- Volume 59 , Number 2
- Page: 229–36
Effect of BCG on the risk of leprosy in an endemic area: a case control study
ABSTRACT
The effect of BCG on the risk of leprosy was measured using a case-control design in an area endemic for the disease. In this study, 397 newly diagnosed cases and 669 controls matched for age, sex and locality were selected f rom a defined population. Information on exposure to BCG, contact with another case of leprosy, and relevant socioeconomic variables were obtained f rom the subjects. Having infectious (multibacillary) and noninfectious (paucibacillary) contacts in the household increased the risk of disease 11.7 times (p < 0.001) and 2.7 times (p < 0.001), respectively. Overall, the protection offered by BCG was not significant (odds ratio = 0.8; p = 0.17). However, BCG appeared to increase the risk for indeterminate leprosy (adjusted odds ratio = 2.7; p = 0.09) while protecting against borderline disease (adjusted odds ratio = 0.39; p = 0.03). It is possible that BCG causes a shift in the overall cell-mediated immune response, thus increasing the risk for milder and transient forms of leprosy while protecting against more serious forms. These findings may have important implications for the design and interpretation of vaccine trials. Namely, trials should be designed to measure the protective efficacy of vaccines against the more serious forms of leprosy, which have the greatest public health significance.RÉSUMÉ
L'influence du BCG sur le risque de lèpre a été mesuré par une étude de type cas-témoin dans une région endémique pour la maladie. Dans cette étude, 397 cas nouvellement diagnostiqués et 669 témoins appariés pour l'âge, le sexe et la localité ont été sélectionnés à partir d'une population définie. Des informations sur l'exposition au BCG, un contact avec un autre cas de lèpre, et des variables socio-économiques pertinentes ont été récoltées chez ces personnes. Le contact domiciliaire avec un malade infectieux (multibacillaire) ou non-infectieux (paucibacillaire) augmentait le risque de maladie respectivement de 11.7 fois (p < 0.001) et 2.7 fois (p < 0.001). Dans l'ensemble, la protection offerte par le BCG n'était pas significative (odds ratio = 0.8; p = 0.17). Cependant, le BCG semblait accroître le risque pour la lèpre indéterminée (odds ratio ajusté = 2.7; p = 0.09), mais protégeait contre la forme borderline de la maladie (odds ratio ajusté = 0.39; p = 0.03). Il est possible que le BCG provoque une modification dans la réponse immunitaire de type cellulaire, augmentant done le risque pour une forme plus bénigne et transitoire de lèpre, mais protégeant contre les formes plus sévères. Ces observations peuvent avoir des implications importantes pour la conception et l'interprétation de'essais de vaccination. Plus précisément, des essais devraient être conçus pour mesurer l'efficacité protectrice des vaccins contre les formes plus sévères de la lèpre, qui ont la plus grande signification du point de vue de la santé publique.RESUMEN
Usando un programa diseñado para el control de casos en un área endémica de lepra, se midió el efecto del BCG sobre el riesgo de desarrollar la enfermedad. Para el estudio, se seleccionaron 397 casos recién diagnosticados y 669 individuos control similares en cuanto a edad, sexo y localidad. De los participantes se obtuvo información sobre exposición al BCG, contacto con otros casos de lepra, y aspectos socioeconómicos relevantes. Los resultados señalaron que el tener contactos infecciosos (multibacilares) y no infecciosos (paucibacilares) dentro de los convivientes, aumentó el riesgo de la enfermedad 1 1.7 veces (p = 0.001) y 2.7 veces (p = 0.001), respectivamente. Aunque en lo general, la protección conferida por el BCG no fue significativa (relación entre grupos = 0.8; p = 0.17), el BCG pareció incrementarei riesgo para lepra indeterminada (relación = 2.7; p = 0.09) al mismo tiempo que pareció proteger contra formas intermedias de la enfermedad (relación = 0.39; p = 0.03). Es posible que el BCG cause un cambio en la respuesta inmune celular general, aumentando el riesgo para las formas leves y transitorias de la lepra y protegiendo contra las formas más severas. Estos hallazgos pueden tener importantes implicaciones en el diseño y en la interpretación de los resultados de los programas de vacunación; esto es, los ensayos de campo deben diseñarse para medir la eficacia protectora de las vacunas contra las formas severas de la lepra, las