- Volume 60 , Number 1
- Page: 71–80
Reflections on the elimination of leprosy
Editorial opinions expressed are those of the writers.
The past decade has witnessed the most dramatic changes in leprosy control since the introduction of dapsone 40 years ago. Research findings, and the implementation of multiple drug regimens, have had farreaching effects on the definitions of leprosy and on the structure and strategy of leprosy control programs. Enthusiasm over the implications of these developments recently led the World Health Assembly to endorse a plan for the "global elimination of leprosy as a public health problem by the year 2000."
What follows are some reflections on how these important developments relate to trends in the perception and the reality of leprosy in the world today. The major issues are discussed under four headings; case definitions, trend analyses, natural history, and the elimination goal.
Case definitions -What is leprosy?
Case definitions of leprosy have evolved in recent years with major implications for carrying out and interpreting research and control. The evolution has occurred at three levels: diagnostic criteria, classification criteria, and administrative criteria.
Diagnostic criteria. The diagnosis of leprosy has sometimes been discussed in dogmatic terms. For example, the 1980 edition of the World Health Organization's (WHO) "A Guide to Leprosy Control" stated: "If there is one diagnosis that should not be established unless there is absolute certainty, it is that of leprosy."' which was later changed to: "The diagnosis should be established after a detailed clinical examination, and only when the signs and symptoms are clear and unequivocal."2
This "absolutely certain" or "clear and unequivocal" diagnosis of leprosy is based upon an assessment of cardinal signs: anesthesia, thickened nerves, typical skin lesions, and acid-fast bacilli (AFB) in the skin. At least two of the first three, or the fourth, are generally required for the diagnosis to be made.
The leprosy research and control communities have shown an increasing appreciation for the difficulty of this diagnostic task. This reflects several things. First, expansion of primary health care and leprosy control activities in endemic communities has meant that an increasing proportion of cases are detected early, when signs are less clear than with advanced disease. Second, there have been increasing efforts in recent years to identify infection with Mycobacterium leprae, as distinct from just overt clinical disease, my making use of various immunological tests. This has encouraged discussion over whether the term "leprosy" implies just infection with M. leprae or whether it should be restricted only to clinical disease attributable to the specific infection, thus further confusing the definition issue. Third, there has been increasing appreciation among leprosy researchers of the statistical implications of misclassification on results of various investigations-for example, the recognition that nonspecific diagnoses (false positives) will lead to underestimates of protective effects of vaccines.3,4 Fourth, a series of published investigations has demonstrated that field workers vary in their interpretations of lesions,5 and that even experienced histopathologists may disagree over whether biopsies reflect leprosy or some other condition.6
There have been varied responses to this increasing concern over basic diagnostic criteria. Some researchers have accepted and tried to quantify the uncertainty inherent in the diagnosis of leprosy. This has involved efforts to measure the sensitivity and specificity of diagnoses4,5 and the attachment of certainty levels to cases included in research studies.7 There are no simple universal rules for dealing with such uncertainty, in particular since diagnostic sensitivity and specificity are often inversely related. It may be important to maximize sensitivity (recognition of all "true" cases) in control programs if there is a compelling need to treat early in order to prevent disability. Or it may be important to maximize specificity (exclusion of all noncases) in control programs if disease stigma is important in a community, or in research programs in order to avoid diluting out the effect of risk factors under investigation.
An appreciable proportion of registered leprosy cases in the world are never seen by a medical officer, let alone biopsied. We must appreciate the implications of the diagnostic difficulties under such circumstances.
