- Volume 59 , Number 2
- Page: 255–61
Four-year follow-up results of a WHO-recommended multiple-drug regimen in paucibacillary leprosy patients in Malawi
ABSTRACT
An evaluation of a World Health Organization-recommended multidrug therapy (WHO/MDT) in 499 paucibacillary leprosy patients is described. Patients were followed for 48 months after completion of treatment. Overall relapse rates after treatment were found to be 6.5 per 1000 person years (95% confidence interval 3.4-11.4). There were 12 relapses. A relative lack of cell-mediated immunity, as suggested by number of lesions, clinical classification and lepromin test results, and poor compliance with the dapsone component of WHO/MDT, appeared to be associated with a marginally increased risk of relapse. Severe type 1 reactions after completion of treatment occurred in 17 (3.5%) patients, 15/17 during the first 12 months of follow-up. Overall, 12 (2.5%) patients developed new disabilities during or after WHO/MDT.RÉSUMÉ
On décrit ici une évaluation de la polychimiothé-rapie telle que recommandée par l'Organisation Mondiale de la Santé (PCT/OMS) réalisée chez 499 patients atteints de lèpre paucibacillaire. Les patients furent suivis durant 48 mois après l'arrêt du traitement. Le taux global de rechute observé après traitement fut de 6.5 pour 1000 personnes-années (intervalle de confiance à 95%: 3.4-11.4). Il y eut 12 rechutes. Un manque relatif d'immunité cellulaire, comme le suggéraient le nombre de lésions, la classification clinique et les résultats du test à la lépromine, ainsi qu'une acceptation faible de la composante dapsone du régime PCT/OMS, semblent être associés à un risque légèrement accru de récidives. Des réactions sévères de type 1 apparurent chez 17 patients (3.5%) après l'arrêt du traitement (dans 15 cas sur 17 au cours des premiers 12 mois du suivi). Globalement 12 (2.5%) patients ont développé de nouvelles incapacités durant ou après la PCT/OMS.RESUMEN
Se describe una evaluación del programa de tratamiento con múltiples drogas recomendado por la Organización Mundial de la Salud (TMD/OMS) aplicado en 499 pacientes con lepra paucibacilar. Los pacientes fueron vigilados durante 48 meses después de completar su tratamiento. Hubieron 12 recaídas, encontrándose que el índice global de recaídas después del tratamiento fue de 6.5 por 1000 personas/año (95% de confianza en el intervalo 3.4-11.4). La falta relativa de inmunidad celular sugerida por el número de lesiones, la clasificación clínica y los resultados de la prueba de la lepromina, así como el pobre cumplimiento con el componente dapsona del TMD/OMS, parecieron estar asociados con el riesgo marginalmente incrementado de recaída. En 17 pacientes (3.5%) ocurrieron severas reacciones de tipo I después de completar el tratamiento, en 15 de los 17 pacientes las reacciones ocurrieron durante los primeros 12 meses de seguimiento. Doce pacientes (2.5%) desarrollaron nuevas incapacidades durante o después del TMD/OMS.In 1983 a study was set up in Malawi, Africa, to evaluate the multiple-drug therapy regimen for paucibacillary leprosy patients recommended by a study group organized by the World Health Organization in 1981 (WHO/MDT 15). A preliminary report of this study, based on data collected up to the end of the first 12 months after completion of treatment, has been published (2). We now present data accumulated during 48 months of follow-up after completion of treatment. Emphasis is placed on the analysis of relapse rates and possible risk factors for relapse.
PATIENTS AND METHODS
The study procedures have been described in detail in the previous publication (2). Originally, 503 newly diagnosed patients were recruited into the study. Of these, 301 were ascertained within the framework of the LEPRA Control Project (LCP) (1) and 202 were recruited in the Karonga District within the LEPRA Evaluation Project (LEP) (11).
During the 4-year follow-up period reported here, lesions in three individuals did not change at all, and we have concluded that they were probably hypopigmented, slightly raised scars rather than paucibacillary leprosy lesions despite initial clinical evidence suggestive of leprosy. In addition, a second histopathologist (Dr. S. Lucas) was asked to review all biopsies from a fourth patient whose clinical course appeared inconsistent with the original histopatholog-ical diagnosis of "BT leprosy (in reaction)." On the basis of this review, and the clinical follow-up information, Dr. S. Lucas concluded that the histopathological findings were more likely to indicate sarcoidosis than leprosy. These four patients have thus been excluded from the present analyses, reducing the total number of study participants to 499 paucibacillary patients. Of these, 484 completed WHO/MDT and were reviewed at regular intervals (usually 6 months) until they either died, could no longer be traced, were declared relapsed, refused further reviews, or completed 48 months of follow-up.
