- Volume 59 , Number 2
- Page: 221–8
Is HIV infection a risk factor for leprosy?
ABSTRACT
A case control study was undertaken during 1988 and 1989 within the framework of the LEPRA Evaluation Project (LEP)/Ka-ronga Prevention Trial (KPT) in Karonga District, northern Malawi, to investigate whether HIV infection is a risk factor for clinical leprosy. Cases were newly ascertained, biopsy-confirmed, incident leprosy patients older than 14 years of age. Controls were selected f rom the computer data base on over 170,000 people who form the basis of LEP/KPT. They were matched for sex, age, and area of residence. HIV seroposi-tivity rates were 1.8% (2/112) for incident leprosy cases and 2.4% (24/1011) for controls. The Mantel Haenszel odds ratio is 0.6 (95% confidence interval 0.1-3.3). Thus, no evidence for an association between HIV infection and leprosy incidence has been observed in this population. In a parallel investigation, an odds ratio of 7.4 (95% confidence interval 3.3-16.7) was found for 102 microscopy- and/or culture-confirmed, incident pulmonary tuberculosis cases in the same population during 1989, a result similar to those obtained elsewhere in Africa. Among leprosy relapses, 16.7% (2/12) were HIV positive.RÉSUMÉ
Une élude cas-témoins a été entreprise en 1988 et 1989 dans le cadre du "Lepra Evaluation Project (LEP/ Karonga Prevention Trial (KPT)," dans le district de Karonga, dans le Nord du Malawi, pour étudier si l'infection VIH est un facteur de risque pour la lèpre clinique. Les cas étaient des patients lépreux nouvellement découverts et confirmés par biopsie, âgés de plus de 14 ans. Les témoins ont été sélectionnés à partir de la base de données informatisées concernant plus de 170.000 personnes qui forment la base de LEP/KPT. Ils ont été appariés pour le sexe, l'âge, et le lieu de résidence. Les taux de séropositivité VIH étaient de 1.8% (2/112) pour les nouveaux cas de lèpre et 2.4% (24/1011) parmi les témoins. L'odds ratio selon la méthode de Mantel Hanszel est de 0.6 (intervalles de confiance à 95%: 0.1-3.3). En conséquence, on n'a observé aucun signe d'association entre l'infection VIH et l'incidence de la lèpre dans cette population. Au cours d'une recherche parallèle, un odds ratio de 7.4 (intervalle de confiance à 95%: 3.3-16.7) fut trouvé parmi 102 nouveaux cas de tuberculose pulmonaire confirmés par microscopic et/ou culture dans la même population en 1989, un résultat similaire à ceux obtenus ailleurs en Afrique. Parmi les récidives de lèpre, 16.7% (2/12) étaient positifs pour le VIH.RESUMEN
Durante 1988 y 1989 se efectuó un estudio de control de casos en el Distrito de Karonga en Malawi del norte para investigar si la infección por el virus de la in-munodeficiencia humana (H1V) es un factor de riesgo para la lepra cínica, el estudio se realizó dentro del marco del Proyecto de Evaluación de LEPRA (LEP)/ Ensayo de Prevención de Karonga (KPT). Los pacientes fueron casos nuevos, mayores de 14 años de edad, con lepra confirmada por biopsia. Los controles, apareados en cuanto a sexo, edad y área de residencia, se seleccionaron a partir de un banco de datos de 170,000 gentes del LEP/KPT. Los grados de seropositividad para HIV fueron del 1.8% (2/112) para los casos de lepra incidente y de 2.4% (24/1011) para los controles. La relación entre grupos de Mantel Haenszel fue de 0.6 (95% de confianza en el intervalo 0.1-3.3). Así, no se observó ninguna asociación entre la infección por HIV y la incidencia de lepra en esta población. En un estudio paralelo durante 1988, se encontró una relación de 7.4 (95% de confianza en el intervalo 3.3-16.7) para 102 casos nuevos de tuberculosis confirmada por microscopía o cultivo, en la misma población, un resultado similar a los obtenidos en Africa. Entre los casos recurrentes de lepra, 16.7% (2/12) fueron HIV positivos.That the human immunodeficiency virus (HIV) has important implications for many mycobacterial infections is well recognized. There is ample evidence that HIV infection constitutes a major risk factor for clinical tuberculosis and for severe illness associated with certain opportunistic nontuber-culous mycobacteria, such as Mycobacterium avium-intracellulare (4,9,17). That HIV might also constitute a risk factor for leprosy has been suggested by several authors (8,19). The question is of particular