de mayor importancia en salud pública.There has been considerable uncertainty regarding the effect of BCG vaccination on the risk of developing leprosy. Even though there is experimental evidence to suggest a protective effect (10, 21-23), major field trials have failed to produce consistent results. The study from Uganda (25) showed 80% protection, while studies from Papua New Guinea (18-20) and Burma (1) showed 46% and 20% protection, respectively. A study from India showed 23% protection (Tri-pathy, S. P. Chingleput trial of the protective effect of BCG against leprosy. Paper presented at the Sixth IMMLEP SWG Meeting, Geneva, June, 1982). More recently, a case-control study from Malawi showed 50% protection (12). The variation in the protective efficacy of BCG in these studies has been postulated to be related, in part, to variations in the intensity of exposure, prevalence of other mycobacteria providing some protection, differing strains of Mycobacterium leprae, and genetic susceptibility of the populations in these studies (15).
At present, several field trials are being planned or arc in progress in different parts of the world to measure the protective effect of various mycobacterial vaccines against leprosy (12). A fresh look at the effect of BCG may help in planning these studies and in interpreting the results. Using a case-control design, we have evaluated the efficacy of BCG in leprosy prevention since it was intraduced, primarily as an antituberculosis vaccine, in a high incidence area for leprosy in the state of Tamil Nadu, India. Our study raises interesting methodologie questions concerning the efficacy of BCG and suggests that the vaccine may have differential efficacy in different types of leprosy.
METHODS
Study area. The state of Tamil Nadu in India is known to be highly endemic for leprosy. The prevalence of leprosy in the Indian states of Tamil Nadu and Andhra Pradesh has been reported to be about 20 per 1000 (5). BCG vaccination against tuberculosis has been done in Tamil Nadu since 1960. Therefore, this area offers an ideal setting for measuring the protective effect of BCG vaccination against leprosy using a case-control design (24).
The study was carried out in the leprosy control project area of the Department of Community Health, Christian Medical College, Vellore. The project has responsibility for a rural population of about 200,000 persons. The annual case detection rate is about 2.5/1000. Leprosy surveillance and diagnosis is done in accordance with the guidelines established by the National Leprosy Eradication Programme of India. Case detection occurs through various surveys and voluntary reporting. By 1983 the entire population had been covered by a general survey at least once. The information pertaining to the members of each household was recorded sequentially on separate pages in the general survey register during the house-to-house survey. These registers, with 200 pages each, played a key role in the design and conduct of our study.
Initially, BCG vaccination (1331 Copenhagen strain) was offered to all Mantoux-negative individuals younger than 20 years of age as part of the National Tuberculosis
Control Programme. Pretesting with Man-toux was subsequently discontinued. During the early 1970s school children formed the main target population of the BCG team. By 1980 the vaccine was being offered mainly to newborns at the hospitals and to the infants at the under-five clinics. The rate of vaccination in the control population was 42%; rates varied from 26% for those 5-9 years of age to 56% in those 20-24 years old.
Study population. All newly detected cases of leprosy, aged 5-24 years, from among the resident population of the project area during July 1986 to June 1988 were included in the study. All individuals whose names appeared in the general survey register by 1983 were considered residents of the area. The cases were subjected to a skin-smear examination and classified by trained physicians using the Ridley and Jopling classification into tuberculoid (TT), borderline tuberculoid (BT), borderline (BB), borderline lepromatous (BL), and lepromatous (LL) leprosy (17). Macular lesions with equivocal loss of sensation were classified as indeterminate leprosy. Histopathological examination was carried out only in doubtful cases. Cases were identified by general survey (86 cases), school survey (141), contact exam (22), voluntary referral (76), and other means (72).
