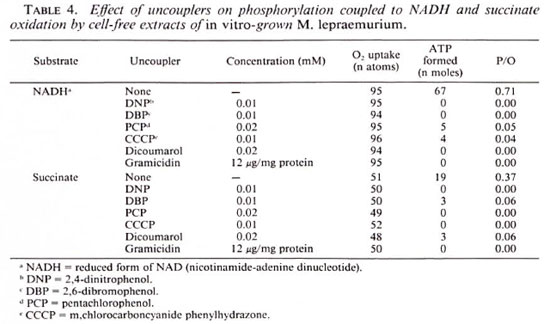
Effect of respiratory chain inhibitors. The data in Table 5 show that NADH oxidation as well as the associated phosphorylation were markedly sensitive to the flavoprotein inhibitors rotenone and amytal. However, neither the oxidation of succinate nor the coupled ATP synthesis was affected by these inhibitors. Interestingly, the oxidation of NADH and the accompanying ATP formation is not inhibited by 0.1 mM TTFA, but oxidation of the succinate and the concomitant ATP generation were completely inhibited. Although the phosphorylations coupled to NADH and succinate oxidation were completely inhibited by antimycin A or HQNO, these inhibitors did not affect ATP generation coupled to ascorbate oxidation. However, complete inhibition of the phosphate csterification was caused by cyanide when NADH, succinate, or ascorbate served as the electron donor. The thiolbinding agents, PCMB and N-ethylmaleimide, were effective inhibitors of both NADH and succinate oxidations as well as the concomitant ATP generation (Table 5).
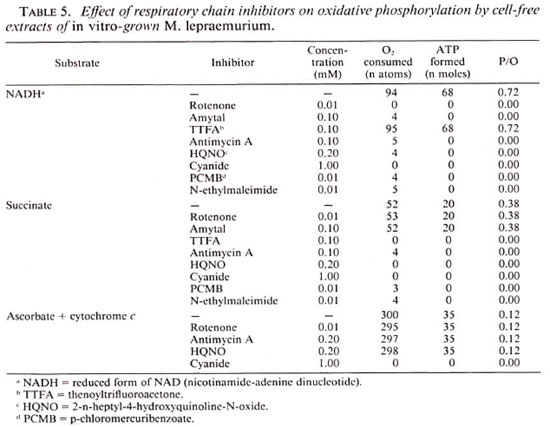
The effect of various inhibitors on the electron transfer reactions when NADH and succinate served as electron donors is shown in Figures 1 and 2, respectively. Cell-free extracts treated with NADH (Fig. 1, trace A) revealed absorption peaks at 562 and 530 nm as well as at 550 and 523 nm which are indicative of alpha and beta peaks of b- and c -type cytochromes, respectively. The gamma peaks of these cytochromes are fused together in a single peak at 428 nm. The absorption peak at 625 nm is of the d type cytochrome. When the cell-free extracts were preincubated with 0.01 mM rotenone for 5 min, no cytochrome was reduced upon addition of NADH (Fig. 1, trace D); whereas in the presence of the same concentrations of rotenone, the cytochromes were completely reduced by succinate (Fig. 2, trace B). On the other hand, 0.1 mM TTFA completely prevented the reduction of respiratory components by succinate (Fig. 2, trace D), but the same system was not affected by TTFA when NADH served as the electron donor (Fig. 1, trace B). When the cell-free extracts containing antimycin A were treated with NADH or succinate, cytochrome b was reduced but cytochrome c remained in the oxidized state (Figs. 1 and 2, trace C).
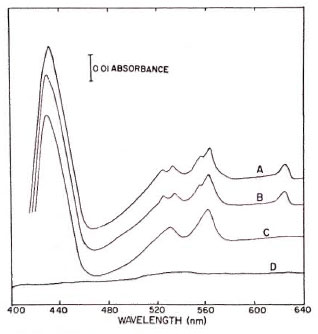
Fig. 1. Effect of different inhibitors on the cyto-chrome system of in vitro-grown . M. lepraemurium reduced with NADH. The reaction mixture in a total volume of 2 ml contained cell-free extracts (3 mg protein) in 500 mM potassium phosphate buffer, pH 6.5.Trace A = NADH reduced minus oxidized difference spectrum; Traces B, C, and D = changes in absorption spectra in presence of 0.1 mM tenoyltrifluoroacetone(TTFA), 0.1 mM antimycin A, and 0.01 mM rotenone, respectively.