importance given the high and increasing prevalence of HIV infection in many leprosy-endemic populations, and the possibility that HIV-induced immunosuppression might increase the prevalence of multibacillary forms of leprosy, which are presumed to be the major sources of infection, and hence lead to increased transmission of M. leprae. Evidence to date is fragmentary. There is a published report of two relapses among HIV-infected leprosy patients (10), and a congress abstract indicating that relapses were more common among HIV-positive than among HIV-negative leprosy cases in Haiti (12). More recently, a small study at a hospital in Zambia found HIV seropositivity to be more frequent among 18 leprosy patients than among 63 blood donor controls (11). There are no published reports of an increase in multibacillary disease in association with HIV infection.
The question of the relationship between HIV infection and leprosy has assumed major importance in the context of an ongoing leprosy vaccine trial in northern Malawi (7). It is recognized that HIV infection is present in the trial population, and its prevalence is presumably rising. Interpretation of the results of the trial will inevitably require information on HIV in the population, insofar as the background susceptibility to leprosy, or any effects of the vaccines, might be influenced by HIV infections. For this reason, an investigation of the association between HIV infection and leprosy was initiated in the trial population in early 1988, with approval of the Malawi Ministry of Health. We report here the findings of the first 2 years of this study.
MATERIALS AND METHODS
The general methods of the LEPRA Evaluation Project (LEP) and Karonga Prevention Trial (KPT) have been reported in detail elsewhere (14). For the purposes of this presentation, we note that the study has involved active surveillance of the total population living in the Karonga District, a rural area in northern Malawi. Approximately 150,000 persons were interviewed and examined for leprosy between 1986 and 1989 as part of the recruitment phase of the vaccine trial. Demographic information collected on all individuals included years of birth, sex, and location of household, both as map coordinates to nearest 10 meters and by ecological zone within the district-i.e., northern hills, northern lakeshore, southern hills, southern lakeshore or semi-urban (in the district capital, a town of 10,000 people). All individuals were examined initially by trained paramedical staff, and all leprosy suspects were reviewed by a medical officer (JMP or SO). One or more 4-mm punch-biopsy specimens were obtained routinely from (more than 98% of) individuals with suspect lesions and read by a single histo-pathologist (SBL) using a standardized reporting protocol (6). Overall diagnostic certainty was assigned according to rigorous criteria and based upon a combination of clinical, histopathological, and bacteriological findings (13). The incidence rate of leprosy in this population (prior to the vaccine trial) was approximately 0.001 per annum, more than 90% of the cases being classified as paucibacillary.
from January 1988, all new leprosy cases aged 15 years and older, as well as all individuals diagnosed as relapses, have been enrolled in the study. New leprosy patients were divided into those who had lived in the district for many years and had been found without any sign of leprosy at a previous examination ("incidence cases") and those who had arrived recently in the district from other parts of Malawi or from neighboring Zambia or Tanzania ("immigrants"). Up to four controls were selected for each leprosy suspect, matched for age (within 5-year age groups up to age 35, within 10-year age groups thereafter); sex; and dwelling location (within 1 km), from computerized files on the trial population. In order to avoid inclusion of co-wives, who might be presumed to share the same HIV status, only one adult female from each household was allowed in each matched set. A blood specimen was collected from leprosy suspects at the time of examination by a medical officer. Controls were visited as soon as identified, and the purpose of the investigation explained to them and to their village headman before their consent to provide a blood specimen was requested.