Controls were chosen from among the resident population and they were matched with a case for age (± 1 year), sex, and locality. Matching for locality was achieved by selecting the controls from the same general survey register to which the cases belonged. A table of random numbers up to 200 was used to identify the page number in the register from which the search for each control was to start. Two controls were chosen for each case younger than 15 and one control for each older case.
Cases and controls were visited at their homes by a team consisting of the investigator, a nonmedical supervisor, and the leprosy paramedical worker. The following information was obtained regarding the cases and controls: a) presence of BCG scar; b) presence of a known case of leprosy in the household; c) presence of previously unknown case in the household; d) presence of a case among the extra household relatives; e) socioeconomic characteristics: i) type of house, ii) occupation, iii) land ownership, iv) number of years spent in school, and v) level of education of the highest educated individual in the family.
Exposure to BCG was ascertained by the nonmedical supervisor by looking for the typical scar over the deltoid region. The information was recorded as positive, negative, or equivocal. Individuals with equivocal BCG scars were excluded from the analysis. Every effort was made to mask the BCG reader regarding the clinical status of the subjects by presenting them as a mixed group and exposing only their deltoid regions to the reader. Controls were examined to rule out any clinical evidence of leprosy. Similarly, all other members of the household were also examined. Information on the presence of an extra-household family member with leprosy and the socioeconomic characteristics of the household were obtained by interviewing the subjects and the adult members of the family.
The data were analyzed on an IBM computer (Model 4381) using the Statistical Analysis System (SAS) package. Unmatched logistic regression was carried out with the Logiest program prepared by Frank E. Harrel, Jr. Matched set logistic regression (2) was done with the McStrat program prepared by James N. Naessins, et al. (SAS Inc., 1986). These procedures indirectly measure the odds ratio (OR), which is the ratio of the odds for the disease among the exposed to that among the unexposed. In a relatively rare disease, such as leprosy, the odds ratio gives a good estimate of the relative risk (1-odds ratio) % gives the protective effect of the vaccine.
RESULTS
During the study period, 421 eligible cases were detected; 405 cases and 694 controls were followed up. BCG was recorded as equivocal for eight cases and 25 controls, and these have been excluded from the following analysis. Thus, there were 397 cases and 669 controls available for unmatched analysis and 380 cases and 625 controls available for matched set analysis.
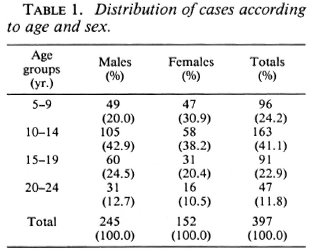
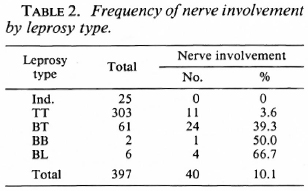
The distribution of cases according to age and sex is shown in Table 1. The distribution of cases according to the type of leprosy and nerve involvement is shown in Table 2. Three of the six BL cases were studied by slit-skin smear and were bacteriologi cally positive.
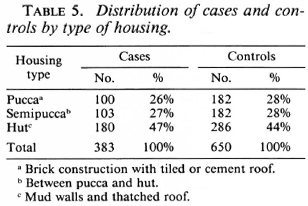
Several socioeconomic and demographic characteristics in the control population were significantly associated with the presence of a BCG scar. Among these were age and sex; 47.2% of males and 33.5% of females had BCG scars. The number of years of education of the individual and the duration of education of the highest educated member of the household were both associated with BCG vaccination. Also, the type of housing and land ownership were surrogates for the socioeconomic level of the family; both correlated with BCG vaccine status. Housing type was classified as "pucca" if it was of brick construction with a tiled or cement roof, hut (or "katcha") if it had mud walls and had a thatched roof, or "semipucca" if it was between the above two in construction.