Classification. Leprologists love to classify. The spectrum of clinical leprosy lends itself to a basic urge to categorize. Thus, we have a long history of discussion over the various Madrid, Indian, and Ridley-Jopling classifications, and over such issues as whether "indeterminate" leprosy is a classification or is even leprosy at all. Since the early 1980s there has been a tendency to simplify leprosy classification into a twogroup system reflecting two basic drug regimens recommended for leprosy control programs. The criteria for even this simple dichotomy into multibacillary and paucibacillary patients have evolved. It got off to a bad start, with a logical inconsistency in the initial publication, multibacillary cases being defined (implicitly) as all individuals with a bacterial index (BI) equal to or greater than 2 at any site and paucibacillary being explicitly defined as all individuals with a BI of less than 2 "at any site"!8 The logic was corrected 3 years later,9 but then, after another 3 years, the criterion itself was reduced to a distinction between smear-negative and smear-positive status.10 We may understand and even sympathize with this evolution; but we must appreciate the confusion engendered by logical fallacies and shifting criteria, let alone an increasing concern that the routine smear services upon which the classification dichotomy is based are often not of sufficient standard to give reliable results.11 Such changes pose considerable problems for efforts to compare disease types or treatment effects between populations or over time.
Administrative criteria. As leprologists love to classify, so we love to collect statistics. This is necessary for research, for fund raising, for monitoring, and for the evaluation of control programs. The past decade has witnessed numerous proposals from WHO, ILEP, OMSLEP, national programs, and research projects recommending various forms for and presentations of statistical data.1,2,9,12,13 In the enthusiasm to collect figures there may have been a danger of forgetting that if the primary data are poor, then no amount of statistical manipulation can compensate for that-quite the opposite, since there may be a tendency to put undue faith in manipulated statistics merely because of impressive packaging.
There are other even more obvious issues at stake relating to routine leprosy statistics. It may be argued that the most important decision promulgated by the famous 1981 WHO Study Group was not to recommend multiple drug combinations, but was to shorten the duration of treatment.8 This was to revolutionize not only the administration of leprosy treatment but also the perception of leprosy as a public health problem. It led to the discharge of very large numbers of individuals who had long been labeled as leprosy patients and who were on leprosy registers throughout the world. The effects were dramatic and far reaching. In Malawi, for example, the number of registered leprosy patients fell by 90%, from 18,862 to 1773, over the years 1980 to 1990 (Fig. 1). Similar changes were reported in many other countries. But they have been misinterprctcd in some quarters. Yes, the fall in numbers of registered cases reflected an improvement in the strategy of leprosy control. But a large proportion of patients discharged in the 1980s as a consequence of the new short duration treatment policies had already been effectively cured and were on registers only because there had been no appropriate discharge policy beforehand. The rapid fall in registered patients is thus largely an artifact, representing a fall in administrative burden but not necessarily a fall in morbidity incidence-or indeed true morbidity prevalence -in leprosy-endemic populations.
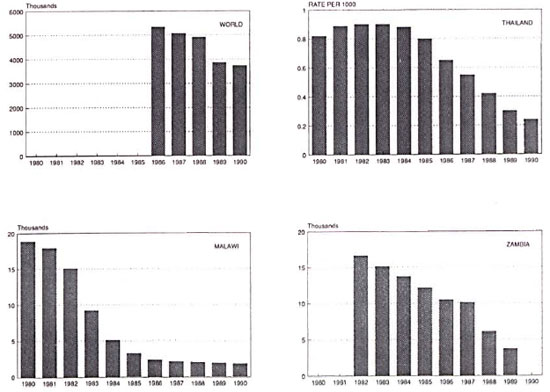
Fig. 1. Prevalence of registered leprosy cases in the world,14 Thailand,15 Malawi,16 and Zambia,17 1980-1990.
Although undoubtedly an improvement in our approach to leprosy control, the shift to short duration regimens has had some ironic, even unfortunate, effects. Advertised widely as a major advance in control of the disease, it may have led to decreased funding in some contexts, for example, to the leprosy component of the WHO TDR program. It would indeed be unfortunate if the statistical effects of a change in administrative case definition were to be interpreted - or misinterpreted -as evidence of total victory! Leprosy is not dead yet! And the change in statistical criteria has made it difficult to see if it is even dying, let alone to measure the speed of its demise.