An additional comparison group was provided by the routine HIV testing of all incident tuberculosis patients diagnosed in the district and admitted to the district hospital at Karonga. Sputum specimens from all of these patients are examined and cultured routinely at the LEP/KPT laboratory in Chilumba. All positive cultures are sent to the PHLS Mycobacterial Reference Laboratory in Cardiff, Wales, for species identification. Only microscopically and/or culture-positive new tuberculosis cases ascertained during 1989 are included in the results reported here.
Five-ml blood specimens were taken to the project laboratory in Chilumba where serum was obtained after centrifugation of clotted specimens. HIV seropositivity status was based upon a protocol involving multiple tests. All sera were screened initially by sandwich ELISA (Organon Virono-stika anti-HTLV-III) and particle agglutination (Edgware modification of the Serodia test 1) assays, and sera negative on both of these tests were considered negative. Those positive on one or both tests were tested further by competitive ELISA (Wellcozyme anti-HTLV-III or Behring Enzygnost anti-HIV) and Fujirebio Serodia particle agglutination assays. Where practical, any test which showed an anomalous result was repeated. Sera which gave repeated anomalous results on more than one test, and sera for which either of the ELISA results differed clearly from the results of the other three tests, were considered indeterminate (16). Quality controls with known positive and negative sera were carried out as recommended for each kit.
All individuals were informed of their HIV test results, unless they had expressly asked not to be told, and were counselled by project or hospital staff.
Data analysis was carried out at the London School of Hygiene and Tropical Medicine, making use of the University of London computer facilities. Stratified and conditional logistic regression analyses were carried out using the SAS (Cary, North Carolina, U.S.A.) and EGRET (SERC, Seattle, Washington, U.S.A.) packages, respectively.
RESULTS
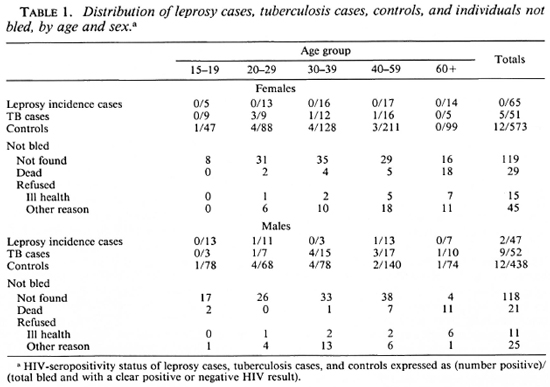
Between January 1988 and December 1989, 112 incident leprosy cases and 1011 controls were recruited into the case-control study. These figures exclude one case and four controls with indeterminate HIV status. In addition, 103 new tuberculosis cases were tested for antibodies to HIV. The distribution of the study population by disease status, age, and sex are shown in Table 1. Also shown are the age and sex distributions of 383 individuals who were selected as controls from the computer files but who either were not found, because they had left their home village (237) or had died (50), or else had refused because of ill health (26), religious conviction (4), or any other reason (66), to provide serum specimens.
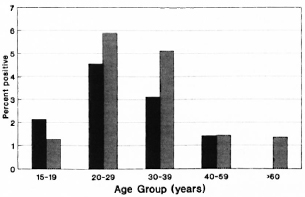
The Figure. HIV-seropositivity rates among controls, by age and sex, Karonga District, northern Malawi, 1988-1989. Males =  ; females =
; females =  .
.
The numbers of individuals seropositive for antibodies to HIV-1 are shown in Table 1 by case and control category. Seropositiv-ity rates in the control population are presented by age and sex in The Figure. Rates were higher in males than in females, peaking in the 20-29 year age group at 4/68 (5.9%) for males and 4/88 (4.5%) for females. They were higher in the semi-urban district capital (4/106, 3.8%) than in the remaining rural areas (20/905, 2.2%) of the district, but small numbers in the urban series prevent meaningful comparison by narrow age and sex categories.