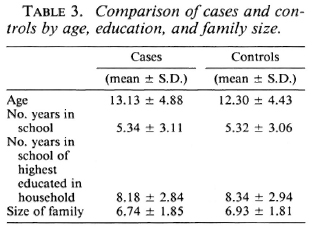
Analysis of the distribution of these characteristics among the cases and controls showed they were well matched by age, education, years of schooling of the most educated in the family, and family size (Table 3). Also, cases and controls were well matched by occupation (Table 4) and by housing type (Table 5).
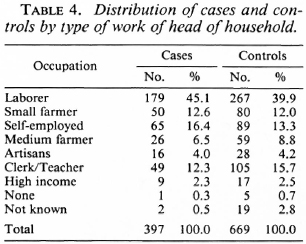
The unmatched analysis showed that BCG was not significantly associated with the risk of leprosy (Table 6). The presence of a known case in the family appeared to increase the risk of the disease considerably (odds ratio = 4.75, x2 = 89.7, p < 0.001).
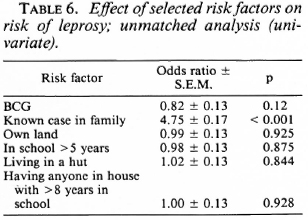
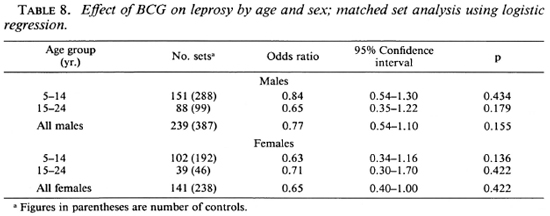
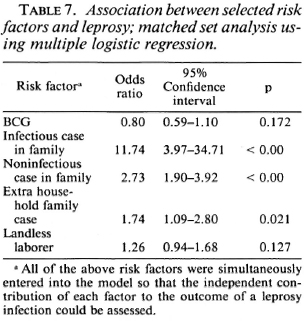
Exposure to noninfectious (I, TT, BT) and infectious (BB, BL, LL) leprosy cases within the family increased the risk for the disease 2.7 times and 11.7 times, respectively, when compared to those having no familial cases in the household (Table 7). Similarly, there was a significant association between having an extra familial case in the household and the risk of leprosy (OR = 1.7). Age and sex did not appear to significantly modify the effect of BCG on the risk for leprosy (Table 8).
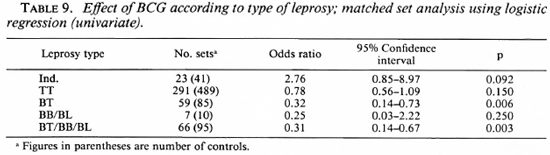
When the effect of BCG on the risk of developing the different types of leprosy was studied, an interesting pattern emerged.
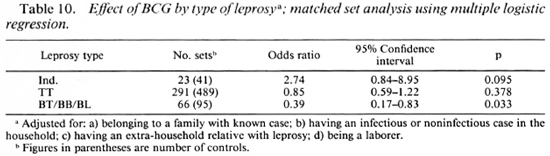
BCG appeared to increase the risk of developing indeterminate leprosy (OR = 2.7), but when one went down the spectrum from tuberculoid to borderline diseases, there was a gradual increase in the degree of protection associated with BCG (Table 9). BCG was found to offer 61% protection against Borderline forms of leprosy after adjusting for significant confounders in a matched set analysis using multiple logistic regression (Table 10). Variables adjusted for this analysis included the following: a) having a known case in the family, b) having an infectious or noninfectious case in the household, c) having an extra-household relative with leprosy, d) being a laborer.
DISCUSSION
Matching the cases and controls for the general locality of the household appears to have created a good balance between cases and controls with respect to many of the socioeconomic factors which could have had a bearing on the chance for receiving BCG, on the one hand, or the risk of disease detection on the other. The magnitude of the association between infectious and noninfectious intra-familial cases and risk of disease is similar to that reported by the other workers (8,9,16).