Leprosy trends
Several major reports have appeared recently describing trends of leprosy in the world.14,18,19 Most of the data presented in these reports are prevalence figures -not prevalence of disease but prevalence of individuals registered for treatment in various programs. The reason for this is obvious, such figures are the most readily available statistics. Declining numbers of registered patients have been reported for most countries and regions of the world over the past several years (Fig. 1) with a few exceptions, such as Brazil.20 As discussed above, these declines are at least in part an artifact of changing administrative case definitions. But the complexity goes deeper than that.
Several recent publications attribute these changes to MDT. This is implicit, for example, in the recent issue of the World Health Statistics Quarterly which is entitled "Progress in Leprosy Control through Multidrug Therapy." Insofar as the declining numbers of registered patients documented in this report reflect the early discharge policy associated with MDT, this is correct. But insofar as much of the decline is an artifact reflecting discharge of cured patients, the implication may be misleading.
Long-term progress in leprosy control should be reflected in declining incidence of disease. Such data are less readily obtained than are prevalence statistics, but some are available. Accepting appropriate caveats about problems of routine data, and the fact that such trends reflect changes in case-finding policies and case definition as well as changes in disease incidence, dramatic declines in leprosy incidence (or "case detection rates") have been documented in many countries of the world for more than a decade. This evidence comes not only from wealthier nations such as Norway,21 Japan,22 and Portugal,23 but from many less wealthy nations as well, such as China,24 Thailand,15 Mexico,25 Venezuela,26 Malawi,16 and Zambia,17 (Fig. 2). Brazil again appears as a prominent exception, for reasons which I do not understand.20
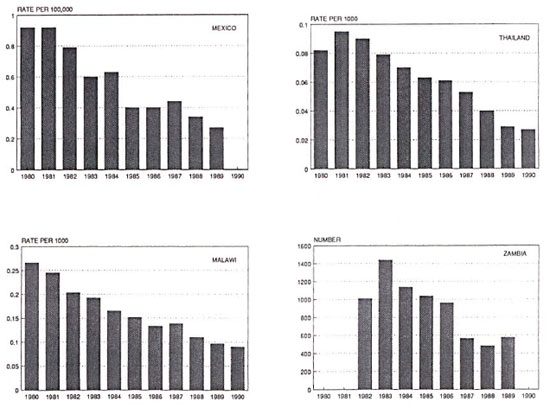
Fig. 2. Trends in leprosy incidence (case detection) in Mexico,25 Thailand,15 Malawi,16 and Zambia,17 1980-1990.
What underlies these declines? It is doubtful that case finding and drug treatment have been responsible for much, if any, of them. The declines certainly began in most countries long before the advent of MDT. In fact there is little, if any, evidence that treatment of leprosy cases has ever been responsible for declines in incidence in any country of the world. On the other hand, we are all aware of evidence that leprosy incidence declines with improving socioeconomic standards-although whether the responsible factor is soap, or nutrition, or living conditions, or crowding, or clothing, or intercurrent infections, no one knows.9,27 Surely the recent secular declines in incidence are due at least in part to these illdefined but indisputably important risk factors.
In addition there is BCG, easily identifiable as a major factor underlying reduced leprosy incidence in many countries. The leprosy community has long been schizophrenic on the subject of BCG. The facts are simple. First, more people have received BCG than have received any other vaccine over the past 30 years-over 2 billion doses administered. Second, everywhere that it has been studied it has been found that BCG imparts some protection against leprosy. The protection appears to be relatively modest in some contexts, e.g., Burma, but quite high in others, e.g., Eastern Africa.28-32 The implication of these two facts is simple-BCG must be having an impact on leprosy worldwide and is undoubtedly responsible, at least in part, for the declines in leprosy incidence observed in many populations. It is probably no coincidence that the highest protective efficacy estimates for BCG against leprosy have come from studies in Africa (e.g., 80% in Uganda) and that some of the greatest recent declines in incidence have also been reported from that continent.