Both of the HIV-seropositive incident leprosy patients were classified as pauci-bacillary. Of the remaining 110 seronegative patients, 8 (7.3%) were multibacillary.
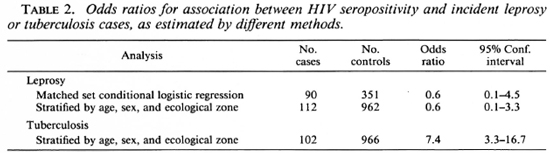
Three-hundred-fifty-one age-sex-area-(square kilometer) matched controls were available for 90 incident leprosy cases. Two (2.2%) of these cases and 11 (3.1%) of the matched controls were positive for antibodies to HIV-1 virus. The odds ratio for the association between leprosy and HIV status in this series was 0.6 (95% confidence interval: 0.06 to 4.5) by matched set conditional logistic regression (Table 2).
In order to take advantage of all available cases and controls, additional analyses were carried out comparing all 112 incident leprosy cases (including those for whom no matched controls were available) with 962 controls (including 611 originally selected as controls for suspects, whose diagnosis was not confirmed). Forty-nine controls were excluded on the single-female-per-house-hold criterion, as explained above. Cases and controls were stratified by age, sex and ecological zone. Similar analyses were carried out for 102 incidence tuberculosis cases (one excluded by the multiple female criterion). Mantel Haenszel odds ratios (Table 2) were: for incidence leprosy cases, 0.6 (95% confidence interval: 0.1 to 3.3); for tuberculosis, 7.4 (3.3 to 16.7).
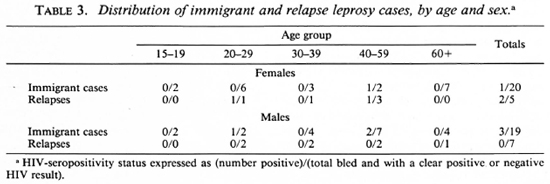
Table 3 shows a breakdown of immigrant and relapse leprosy cases by age, sex and HIV status. Although the numbers of cases are small, there is a suggestion of higher HIV-seropositivity rates in these groups compared to the control series (Table 1, The Figure).
DISCUSSION
No evidence for an association between incident leprosy and HIV infection has been observed in this rural African population. In contrast, a strong association is observed between tuberculosis and HIV infection. Although the numbers are very small, there is a suggestion of a possible association between HIV infection and relapses. We discuss first the validity of our findings, and then consider their implications.
The strong association between HIV infection and tuberculosis observed in this population is consistent with many published reports (4,17) and provides a validation of the methods employed in this investigation. The sevenfold relative risk estimate observed here is similar to that observed in several studies in Africa (Tuberculosis Programme/Global Programme on AIDS. Preventive tuberculosis chemotherapy among persons infected with human immunodeficiency virus. Report of the Informal Consultation, Geneva, 6-8 February 1990, WHO/TUB/AIDS/90.1).
In contrast with tuberculosis, we find no evidence of an association between HIV infection and incident leprosy in this population. Three features of this finding deserve emphasis. First, the prevalence of HIV infection is low and thus the confidence intervals for the odds ratios are, of necessity, broad. Second, it should be emphasized that most (86/112, 77%) of these leprosy cases were actively detected in a total population survey. Only six (5.4%) had any disability at all, and none of these was HIV seropositive. This is in contrast to the cases described in the published Zambian investigation (11), all of whom had self-reported or been referred to hospital and were described as tending "to have serious symptoms, such as paralysis or neuritis, rather than a single skin lesion." The method of ascertainment of the Zambian patients could itself explain the observed association between leprosy and HIV infection in that investigation. Even among our self-reporting (incident and immigrant) patients, the HIV seropositivity was high (4/35, 11%). For both of these reasons, the Zambian cases were thus not representative of all new leprosy patients in the community. Third, we note that HIV infection is relatively recent in this rural northern Malawi population. Evidence for this recent arrival is seen in the comparatively low overall HIV-seropositivity rate among pulmonary tuberculosis patients, which was only 14%, in contrast to 49% reported from Lusaka, capital of neighboring Zambia (5). It is likely that, with time, the proportion of HIV-infected individuals with severe immunosuppression will increase, and a consequent association with leprosy may then emerge.