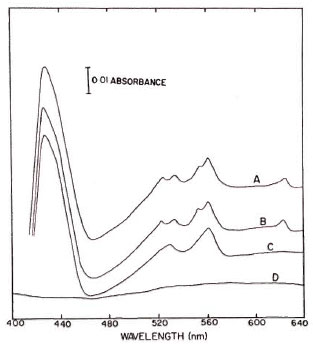
Fig 2. Effect of different inhibitors on cytochromesystem of in vitro -grown M. lepraemurium reduced with succinate. Reaction mixture was the same as in Figure1. Trace A = succinate reduced minus oxidized difference spectrum; Traces B, C, and D = changes in absorption spectra in presence of 0.1 mM rotenone, 0.1mM antimycin A, and 0.1 mM tenoyltrifluoroacetone (TTFA), respectively.
DISCUSSION
Although components of the respiratory chain, mechanisms of electron transport and the energy production processes have been studied extensively in several bacterial systems, relatively very little is known about such systems in leprosy-causing mycobacteria, namely, M. leprae and M. lepraemurium. The reason for this has undoubtedly been the inability to cultivate these mycobacteria in vitro. M. lepraemurium can now be cultivated in vitro and an adequate amount of pure cells can be obtained for metabolic studies using the mass bacterial inoculation technique described by Ishaque(7). It has been shown previously (9) that in vitro -grown M. lepraemurium contained flavoproteins and cytochromes of the b, c, d 2 and o types. It has also been shown (8) that glycerol, succinate, Tween 80, and some sulfhydryl compounds were oxidized by the cell-free extracts prepared from in vitro -grown M. lepraemurium. Energy coupling mechanisms in in vivo -grown M. lepraemurium have previously been reported (10). The results presented in this report show for the first time that cell-free preparations from in vitro -grown M. lepraemurium catalyzed phosphorylation coupled to the oxidation of generated NADH, added NADH, succinate, and ascorbate (plus cytochrome c), yielding P/O ratios of 0.75, 0.52, 0.36 and 0.12, respectively (Table 1). That the phosphate esterification resulted from oxidative phosphorylation is supported by the data which show that the classical uncoupling agents, at concentrations which do not inhibit oxidation, completely uncoupled the process when cither NADH or succinate served as the electron donor (Table 4).
Although the rate of oxidation of added NADH was nearly the same as that obtained by the generated NADH, consistently much lower P/O ratios resulted with exogenously added NADH. Such a phenomenon has also been observed in the in vivo -grown M. lepraemurium (10). Added NADH was possibly partly oxidized by the cell-free extracts of M. lepraemurium through the nonphosphorylative pathway as suggested in M. phlei (2,4,19). Similar results, about NADH oxidation, have been reported in other bacterial systems (5,12) and in mammalian mitochondria (15,16'). The soluble fraction alone failed to carry out ATP synthesis with any substrate. Thus, similar to the in vivo -grown bacilli (10), the generation of high energy phosphate bonds by the in vitro -grown M. lepraemurium required the combined participation of both particulate and soluble components (Table 3).
The localization of the phosphorylation sites in the electron transport chain by the use of respiratory chain inhibitors in conjunction with substrates which enter the chain at different but specific loci indicates the presence of three functional energy-coupling sites in invitro -grown M. lepraemurium. The high sensitivity of phosphorylation coupled to the oxidation of NADH, but not to succinate, to rotenone or to amytal, is an accepted indication of a first energy coupling site. Antimycin A or HQNO, specific inhibitors of the second energy coupling site, are both very effective inhibitors of phosphorylations coupled to NADH or succinate oxidations in this organism (Table 5). Moreover, the presence of the first and second energy coupling sites is also supported by the observed effect of rotenone and antimycin A (Figs. 1 and 2) on cytochrome reduction.
Quiñones or vitamin K have been reported to be involved in the electron transport chain of M. phlei (1) and other bacteria (11,22). The marked inhibitory effect of dicoumarol on NADH and succinate-linked ATP synthesis in the present (Table 4) as well as in the earlier studies (10) indicates the involvement of menaquinone (a vitamin K. derivative) in the electron transport chain of M. lepraemurium.