Closely related to the issue of BCG and its impact on leprosy trends is the question of its effectiveness against multibacillary disease. This is important insofar as multibacillary disease is the more important clinically, and thus there is a special premium attached to its prevention. In addition, multibacillary cases may be of importance as sources of infection in the community, and thus their prevention could have the additional effect of reducing transmission, and hence overall incidence of infection in the community.27 There is a widespread conception, or misconception, that BCG is not effective in protecting against multibacillary disease. This has three origins. First is a widely held view that Lepromatous disease occurs in individuals who are somehow inherently immunologically defective, hence, by implication, they should not be able to respond to BCG. But this is just an hypothesis; whereas in fact we understand little about the nature of immunity in leprosy and as yet have very little idea why some individuals contract multibacillary disease and others contract paucibacillary disease.
Second, there is a mistaken view that the four famous controlled trials of BCG against leprosy failed to find protection against multibacillary disease. This opinion, traceable in its origin to early results of the vaccine trials when they had too few multibacillary cases to analyze, is inconsistent with the published data. In fact, the most up-todate published evidence from the three trials which accumulated sufficient cases for analysis (the Uganda trial being the exception, with only a single incident lepromatous case -in a vaccinated individual!) has shown no differences between the protection imparted against multibacillary as distinct from paucibacillary disease (The Table). (Recent unpublished data from the South India trial raise questions about the published results on this aspect of that trial. It is difficult to judge findings in the absence of full published results, however, and this once again highlights the necessity for formal publication of the valuable data inherent in the South India vaccine trial experience).
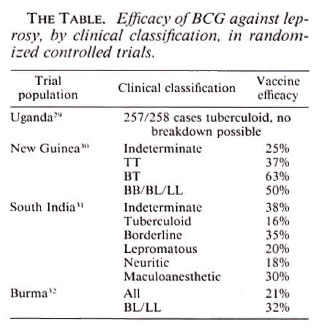
The third line of evidence is represented by two small observational studies (reported only as letters) which claimed to show protection against paucibacillary but not multibacillary disease.33,34 On the other hand, a case-control study recently reported from South India found higher protection by BCG against borderline and lepromatous than against tuberculoid disease.35 And recent analyses of data from Malawi show that routine BCG vaccination has provided approximately 54% protection against paucibacillary disease and 84% protection against multibacillary disease in that country. The confidence limits are broad, in particular due to the small numbers of multibacillary cases, but there is at least no evidence that protection against multibacillary disease is any less than against paucibacillary disease.36
Taken all together, the available evidence demonstrates that BCG provides some protection against multibacillary, as well as against paucibacillary, disease in most populations of the world. This may be mediated, or complicated, by a tendency for BCG to induce some cell-mediated immunity, sufficient to shift potentially lepromatous individuals toward the paucibacillary pole. Whatever the mechanism, it means that BCG is not only reducing leprosy disease incidence overall, but it is also reducing the numbers of sources of infection in the community and, hence, presumably the incidence of infection. These effects have important implications, not only for the practice of leprosy control but also for the theoretical basis of research on vaccines against leprosy. This said, it is interesting that the recent World Health Statistics Quarterly volume on leprosy trends in the world should make not a single mention of BCG. We may suspect this is because BCG has traditionally been considered the provenance and property of tuberculosis programs, and leprologists find it hard to accept that tuberculosis control could be or should be having an impact on leprosy. But it is. And it will continue to do so-until the leprosy community wakes up to the facts and recognizes that current BCG programs, paid for by national health ministries, are not just tuberculosis control programs, they are leprosy control programs too. Why not take some credit for them?
Another reason for the neglect of BCG in leprosy institutions today is the strong lobby supporting MDT as a mainstay of leprosy control -a lobby whose crusading zeal has difficulty in accepting that a vaccine may also be having immense effect in controlling leprosy.37 Crusading zeal as such is wonderful, but when it leads to neglect or distortion of facts it may be dangerous. If we wish really to understand leprosy trends in the world today, BCG must be high on our agenda list of determinants. BCG is not everything, and it is certainly not perfect, but it is there. Information on BCG coverage must be included in any serious effort to explain leprosy patterns by age, space, or time.