A relapse was defined as the appearance of a new skin lesion or the increase in size of a pre-existing skin lesion (after completion of WHO/MDT and in the absence of signs of type 1 reaction), provided there was either strong clinical or definite histopathological evidence (or both) of leprosy in such a lesion. All relapses were given a second course of WHO/MDT for paucibacillary patients (15).
Renewed inflammation in previously existing skin lesion(s) and/or any sign of neuritis were interpreted as evidence of a type 1 reaction. If the renewed inflammation was mild and restricted to skin lesions only, prednisolone treatment was not instituted. In severe cases of type 1 reaction, a course of prednisolone was given as described previously (2).
Most of the routine follow-up examinations were carried out by trained paramedical workers, leprosy control assistants (LCAs) only. However, all review examinations of patients in whom the LCAs suspected a relapse or a type 1 reaction after completion of WHO/MDT were carried out by medical officers (GB and/or JMP).
Lepromin tests were performed in the LCP area at the time of intake and 6 months after completion of treatment. In the Karonga District, most patients were lepromin tested only at 6 months after completion of treatment. After obtaining permission from the patient, 0.1 ml of lepromin (4.0 x 106 armadillo-derived killed Mycobacterium leprae provided by Dr. R. J. W. Rees, London) was injected intradermally on the volar side of the left forearm. Induration, if any, was measured 4 weeks later by an LCA. Two diameters were recorded, along and across the arm, their average being used in the analyses.
Urine samples were collected at surprise visits during the course of treatment, and dapsone/creatinine ratios were measured in the LEP laboratory as previously described (2).
Four-mm-punch skin biopsies were taken from all suspected relapses, with the exception of one patient who refused permission. These specimens were examined by Dr. A. C. McDougall or Dr. S. Lucas, and reported according to a standardized protocol (3).
In addition to providing a clinical classification, we also grouped patients according to what we would call the "stage" of their disease at the time of diagnosis: stage 1 referring to patients with nonanesthetic skin lesions only; stage 2 referring to patients with anesthetic skin lesions only; stage 3 consisting of patients with definitely enlarged nerves and with or without minimal signs of nerve damage; and stage 4 consisting of patients with overt disabilities. This is a simplified version of a system which originally consisted of five "stages," and separated patients with enlarged nerves only (previous "stage 3") from those with enlarged nerves plus minimal signs of peripheral nerve damage (previous "stage 4") (10).
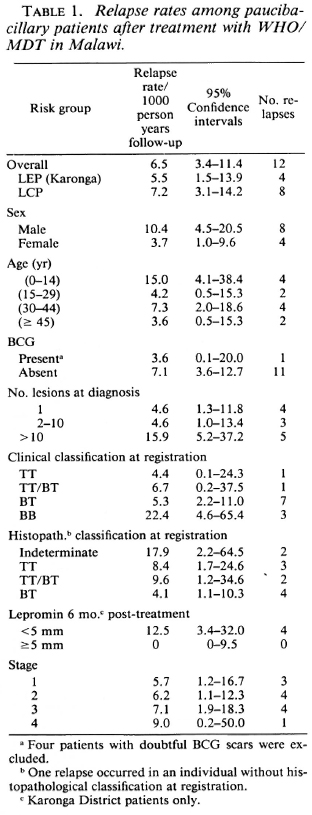
Relapse rates were calculated with person-time denominators up to relapse onset or last examination. Confidence intervals were calculated using the Poisson distribution. Since there was no significant difference in the overall relapse rates between the largely actively found patients in Karonga District and the largely self-reporting patients in the LCP area (Table 1), the data are combined for the purpose of this presentation.
RESULTS
Of the 499 patients, 4 died during treatment and 24 died during the follow-up period. The distribution of deaths over time (Fig. 1) shows no association between the WHO/MDT treatment period and time of death.
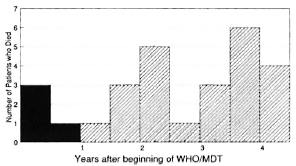
Fig. 1. Temporal distribution of deaths of pauci-bacillary leprosy patients after beginning treatment with WHO/MDT-28/499 (5.6%) died within 4.5 years.  = death during WHO/MDT;
= death during WHO/MDT;  = death after WHO/ MDT.