The electron transport chain of in vitro- grown M. lepraemurium is very similar to the in vivo -grown mycobacterium (10) in being sensitive to inhibitors of the electron transfer reactions and to uncouplers of oxidative phosphorylation. Also as in in vitro- grown M. lepraemurium, the first two energy conservation sites were found to be operative in the in vivo -grown M. lepraemurium. However, of interest is the observation that, contrary to in vivo -grown M. lepraemurium, ATP generation coupled to ascorbate oxidation in the in vitro -grown mycobactcrium occurred in the presence of added cytochrome c (Table 1). The failure of the cell-free preparations from in vivo -grown M. lepraemurium to generate ATP(10) in the presence of added cytochrome c led us to conclude that this mycobactcrium is deficient in the terminal energy coupling site. This variation in the in vivo- versus in vitro -grown cells could possibly be due to the difference in the respiratory components) in the cytochrome c: O2 oxido-reductase region of the electron transport chain. It may be emphasized that cytochrome a+a 3 is present in in vivo -grown M. lepraemurium (13); whereas in vitro -grown cells were found to be deficient in cytochrome a+a 3 and instead contained cytochrome d 2 (9). These findings might have some significance to the fact that a functional third site was detected in the in vitro but not in the in vivo -grown M. lepraemurium. More detailed studies are necessary to explain this variation but it is tempting to speculate that the presence of an d 2-type cytochrome in the in vitro -grown M. lepraemurium may be the reason for the presence of the terminal energy coupling site. The presence of a third phosphorylation site is also supported by the effect of inhibitors (Table 5) on ascorbate oxidation. Cytochrome o has been shown to be present in in vitro-grown M. lepraemurium (9). Whether cytochrome o is involved in energy conservation remains to be investigated.
We have shown previously that a number of sulfhydryl compounds were oxidized by in vivo- (14) as well as by in vitro -grown (8) lepraemurium (10), the oxidations of NADH and succinate as well as the associated phosphorylations catalyzed by the cell-free extracts of in vitro -grown cells were markedly inhibited by the thiol-binding agents PCMB and N-ethylmaleimide (Table 5). These results suggested the involvement of sulfhydryl groups in the enzymatic oxidation of these substrates. The present results obtained by in vivo -grown cells clearly indicate that the results obtained in earlier studies with in vivo -grown M. lepraemurium (10) were indeed due to pure cells, devoid of any host tissues.
Based on experimental observations the probable pathways of electron and energy transfer reactions are presented in Figure 3.
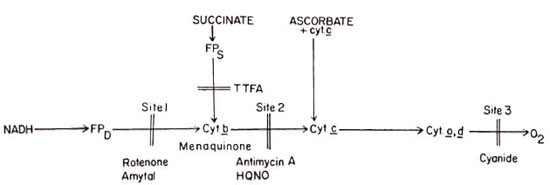
Fig. 3. Proposed pathways of energy and electron transfer reactions in cultivated M. lepraemurium. The electron transport from NADH to O2 involved entire respiratory chain. Electrons derived from oxidation of thesuccinate couple with the respiratory chain mainly at cytochrome c level. The entry of electrons from ascorbateoccurs at cytochrome c level. The third site has been found functional only in the presence of added cytochrome c . TTFA = tenoyltrilluoroacetone; HQNO = 2-n-heptyl-4-hydroxyquinolone-N-oxide.
Acknowledgment. This investigation was generously supported by Le Secours aux Lépreux, Canada, Inc.
REFERENCES
1. ASANO , A. and BRODIE, A. F. Oxidative phosphorylation in fractionated bacterial systems. XVIII. Phosphorylation coupled to different segments of the respiratory chains of Mycobacterium phlei. J. Biol. Chem. 240(1965)4002-4010.
2. BOGIN, E., HIGASHI, T. and BRODIE, A. F. Exogenous NADH oxidation and particulate fumarate reductase in Mycobacterium phlei. Arch. Biochem. Biophys. 129(1969)211-220.
3. Boos, W. Bacterial transport. Ann. Rev. Biochem. 43(1974)123-146.
4. BRODIE, A. F. Oxidative phosphorylation in fractionated bacterial systems. 1. Role of soluble factors. J. Biol. Chem. 234(1959)398-404.