While appreciating the role of BCG on leprosy today, we should not neglect its idiosyncrasies, in particular the fact that its effects differ between populations for reasons we do not understand.28,38 It is unfortunate that the effectiveness of BCG appears to be less in India than in Africa, given that there is much more leprosy in India than in Africa and, hence, an effective preventive measure is especially needed there. And what about Brazil? Why is leprosy apparently increasing there, in spite of socioeconomic improvements as well as widespread use of BCG?
Natural history- the infection and the disease
Some of us had high hopes a decade ago that we were about to solve many of the fundamental riddles of leprosy. This was going to happen because of advances in basic immunology, and the availability of armadillo-derived M. leprae, which were to provide us with tests for infection with the leprosy bacillus. We were going to be able to study the pattern of infection in endemic communities, to identify infected individuals, and to learn reasons why some individuals became infected and others did not, and why some infected individuals became diseased and others did not. To me, the great leprosy disappointment of the 1980s is that these magic tools never materialized, and that we are still abysmally ignorant of the fundamental natural history of the leprosy bacillus.
With no means of knowing who is infected, epidemiological studies remain based on overt clinical disease. The list of risk factors has not changed over the decade: age, sex, BCG, household contact, genetics. Important data sets have been assembled in Chingleput/South India, Karonga District/ Malawi, and in Venezuela; but these have yet to be analyzed thoroughly, let alone published. We can at least hope that new insights will emerge from these data. In addition there is a new factor in the equation . . .
HIV. The most important event affecting human health over the past decade has undoubtedly been the advent and spread of HIV infection and AIDS. Leprologists may note analogies between AIDS and leprosy, not in transmission but in the long incubation period and associated social stigma. Because of the effect of HIV virus on cell mediated immunity, some authors have predicted that the HIV epidemic might have dire consequences for leprosy, that HIV-infected individuals might be at increased risk of leprosy, in particular of multibacillary disease, and that these effects could lead to increases in leprosy incidence in HlV-endemic countries.39,40 Interestingly -and fortunately-enough, there is as yet little or no evidence for any such effect, despite the co-existence of the two infections in large populations, particularly in Africa. The only evidence favoring an association, in addition to a few case reports, comes from a study in Zambia reporting an association between HIV and leprosy patients seen in a hospital clinic.41 But these patients had relatively severe and complicated disease, and were thus not representative of all leprosy cases. A much larger and carefully controlled study has now been carried out in Malawi, and failed to find any association between HIV infection and incident leprosy.42 It is interesting that the Malawi study found that leprosy cases who had recently arrived in the study area were more likely to be HIV positive than were the resident cases. This undoubtedly reflected higher HIV prevalence rates in surrounding populations and the tendency for people to return home when they become ill-and it points to the need for extreme care in epidemiological studies of this association, to control for social factors (in this case, migration) which could lead to apparent but spurious associations. Although negative results are always difficult to interpret, the continued absence of evidence-even anecdotes-from Africa indicating any change in leprosy in conjunction with the very high coincident prevalence of both HIV and leprosy-in contrast to the many reports of devastating increases in tuberculosis attributable to HIV infection -suggests that the immunology of leprosy is once again not as we thought it would be. This may be an example of a situation in which a negative or absent association is more interesting than a positive, insofar as it may reflect an important fundamental difference between the immunological mechanisms underlying leprosy and tuberculosis. Here is an example of an epidemiological finding which may provide an important clue to laboratory immunologists working on these three important diseases: leprosy, tuberculosis, and AIDS.
The elimination initiative
Only weeks ago, the 44th World Health Assembly approved a statement committing WHO "to attain the global elimination of leprosy as a public health problem by the year 2000." That is a momentous statement. It is a political statement, but it has important scientific implications which all leprosy workers-and patients-are going to have to live with over the coming years.