= death after WHO/ MDT.
Figure 2 shows the cumulative percentages of total patients "lost" for reasons other than death. After 48 months of follow-up the cumulative percentage of patients lost was 14.4% (68/471).
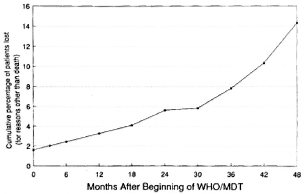
Fig. 2. Cumulative percentage of patients lost from the study, for reasons other than death, over time after beginning of treatment with WHO/MDT.
Twelve patients relapsed. Eleven of them manifested only one or two new skin lesions and no new, or worse, disabilities. The twelfth patient, who had developed a disability (anesthesia in one finger) by the time he completed WHO/MDT, relapsed 42 months later with four new skin lesions.
Figure 3A shows the distribution of relapses over time. Relapse rates for the entire study population and for various subgroups are shown in Table 1. The overall rate is 6.5 per 1000 person years of follow-up. None of the differences in relapse rates between the various subgroups were statistically significant. Nevertheless, there may be suggestions that males, younger individuals, individuals with more than 10 skin lesions at the time of diagnosis, those with a clinical classification of borderline (BB) leprosy and/ or with a histopathological classification of indeterminate leprosy, and/or those with a minimal reaction to lepromin after completion of treatment might be at increased risk of relapse. There is a trend for relapse rates to rise by stage of disease at the time of diagnosis, but this rise is small and does not quite reach statistical significance (p = 0.054).
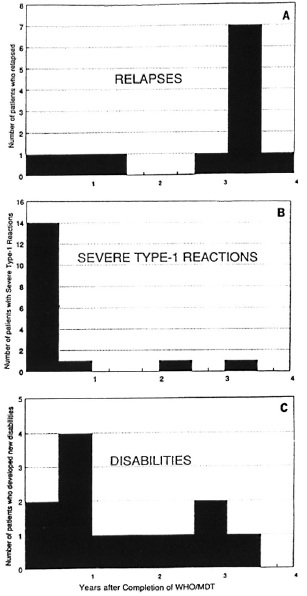
Fig. 3. Temporal distribution of relapses (A) and severe type 1 reactions (B) and new disabilities (C) since completion of WHO/MDT.
The relationship between the relapse rate and the lepromin reaction could only be analyzed for the 199 patients ascertained in Karonga District, because it became apparent from the overwhelmingly negative results that the LCAs in the LCP area had not been trained sufficiently to read small induration sizes accurately. Frequency distributions of lepromin-test results carried out 6 months after completion of treatment in the LEP area are presented in Figure 4. The mean induration size among individuals who relapsed was 2.5 mm, compared to 4.84 mm among the other patients (p < 0.03).
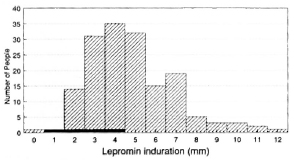
Fig. 4. Frequency distribution of results of lepromin tests carried out in LEP area 6 months after completion of WHO/MDT.  = relapses;
= relapses;  = nonrelapses.
= nonrelapses.
Poor compliance during the course of WHO/MDT was defined as a dapsone/cre-atinine ratio equal to or less than 12. The age-sex standardized incidence rate of relapse was 2.2. times higher in patients with poor compliance than in those with better compliance, but this was based on small numbers and was not significant (p > 0.2).
The temporal distribution of severe type 1 reactions after completion of WHO/MDT was very different from that of the relapses, as shown in Figure 3B. During the follow-up period, 17 severe type 1 reactions were observed, 15 (88%) within the first 12 months after completion of treatment. In all 17 patients, the inflammation subsided following initiation of prednisolone treatment.
There was a clear association between the stage of the disease at the time of diagnosis and the percentage of patients in whom the skin lesions were no longer evident 48 months after completion of treatment. As can be seen in Figure 5, this percentage ranged from 79% for patients initially in stage 1 at intake to 52% for patients in stage 4 at intake.
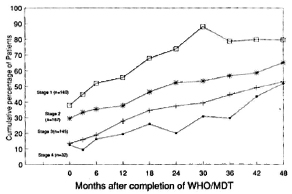
Fig. 5. Cumulative percentages of patients, over time, whose lesions were no longer evident after completion of WHO/MDT, by stage of disease at time of diagnosis.