On the other hand, it is possible that the relationship between HIV infection and leprosy is such that clinical signs of leprosy appear only relatively late in the course of the immunosuppression. We are aware of only three case reports of leprosy in association with induced immunosuppression-each in individuals who were im-munosuppressed following kidney transplantation (18). If immunosuppression must be very severe before it has implications for leprosy pathogenesis, then one might predict that many individuals in rural African populations would die of other infections, including tuberculosis, before leprosy reveals itself.
It is unlikely that our failure to observe an association between HIV infection and leprosy onset is attributable to exaggerated seropositivity rates in the controls due to a bias in control recruitment. Approximately 27% (383/1394) of computer-selected controls either could not be found or refused to provide blood for this investigation. Of these, 76 were not bled because they were either dead or unwilling because of ill health, and it might be presumed that HIV seropositivity would be particularly high in such individuals. But even if all of those refusing had been bled, and all were seronegative, the odds ratio would still have been effectively unity (1.1, 95% confidence limit: 0.25 to 5.0). On the other hand, given the inevitable time lag between onset of disease, ascertainment of diagnosis and recruitment of controls, the conjectured rise in the HIV-seroprevalence rate in the population could have led to artificially high seropositivity rates among controls and, hence, to a slight underestimate of the relative risk.
Although our numbers are very small, it is of interest that 2 out of 12 (17%) relapses in our study were HIV positive. It is improper to calculate the odds ratios for such cases because appropriate controls (which should be cases matched for date of onset, type of disease and treatment) are not yet available-let alone the fact that the sample size is small. On the other hand, this observation of a "high" prevalence of HIV in relapse cases is at least consistent with two published reports of an association between HIV and leprosy relapse (10,12). We might even speculate that the pathogenesis of relapse in leprosy is more analogous to that of pulmonary tuberculosis in adults (in being attributable to endogenous reactivation) than is the pathogenesis of primary/incident leprosy.
Our data also show a higher HlV-posi-tivity rate among immigrant than among resident incidence leprosy patients. This, again, is consistent with a relatively recent arrival of HIV infection in the Karonga District. More importantly, it points to a serious problem in investigations of this sort. Immigrant status is potentially an important confounder in circumstances in which the HIV-seropositivity rate is different in a study population from that in surrounding populations.
Several authors have wondered whether HIV infection might not be associated with downgrading in leprosy and shifts toward multibacillary disease. We find no evidence of such a shift. The very small proportion of multibacillary cases in our series is consistent with the pattern reported in this population and in much of Africa (15). We are aware of no reports or even anecdotes indicating a shift toward increased multibacillary disease anywhere in Africa, despite a high prevalence of HIV infection in many leprosy-endemic populations.
Our evidence to date suggests that the association between HIV and leprosy is slight, if present at all. This may be surprising, given that clinical leprosy reflects a chronic mycobacterial infection, and HIV is associated strongly with clinical tuberculosis and with systemic disease due to several "atypical" mycobacterial infections (4,5,9,17). It is recognized that different opportunistic infections tend to arise at different periods during the course of HIV-induced immunosuppression (3). It may be that leprosy, perhaps because of the very slow proliferation of the bacilli or perhaps because of particular cellular mechanisms involved in its pathogenesis, will be affected relatively little by HIV infection. Time will tell. For the present, workers in leprosy control programs may be relieved to know that some of the more dire predictions of dramatic increases in leprosy in conjunction with the HIV pandemic may have been overly pessimistic (2). On the other hand, they should take note of the possibility of an increased incidence of relapses as a consequence of HIV-1 infection. In addition, basic scientists may find the absence of a strong association between leprosy and HIV infection to be a useful clue in our attempts to understand the interaction of HIV-1, M. leprae, and the human immune response.