The elimination commitment bears close examination. For its meaning one must look to a footnote of the resolution, which reads: "Elimination of leprosy as a public health problem is defined as the reduction of prevalence to a level below one case per 10,000 population." This encourages four comments.
First, we note that the elimination criterion is phrased in terms of prevalence, not incidence. Just what this prevalence is of, is not stated, although we may assume this means the prevalence of persons "having clinical signs of leprosy . . . and requiring chemotherapy," as recommended in the latest (1988) edition of the WHO "Guide to Leprosy Control."2 As noted above, the great fall in leprosy prevalence in recent years has come from shortening the recommended period of treatment. But some may find it misleading to speak of the "elimination" of leprosy without requiring that the incidence, the numbers of new cases, are reduced, preferably to zero. Unless incidence is reduced, all the problems of case finding, diagnosis, and registration remain unchanged. We may note that the easiest way to reduce the prevalence of cases on treatment is, aside from just not putting them on treatment in the first place, to shorten their course of treatment. If some new drug were recommended as a one-month course (as has been suggested of ofloxacin), the "prevalence" would be reduced by more than sixfold, but some might consider it misleading to call this "elimination." Control, yes. Elimination, no.
Secondly, we note that the "global elimination" target is phrased as "below one case per 10,000 population." We may ask what population is implied. From the context of the World Health Assembly resolution, and the accompanying press release, it appears that the reference is to the total global population. This is a strange denominator to use for a disease which is very unevenly distributed throughout the world. According to the WHO press release, the current world prevalence rate of registered cases is 7/10,000. Thus the goal would seem to imply a sevenfold reduction overall (actually somewhat more, as unregistered cases should be considered in the numerator). But would a sevenfold decrease in India mean elimination in that country? Would it mean elimination from the world -but not from India? There will be much number-dancing over the next decade, as different people try to interpret the target and as different organizations twist it in various ways in order to suit their own publicity needs.
Thirdly, it is notable how strongly the World Health Assembly resolution emphasizes multiple drug therapy as the key to the elimination strategy. The phrase "multiple drug therapy" appears no less than six times in the resolution. This emphasis is now familiar, continuing a publicity effort which has dominated the leprosy world since 1982. To question this publicity effort should not be interpreted as a denial that multiple drug theragy is good (if it can be afforded) or that short-term regimens are good. But dogma and inflexibility can be dangerous. They lead to falsification and blindness. If elimination is to be meaningful, it must entail a reduction in leprosy incidence and, as noted above, we have no evidence that MDT has or will have any appreciable impact on incidence at all. Beyond that, we have every evidence that socioeconomic improvements and BCG-despite the fact that they are not mentioned even once in the World Health Assembly resolution-are having a sizable impact on incidence in most countries. We should not forget this, since it opens up a variety of approaches to leprosy control beyond case finding and treatment alone. If elimination is to be achieved, it will not be by MDT alone.
Finally, a word on the overall implication of the elimination goal. Elimination sounds wonderful. After the success of global smallpox eradication, there has been a spate of elimination and eradication goals-polio, measles, guinea worm. But we should not neglect the deeper lesson of the smallpox program -that goals should be ambitious, but achievable. If they are not, they may carry more trouble than they are worth.43 We hope that leprosy will be eliminated by the year 2000. But we may note the challenging position in which many leprosy workers now find themselves -cither they are failures by the year 2000, or they're out of a job, or both!
- Paul E. M. Fine, V.M.D., Ph.D.
Communicable Disease Epidemiology Unit
Department of Epidemiology and Population Sciences
London School of Hygiene and Tropical Medicine
Keppel Street
London We1E 7HT, England
Acknowledgment. This paper is adapted from a presentation at the World Health Organization/Sasakawa Memorial Health Foundation International Meeting on Epidemiology of Leprosy in Relation to Control, Jakarta, Indonesia, 17-21 June 1991. The author is indebted to Helen Berrett for preparation of the manuscript.