5. GIBSON, J. and MORITA, S. Changes in adenosine nucleotides of intact Chromatium D produced by illumination. J. Bacteriol. 93(1967)1544-1550.
6. HAROLD , F. M. Conservation and transformation of energy by bacterial membranes. Bacteriol. Rev. 36(1972)172-230.
7. ISHAQUE , M. in vitro cultivation of Mycobacterium lepraemurium and its identification by animal inoculation. Can. J. Microbiol. 27(1981)788-794.
8. ISHAQUE , M. Respiratory activities of in vitro- grown Mycobacterium lepraemurium. Int. J. Lepr. 51(1983)64-71.
9. ISHAQUE , M. Cytochrome system in cultivated Mycobacterium lepraemurium. Cytobios 39(1984)165-170.
10. ISHAQUE, M., ADAPOE , C. and KATO, L. Energy coupling mechanisms in host grown Mycobacterium lepraemurium. Can. J. Biochem. 59(1981)75-82.
11. ISHAQUE , M. and ALEEM, M. I. H. Energy coupling in Hydrogenomonas eutropha. Biochim. Biophys. Acta 223(1970)388-397.
12. ISHAQUE , M., DONAWA , A. and ALEEM, M. I. H. Electron transport and energy generation in Pseudomonas saccharophila. Can. J. Biochem. 49(1971)1175-1182.
13. ISHAQUE, M. and KATO, L. The cytochrome system in Mycobacterium lepraemurium. Can. J. Microbiol. 20(1974)943-947.
14. KATO, L., ADAQUE , C. and ISHAQUE , M. The respiratory metabolism of Mycobacterium lepraemurium. Can. J. Microbiol. 22(1976)1293-1299.
15. LEHNINGER , A. L. Phosphorylation coupled to oxidation of dihydrodiphosphopyridine nucleotide. J. Biol. Chem. 190(1951)345-359.
16. LESTER, R. L., ZIEGLER, D. M. and GREEN, D. E. Studies on the mechanism of oxidative phosphorylation. II. Role of bound pyridine nucleotide in phosphorylation. 24(1957)155-160.
17. LOWRY, O. H.. ROSEBROUOH, N. J., FARR, A. L. and RANDALL, R. J. Protein measurement with Folin phenol reagent. J. Biol. Chem. 193(1951)265-275.
18. MORI. T. Biochemical properties of cultivated Mycobacterium lepraemurium. Int. J. Lepr.43(1975)210-217.
19. MURTHY, P. S. and BRODIE, A. F. Oxidative phosphorylation, in fractionated bacterial systems. XV. Reduced nicotinamide adenine dinucleotide phosphate-linked phosphorylation. J. Biol. Chem. 239(1964)4292-4297.
20. OGAWA, T. and MOTOMURA. K. Studies of murine leprosy bacillus. 1. Attempt to cultivate in vitro the Hawaiian strain of Mycobacterium lepraemurium. Kitasato, Arch. Exp. Med. 43(1970)21-36.
21. PATTYN, S. R. and PORTAELS, F. in vitro cultivation and characterization of Mycobacterium lepraemurium. Int. J. Lepr. 48(1980)7-14.
22. SCHOLES, P. B. and SMITH. L. Composition and properties of membrane-bound respiratory chain system of Micrococcus denitrificans. Biochem. Biophys. Acta 153(1968)363-375.
23. STEFANSKY, W. K. Eine lepra-ähnliche Erkrankung der Haut und der lymphdrusen bei Wanderratten. Zentrallel. Bakteriol. parasitenkd. Infektionskr. Hyg. Abt. 1: Orig. 33(1903)481-487.
24. STREHLER, B. M. Adenosine-5'-triphosphate and creatine phosphate; determination with luciferasc. In: methods of Enzymatic Analysis. Bergmeyer, H. U., ed. New York: Academic Press, 1965, p. 563.
1. Ph.D., Associate Professor, Applied Microbiology Research Center, Institut Armand-Frappier. Université du Québec, Case Postale 100, Laval. Québec, Canada H7V IB7.
Received for publication on 22 February 1991.
Accepted for publication in revised form on 9 October 1991.