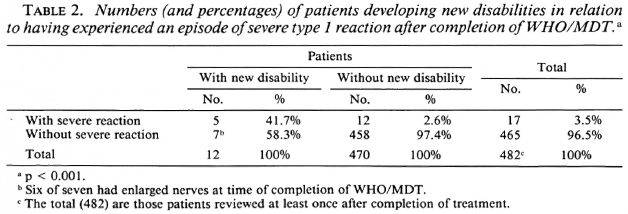
Twelve patients developed new disabilities, and 16 patients experienced a worsening of their disabilities during the study. The distribution over time is shown in Figure 3C. The risk of developing disabilities was significantly higher (p < 0.003) among patients who experienced a severe type 1 reaction after completion of WHO/MDT than among those who did not (Table 2). On the other hand, the majority (7/12, 58%) of new disabilities occurred in patients in whom no severe type 1 reaction was seen.
No relapses were observed in any of the patients who received prednisolone at any time during the study.
DISCUSSION
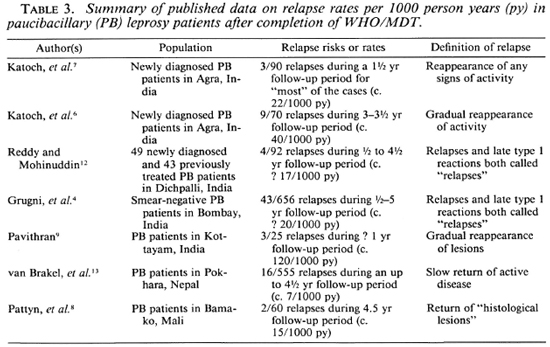
The issue of relapses following WHO/ MDT is obviously of great importance, and several studies have been initiated to provide estimates of the risk involved. Data published to date are summarized in Table 3.
According to these data, the overall relapse rate in Malawi, 6.5 per 1000 person years (95% confidence interval 3.4-11.4), is lower than that reported from India, and similar to that reported from Nepal. The reasons for these differences are not clear, but certainly include differences between the studies in diagnostic criteria for relapses. They may also include differences in the immunological status of the cases involved. A thorough comparison should take many features of the cases into account, including demographic variables, disease type and extent, disease severity, immunological status, the use of lepromin, treatment compliance, etc., but the data required for such a metaanalysis are not readily available.
Although none of the risk factors analyzed in this investigation were statistically significantly associated with the risk of relapse, the trends suggest that patients with a relative lack of cell-mediated immunity (as manifested by weak lepromin response, larger numbers of lesions, and clinical classification) are particularly prone to relapse after the completion of WHO/MDT. Even the slightly higher relapse rate among males may be a reflection of this trend, because multibacillary disease is more frequent in males than in females. Such findings are consistent with reports by other investigators (5).
In contrast to relapses, most of which occurred in the latter part of the 4-year follow-up in our investigation, most (15/17, 88%) of the severe type 1 reactions were observed soon after completion of WHO/MDT (Fig. 3 A and B). This has also been reported by other investigators (14). Given the low overall relapse rate, the observed early appearance of severe type 1 reactions has led us to reduce the period of active surveillance after completion of WHO/MDT to 1 year for uncomplicated paucibacillary patients in Malawi.
The development of disabilities remains a major concern since 2.5% of the patients in our study developed new or worse disabilities during or after WHO/MDT. The risk of developing disabilities was statistically significantly higher among patients with a severe type 1 reaction after completion of treatment than among those without this complication. However, most (7/12, 58%) of the disabilities occurred in patients who never had a recognized type 1 reaction and in 6/7 of these individuals the disabilities developed in areas supplied by a nerve already enlarged at the time of completion of WHO/MDT. It is thus not clear what can be done to prevent such unsatisfactory outcomes.
On the whole, in spite of this problem of disabilities, WHO/MDT appears to be a satisfactory treatment regimen for pauci-bacillary leprosy patients. The regimen is well tolerated and the subsequent relapse rates are, in our experience, low. It could even be argued that with less frequent surveillance some of the relapses we observed in our study might have self-healed and never come to our attention.
Acknowledgment. This investigation received financial support from the THELEP component of the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases. Basic funding for the LEPRA Evaluation Project and the LEPRA Control Project is provided by the British Leprosy Relief Association and other ILEP members. We wish to thank the LCAs, Mr. P. Nkhosa, J. Kapindira, J. Mkongojo and H. Kambcwa, for their dedication in tracing and examining patients regularly, and we thank the patients for their cooperation. We are grateful to Dr. R. J. W. Rees for providing the lepromin and to Drs. A. C. McDougall and S. Lucas for histopatholog-ical support. Our thanks go to the Ministry of Health of the Republic of Malawi for permission to publish this paper.