1. World Health Organization. "A Guide to Leprosy Control." Geneva: World Health Organization, 1980.
2. World Health Organization. "A Guide to Leprosy Control." 2nd ed. Geneva: World Health Organization, 1988.
3. Rothman, K. J. Modern Epidemiology. Boston: Little Brown and Co., 1986.
4. Ponnighaus, J. M. and Fine, P. E. M. Sensitivity and specificity of the diagnosis and the search for risk factors from leprosy. Trans. R. Soc. Trop. Med. Hyg. 82(1988)803-809.
5. Gupte, M. D., Valli Shayee, R. S., Nagaraju, B., Ramalingam, A., Lourdusamy, G. and Kannan. S. Inter-observer agreement and clinical diagnosis of leprosy for prophylaxis studies. Indian J. Lepr. 62(1990)281-295.
6. Fine, P. E. M., Job, C. K., McDougall, A. C, Meyers, W. M. and Pönnighaus, J. M. Comparability among histopathologists in the diagnosis and classification of lesions suspected of leprosy in Malawi. Int. J. Lepr. 54(1986)614-625.
7. Pönnighaus, J. M., Fine, P. E. M. and Bliss, L. Certainty levels in the diagnosis of leprosy. Int. J. Lepr. 55(1987)454-462.
8. WHO Study Group. Chemotherapy of leprosy for control programmes. Geneva: World Health Organization, 1982. Tech. Rep. Ser. 675.
9. WHO Study Group. Epidemiology of leprosy in relation to control. Geneva: World Health Organization, 1985. Tech. Rep. Ser. 716.
10. WHO Expert Committee on Leprosy. Sixth report. Geneva: World Health Organization, 1988. Tech. Rep. Ser. 768.
11. Georgiev, G. D. and McDougall, A. C. The bacteriological examination of slit-skin smears in leprosy control programmes using multi drug therapy: a plea for radical changes in current operational methodology. Indian J. Lepr. 59(1987)373-385.
12. International Federation of Anti-Leprosy Associations. ILEP Statistics 1989. London: ILEP.
13. Declercq, E., Gelin, C. and Lechat, M. F. Global evaluation of the introduction of multidrug therapy. Lepr. Epid. Bull. 6(1991)1-58.
14. Noordeen, S. K. A look at world leprosy. Lepr. Rev. 62(1991)72-86.
15. Piravaraporn, C. and Peerapakorn, S. Leprosy control in Thailand. In: "Leprosy profiles with special attention to MDT implementation." Tokyo: Sasakawa Memorial Health Foundation. 1991.
16. Data courtesy of British Leprosy Relief Association (LEPRA) and Dr. Gjalt Boerrigter.
17. Steenbergen, G. J. Leprosy control in Zambia. World Health Stat. Q. 44(1991)30-35.
18. Various authors. Progress in leprosy control through multidrug therapy. World Health Stat.' Q. 44(1991)1-46.
19. Various authors. "Leprosy profiles with special attention to MDT implementation." Tokyo: Sasakawa Memorial Health Foundation. 1991.
20. Penna. G. O. and Pereira, G. F. M. Leprosy control in Brazil. In: "Leprosy profiles with special attention to MDT implementation." Tokyo: Sasakawa Memorial Health Foundation, 1991.
21. Irgens, L. M. Leprosy in Norway. Lepr. Rev. 51 Suppl.(1980)1-130.
22. Ito, T. The epidemiological situation in South East Asia. Lepr. Rev. 52 Suppl. 1(1981)43-51.
23. Irgens, L. M., Melo Caciro, F. and Lcchat, M. F. Leprosy in Portugal 1946-1980: epidemiological patterns observed during declining incidence rates. Lepr. Rev. 61(1990)32-49.