Acknowledgment. The authors wish to thank the government of the Republic of Malawi for its interest in the LEPRA Evaluation Project/Karonga Prevention Trial and their permission to undertake this study. Basic funding for the LEP/KPT is provided by the British Leprosy Relief Association (LEPRA) and other I LEP members. The study was supported by a grant from the Special Programme on Research and Training in Tropical Diseases (TDR) of the UNDP/World Bank/ World Health Organization. Particular thanks are expressed to Mr. Mwakasungulu and Mr. Mugabe for collecting serum specimens from the people selected as controls. Drs. M. Pit and N. Vons were helpful in obtaining specimens from tuberculosis patients.
REFERENCES
1. Barbara, J. A. J., Salker, R., Challis, P. and Contreras, M. Gelatin particle agglutination assay for HIV antibodies: a rapid, economical modification with increased sensitivity. Med. Lab. Sei. 46(1989)135-140.
2. Baskin, G. B., Gormus, B. J., Martin, L. N., Murphy-Coru, M., Walsh, G. P. and Meyers, W. M. Pathology of dual Mycobacterium leprae and simian immunodeficiency virus infection in rhesus monkeys. Int. J. Lepr. 58(1990) 358-364.
3. Blaser, M. J. and Cohn, D. L. Opportunistic infections in patients with AIDS: clues to the epidemiology of AIDS and the relative virulence of pathogens. Rev. Infect. Dis. 8(1986)21-30.
4. Collins, F. M. Mycobacterial disease, immunosuppression, and acquired immunodeficiency syndrome. Clin. Microbiol. Rev. 2(1989)360-377.
5. Elliott, A. M., Luo, N., Tembo, G., Halwiindi, B., Steenbergen, G., Machiels, L., Pobee, J., Nunn, P., Hayes, R. and McAdam, K. P. W. j. Impact of HIV on tuberculosis in Zambia: a cross sectional study. Br. Med. J. 301(1990)412-415.
6. Fine, P. E. M., Job, C. K., McDougall, A. C, Meyers, W. M. and Ponnighaus, J. M. Comparability among histopathologists in the diagnosis and classification of leprosy. Int. J. Lepr. 54(1986)614-625.
7. Fine, P. E. M. and Ponnighaus, J. M. Background, design, and prospects of the Karonga Prevention Trial, a leprosy vaccine trial in northern Malawi. Trans. R. Soc. Trop. Med. Hyg. 82(1988)810-817.
8. Fleming, A. F. Opportunistic infections in AIDS in developed and developing countries. Trans. R. Soc. Trop. Med. Hyg. 84Suppl.(1990)1-6.
9. Horsburgh, C. R., Mason, U. G., Farhi, D. C. and Iseman, M. D. Disseminated infection with Mycobacterium avium-intracellulare: a report of 13 cases and a review of the literature. Medicine. 64(1985)36-48.
10. Janssen, F., Wallach, D., Khuong, M. A., Pennec, J., Pradinaud, R., Said, G. and Cottenot, F. Association dc maladie dc Hansen et d'infection par lc virus dc 1'immunodeficiencc humaine. Deux observations. Presse Med. 17(1988)1652-1653.
11. Meeran, K. Prevalence of HIV infection among patients with leprosy and tuberculosis in rural Zambia. Br. Med. J. 298(1989)364-365.
12. Pean, C, Pape, J. W., Deschamps, M. M. and Dambreville, M. Prevalence et evolution dc l'infection au virus humain d'immunodeficience (VIH) chez les lepreux en Haiti. (Abstract) Int. J. Lepr. 51 Suppl. 1(1988)306-307.