BCG was found to increase the risk of indeterminate leprosy while offering protection against the borderline forms. The point estimates of the odds ratio suggest increasing protection as one goes down the spectrum from the indeterminate to paucibacillary to multibacillary leprosy. There was 61 % protection against borderline types. Although the finding appears to be paradoxical at first glance, this may offer a new insight into the manner in which BCG affects the natural history of the disease. M. leprae infections elicit a highly variable response in the host, ranging from subclinical infection to polar lepromatous leprosy. A majority of the indeterminate and some of the tuberculoid cases may heal spontaneously, and may not contribute substantially to the public health importance of the disease (4,13,14). Following vaccination with BCG, the host immune response may be shifted to the left (The Figure), resulting in a greater proportion of individuals responding to infection with subclinical disease and indeterminate leprosy and a smaller proportion manifesting borderline forms of the disease. This would explain the variability in the protection offered by BCG with respect to the different types of leprosy.
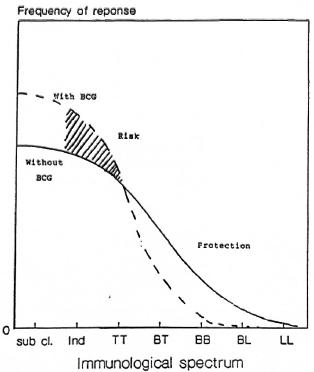
The figure. Hypothesized effect of BCG on theimmunological spectrum of leprosy.
The results of the BCG trial in New Guinea had some similarities to our findings (20), namely, the protective efficacy against indeterminate, tuberculoid, and borderline tuberculoid forms in the New Guinea trial were 20%, 27% and 69%, respectively. However, the protective efficacy against what probably was BB/BL and LL disease was 39% and 40%, respectively. The Uganda trial found 80% protection; all cases but one in this study were tuberculoid.
The findings of this study also offer a possible new explanation for the variation in the protective effect of BCG reported by the major field trials. The study from Uganda, which reported the greatest protective efficacy of BCG, was designed in such a way that the duration between examinations was about 3 years (25). This implies that the investigators would have missed many of the self-healing forms, and were dealing with a higher proportion of persistent cases. In the Burmese study, on the other hand, the subjects were examined annually and a greater proportion of earlier and transient forms would have been detected (1). If BCG causes a shift in the immune response, it is conceivable that the vaccinated population may manifest a higher risk for these transient forms of leprosy (4,11,13,14). This hypothesis is also consistent with the reports on "BCG-induced Leprosy" (26).
from a public health point of view, leprosy cannot be considered to be simply a dichotomous phenomenon. A vaccine that protects against the more serious forms of leprosy might be recommended even if it increased the risk for milder transient forms of the disease. This study also highlights an important issue related to designing field trials of vaccines against leprosy. The emphasis should be on type-specific protection, rather than on overall protection. Since classification of early lesions may be difficult and since one is ethically obliged to treat all detected cases promptly, too frequent a follow up of subjects may provide misleading information on the true impact of the vaccine (14). Another issue in vaccine trials relates to the duration of follow up required to reliably estimate vaccine efficacy. Since multibacillary leprosy may have generally longer incubation periods than indeterminate or paucibacillary types (11), a study that is not continued for a sufficient length of time may underestimate vaccine efficacy.
Since there were no cases of lepromatous leprosy in this series among patients aged 5-24 years, we cannot draw any conclusions on the effect of BCG with respect to this type of disease. The age at vaccination of the subjects in our study was not known. It is extremely unlikely that any of our cases were vaccinated after the onset of leprosy, since by 1980 BCG was used exclusively among newborns and in the under-five clinics. Unfortunately, since they were not available we could not verify the time of BCG vaccination with medical records. The effect of the temporal relationship between vaccination and exposure to M. leprae to the degree of protection offered by the vaccine needs to be studied further. Convit's experiments with the immunotherapy of cases appear to suggest that immunization with some M. leprae-derived vaccines may be useful even after exposure to M. leprae (6,7).