REFERENCES
1. Boerrigter, G. and pónnighaus, J. M. Ten years' leprosy control work in Malawi (Central Africa). 1. Methods and outcome after treatment. Lepr. Rev. 57(1986)199-219.
2. Boerrigter, G., Põnnighaus, J. M. and Fine, P. E. M. Preliminary appraisal of a WHO-recom-mended multiple drug regimen in paucibacillary leprosy patients in Malawi. Int. J. Lepr. 56(1988)408-417.
3. Fine, P. E, M., Jou, C. K., McDougall, A. C, Meyers, W. M. and Ponnighaus, J. M. Comparability among histopathologists in the diagnosis and classification of lesions suspected of leprosy in Malawi. Int. J. Lepr. 54(1986)614-625.
4. Grugni, A., Nadkarni, N. J., Kini, M. S. and Mehta, V. R. Relapses in paucibacillary leprosy after MDT-a clinical study. Int. j. Lepr. 58(1990)19-24.
5. Jesudasan, K. and Christian, M. Risk of paucibacillary leprosy patients released from control relapsing with multibacillary leprosy. Int. J. Lepr. 53(1985)19-21.
6. Katoch, K., Ramanathan, U., Natrajan, M., Bagga, A. K., Bhatia, A. S., Saxena, R. K. and Ramu, G. Relapses in paucibacillary patients after treatment with three short-term regimens containing rifampin. Int. J. Lepr. 57(1989)458-464.
7. Katoch, K., Ramu, G., Ramanathan, U. and Desikan, K. V. Comparison of three regimens containing rifampin for treatment of paucibacillary leprosy patients. Int. J. Lepr. 55(1987)1-8.
8. Pattyn, S. R., Husser, J. A., Baquillon, G., Mai-ga, M. and Jamet, P. Evaluation of five treatment regimens, using cither dapsone monotherapy or several doses of rifampicin in the treatment of paucibacillary leprosy. Lepr. Rev. 61(1990)151-156.
9. Pavithran, K. Relapse of paucibacillary leprosy after short course multidrug therapy. Indian J. Lepr. 60(1988)225-229.
10. Ponnighaus, J. M. and Fine, P. E. M. Leprosy in Malawi. 1. Sensitivity and specificity of the diagnosis and the search for risk factors for leprosy. Trans. R. Soc. Trop. Med. Hyg. 82(1988)803-809.
11. Ponnighaus, J. M., Fine, P. E. M., Buss, L., Sliney, I. J., Bradley, D. J. and Rees, R. j. W. The Lepra Evaluation Project (LEP) an epidemiological study of leprosy in northern Malawi. 1: Methods. Lepr. Rev. 58(1987)359-375.
12. Reddy, P. K. and Mohinuddin, S. K. Pattern of relapses in pauci-bacillary leprosy patients treated with M.D.T. (W.H.O. 1982). Indian J. Lepr. 60(1988)581-588.
13. van Brakel, W., Kist, P., Noble, S. and O'Toole, L. Relapses after multidrug therapy for leprosy: a preliminary report of 22 cases in West Nepal. Lepr. Rev. 60(1989)45-50.
14. Waters, M. F. R., Ridley, D. S. and Ridley, M. J. Clinical problems in the initiation and assessment of multidrug therapy. Lepr. Rev. 57 Suppl. 3(1986)92-100.
15. World Health Organization Chemotherapy of Leprosy for Control Programmes. Geneva: World Health Organization, 1982. Tech. Rep. Ser. 675.
1. M.D., LEPRA Control Project, P.O. Box 148, Lilongwe, Malawi.
2. Dr. Med., D.T.P.H., LEPRA Evaluation Project, P.O. Box 46, Chilumba, Malawi.
3. V.M.D., Ph.D.; London School of Hygiene and Tropical Medicine, Keppel Street, London WC1E 7HT, England.
4. B.Sc, London School of Hygiene and Tropical Medicine, Keppel Street, London WC1E 7HT, England.
Reprint requests to Dr. Ponnighaus, London School of Hygiene and Tropical Medicine, Keppel Street, London WC1E 7HT, England.
Received for publication on 29 October 1990.
Accepted for publication in revised form on 11 January 1991.