13. Ponnighaus, J. M., Fine, P. E. M. and Bliss, L. Certainty levels in the diagnosis of leprosy. Int. J. Lepr. 55(1987)454-462.
14. Ponnighaus, J. M., Fine, P. E. M., Bliss, L., Sliney, I. J., Bradley, D. J. and Rees, R. J. W. The Lepra Evaluation Project, an epidemiological study of leprosy in northern Malawi. I. Methods. Lepr. Rev. 58(1987)359-375.
15. Ponnighaus, J. M., Fine, P. E. M., Maine, N., Bliss, L., Kalambo, M. and Ponnighaus, I. The Lepra Evaluation Project (LEP), an epidemiological study of leprosy in northern Malawi. II. Prevalence rates. Lepr. Rev. 59(1988)97-112.
16. Shaw, M. A., Turner, A. C, Blackwell, J. M., Fine, P. E. M. and Ponnighaus, J. M. Setting up HIV serology for the Karonga leprosy vaccine trial in Malawi. Lepr. Rev. (in press).
17. Slutkin, G., Leowski, J. and Mann, J. The effects of the AIDS epidemic on the tuberculosis problem and tuberculosis programmes. Bull. Int. Union Tuberc. Lung Dis. 63(1988)21-24.
18. Ter el, J. L., Liano, F., del Hoyo, M., Rocamo-ra. A., Mompaso, E. G., Quereda, C. and Ortuno, j. Successful kidney transplantation in leprosy and transitory recurrence of the disease. Int. J. Lepr. 53(1985)410-411.
19. Turk, J. L. and Rees, R. J. W. AIDS and leprosy. Lepr. Rev. 59 (1988) 193-194.
Added in proof: Since submission of this paper two other recent investigations on HIV and leprosy have been brought to our attention. Neither found any evidence for an association between the two conditions.
Leonard, G., Sangare, A., Verdilr, M., Sas-sou-Guesseau, E., Petit, G., Milan, J., M'Bouip, S., Rey, J.-L., Dumas, J.-L., Hugon, J., N'Gaporo, I. and Denis, F. Prevalence of HIV infection among patients with leprosy in African countries and Yemen. J. Acq. Immun. Defic. Syndr. 3 (1990) 1109-1113.
Tekle-Haimanot, R., frommel, D., Tadesse, T., Abebe, M., Verdier, M. and Denis, F. A survey of human T-lymphotrophic virus type 1 and human immunodeficiency viruses in Ethiopian leprosy patients. AIDS (in press).
1. Dr. Med., D.T.P.H.; LEPRA, P. O. Box 46, Chilumba, Malawi.
2. LEPRA, P. O. Box 46, Chilumba, Malawi.
3. M.B.B.S., LEPRA, P. O. Box 46, Chilumba, Malawi.
4. V.M.D., Ph.D.; London School of Hygiene and Tropical Medicine, Keppel Stret, London WC1E 7HT, England.
5. Ph.D.; London School of Hygiene and Tropical Medicine, Keppel Stret, London WC1E 7HT, England.
6. B.Sc.(Hons); London School of Hygiene and Tropical Medicine, Keppel Stret, London WC1E 7HT, England.
7. F.R.C.Path.; London School of Hygiene and Tropical Medicine, Keppel Stret, London WC1E 7HT, England.
8. Ph.D.; London School of Hygiene and Tropical Medicine, Keppel Stret, London WC1E 7HT, England.
9. London School of Hygiene and Tropical Medicine, Keppel Stret, London WC1E 7HT, England.
10. Ph.D., PHLS Mycobacterium Reference Laboratory, University Hospital Wales, Heath Park, Cardiff CF4 4XW, U.K.
Reprint requests to Dr. Fine.
Received for publication on 22 October 1990.
Accepted for publication in revised form on 4 December 1990.