A case-control study of the type we performed is more easily done in developing countries with limited resources than is a placebo-controlled vaccine trial. Of course, there are several sources of bias that need to be considered in interpreting case-control studies of this type. Most importantly, the controls should be selected from a population having a similar risk of exposure to M. leprae, of diagnosis of leprosy, and access to vaccination. In order to obtain unbiased estimates, it is important that the probability of selection on the basis of outcome is independent of the probability of selection by vaccination status.
We attempted to minimize bias by selecting controls from the same population as the cases. When we examined the controls and cases stratified by various socioeconomic variables, namely, occupation, education, education of household head, type of house, and land ownership, the two groups were similar in distribution. As we expected, many of these socioeconomic variables were correlated with BCG vaccination status. Controls and cases were matched by age, sex, and geographic area of residence. The matched analysis reduced or eliminated bias related to several demographic and socioeconomic characteristics. Residual bias, such as the presence of a case in the household, was adjusted for a multivariate analysis (Table 10).
Random misclassification could have occurred in exposure ascertainment and in selection of cases and controls. This, however, would have the effect of moving the odds ratios closer to unity (13), thus providing a lower estimate of risk for indeterminate leprosy and protection against borderline forms. Finally, had there been self-selection bias with respect to vaccination, the association would have been unidirectional for the entire spectrum.
In conclusion, our study found that BCG offers about 60% protection against borderline forms of leprosy probably by bringing about a shift in the immune response to a higher level of cell-mediated immunity. This shift appeared to cause an increase in the risk for milder forms of the disease. from a public health point of view, BCG should be recommended for the prevention of leprosy until a better vaccine is available. In designing field trials to measure the protective effects of other mycobacterial vaccines against leprosy, efforts need to be made to demonstrate type-specific protection against the various types of leprosy in addition to overall protection.
Acknowledgment. We are grateful to the Damien Foundation Belgium and to the Ford Foundation, U.S.A., for supporting this study and to Professor Abraham Joseph, Head of the Department of Community Health, Christian Medical College, for his encouragement.
REFERENCES
1. Bechelli, L. M., Lwin, K., Garbajosa, G., Gyi, M. M., Vemara, K. and Sundaresan, T. BCG vaccination of children against leprosy: nine year findings of the controlled WHO trial in Burma. Bull. WHO 51(1974)93-99.
2. Breslow, N. E., Day, N. E., Halvorsen, K. T., Prentice, R. L. and Saba, C. Estimation of multiple relative risk functions in matched case-control studies. Am. J. Epidemiol. 108(1978)299-307.
3. Bross, I. D. J. Misclassification in 2 x 2 tables. Biometrics 10(1954)478-486.
4. Browne, S. G. Self-healing leprosy: report on 2749 patients. Lepr. Rev. 45(1974)104-111.
5. Christian, M. The epidemiological situation of leprosy in India. Lepr. Rev. 52 Suppl. 1(1981)35-42.
6. Convit, J., Aranzazu, N., Pinardi, M. and Ulrich, M. Immunological changes observed in indeterminate and lepromatous leprosy patients and Mitsuda-negative contacts after the inoculation of a mixture of Mycobacterium leprae and BCG. Clin. Exp. Immunol. 36(1979)214-220.
7. Convit, J., Aranzazu, N., Ulrich, M., Pinardi, M. E., Reyes, O. and Alvarado, J. Immunotherapy with a mixture of Mycobacterium leprae and BCG in different forms of leprosy and in Mitsuda-negative contacts. Int. j. Lepr. 50(1982)415-424.