24. Li. H.-L., Pan, Y.-L. and Wang, Y. Leprosy control in Shandong Province, China, 1955-1983; some epidemiological features. Int. J. Lepr. 53(1985)79-85.
25. Dominguez, J. R. and Garcia, F. C. Leprosy control programme in Mexico. In: "Leprosy profiles with special attention to MDT implementation." Tokyo: Sasakawa Memorial Health Foundation, 1991.
26. Zuniga, M. and Hernando, F. Modificaciones de la distribución urbano-rural de la lepra en Venezuela 1949-1979. Unpublished typescript, 1981.
27. Fine, P. E. M. Leprosy-the epidemiology of a slow bacterium. Epidemiol. Rev. 4(1982)161-188.
28. Fine, P. E. M. and Rodrigues, L. C. Mycobacterial vaccines. Lancet 1(1990)1016-1020.
29. Stanley, S. J., Howland. L., Stone, M. M. and Sutherland, I. BCG vaccination of children against leprosy in Uganda-final results. J. Hyg. (Camb.) 87(1981)233-248.
30. Bagshawe. A., Scott, G. C. Russell, D. A., Wigley, S. C, Merianos, A. and Berry, G. BCG vaccination in leprosy: final results of the trial in Karimui. Papua New Guinea. Bull. WHO 67(1989)389-399.
31. Tripathy, S. P. The case for BCG. Ann. Natl. Acad. Med. Sci. India 19(1983)11-21.
32. Lwin, K., Sundarsan, T., Gyi, M. M., Bechelli, L. M., Tamondong, C, Garbajosa. P. G.. Sansarricq, H. and Noordeen, S. K. BCG vaccination of children against leprosy: fourteen-year findings of the trial in Burma. Bull. WHO 63(1985)1069-1078.
33. Abel. L., Cua, V. V., Oberti, J., Lap, V. D., Due, L. K., Grosset, J. and Lagrange P. N. Leprosy and BCG in southern Vietnam. (Letter) Lancet 1(1990)1536.
34. Kaklamani, E., Koumondaki, Y., Katsouyanni, K. and Trichopoulos, D. BCG, tuberculosis, and leprosy. (Letter) Lancet 1(1991)304.
35. Muliyil. J.. Nelson, K. E. and Diamond, E. L. Effect of BCG on the risk of leprosy in an endemic area: a case control study. Int. J. Lepr. 59(1991)229-236.
36. Pönnighaus, J. M., Fine, P. E. M., Sterne, J. A. C, Wilson, R., Msosa, E., Gruer, P., Jenkins. P. A., Lucas, S. B., Liomba, G. and Bliss, L. BCG imparts greater protection against leprosy than against tuberculosis in northern Malawi (submitted for publication).
37. Ellard, G. A. The chemotherapy of leprosy. Part I. Int. J. Lepr. 58(1990)704-716.
38. Fine, P. E. M. The BCG story': lessons from the past, implications for the future. Rev. Infect. Dis. 11(1989)353-359.
39. Turk, J. L. and Rees. R. J. W. AIDS and leprosy. Lepr. Rev. 59(1988)193-194.
40. Baskin, G. B., Gormus, F. J., Martin, L. N., Murphy-Corb, M., Walsh, G. P. and Meyers. W. M. Pathology of dual Mycobacterium leprae and simian immunodeficiency virus infection in rhesus monkevs. Int. J. Lepr. 58(1990)356-364.
41. Meeran, K. Prevalence of HIV infection among patients with leprosv and tuberculosis in rural Zambia. Br. Med. J. 298(1989)364-365.
42. Pönnighaus, J. M., Mwanjasi, L. J., Fine, P. E. M., Shaw, M. A., Turner, A. C, Oxborrow, S. M., Lucas, S. B., Jenkins, P. A., Sterne, J. A. C. and Bliss, L. Is HIV infection risk factor for leprosy? Int. J. Lepr. 59(1991)221-228.
43. Henderson, D. A. Global measles eradication. Lancet 2(1982)208.