8. Dominguez, V. M., Garbajosa, P. G., Gyi, M. M., Tamondong, C. T., Sundaresan, T., Bechelli, L. M., Lwin, K., Sansarricq, H., Walter, J. and Noussitou, F. M. Epidemiologic information on leprosy in the Singu area of upper Burma. Bull. WHO 58(1980)81-89.
9. Doull, J. A., Rodriguez, J. M., Guinto, R. and Plantilla, F. C. A field study of leprosy in Cebu. Int. J. Lepr. 4(1936)141-170.
10. Fernandez, J. M. M. Comparative study of the Mitsuda reaction with tuberculin reaction. Rev. Argent. Dermatosifil. 23(1939)425-453.
11. Fine, P. E. M. Leprosy: epidemiology of a slow bacterium. Epidemiol. Rev. 4(182)161-188.
12. Fine, P. E., Ponnighaus, J. M., Maine, N., Clarkson, J. A. and Bliss, L. Protective efficacy of BCG against leprosy in northern Malawi. Lancet 2(1986)499-502.
13. Jesudasan, K. and Christian, M. Spontaneous healing in paucibacillary leprosy. Indian J. Med. Res. 81(1985)119-122.
14. Jesudasan, K., Bradley, D., Smith, P. G. and Christian, M. The effect of intervals between surveys on the estimation of incidence rates of leprosy. Lepr. Rev. 55(1984)353-359.
15. Noordeen, S. K. BCG vaccination in leprosy. Develop. Biol. Standard. 58(1986)287-292.
16. Rao, P. S. S., Karat, A. B. A., Kalieperumal, V. A. and Karat, S. Transmission of leprosy within the household. Int. J. Lepr. 43(1975)4554.
17. Ridley, D. S. and Jopling, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966) 255-273.
18. Russell, D. A. BCG vaccination in the prophylaxis of leprosy. The Karimui Leprosy Research Group. (Abstract) Int. J. Lepr. 41(1973)617.
19. Russell, D. A., Scott, G. C. and Wigley, S. C. BCG and prophylaxis - the Karimui trial. (Abstract) Int. J. Lepr. 36(1968)618.
20. Scott, G. C, Russell, D. A., Boughton, C. R. and Vincin, D. R. Untreated leprosy: probability for shifts in Ridley-Jopling classification. Development of "flares," or disappearance of clinically apparent disease. Int. J. Lepr. 44(1976)110-122.
21. Shepard, C. C. A comparison of the effectiveness of two freeze-dried BCG vaccines against Mycobacterium leprae in mice. Bull. WHO 38(1968)135-140.
22. Shepard, C. C. Vaccination against human leprosy bacillus infections of mice: protection by BCG given during the incubation period. J. Immunol. 96(1966)279-283.
23. Shepard, C. C. Vaccination against experimental infection with Mycobacterium leprae. Am. J. Epidemiol. 81(1965)150-163.
24. Smith, P. G. Evaluating interventions against tropical diseases. Int. J. Epidemiol. 16(1987)159- 166.
25. Stanley, S. J., Howland, C, Stone, M. M. and Sutherland, I. BCG vaccination against leprosy in Uganda: final results. J. Hyg. (Camb.) 87( 1981 )233-248.
26. Wade, H. W. BCG induced activations. Int. J. Lepr. 28(1960)179-181.
27. Watson, J. D. Prospects for new generation vaccines for leprosy: progress, barriers, and future strategies. Int. J. Lepr. 57(1989)834-842.
1. M.D., Dr.P.H.(Epid.), Associate Professor, Community Health Department, Christian Medical College, Vellore 632 002, South India.
2. M.D., Professor of Epidemiology, School of Hygiene and Public Health, The Johns Hopkins University, Baltimore, Maryland 21205, U.S.A.
3. Ph.D., Professor of Epidemiology, School of Hygiene and Public Health, The Johns Hopkins University, Baltimore, Maryland 21205, U.S.A.
Reprint requests to Prof. Nelson.
Received for publication on 4 September 1990.
Accepted for publication in revised form on 7 January 1991.