- Volume 59 , Number 2
- Page: 271–7
Essential fatty acids in plasma of patients with leprosy
ABSTRACT
We have investigated the fatty-acid composition of plasma phospholipids in 61 patients with leprosy of various clinical types with either a short or long duration of treatment. All patients had significantly decreased levels of linoleic acid and alpha-linoleic acid, the parent fatty acids of the n-6 and n-3 families, respectively. Patients with a treatment duration of more than 6 months had significantly low levels of arachidonic acid and eicosapentaenoic acid compared to controls or to patients with a treatment duration of less than 6 months. There were no differences in the fatty-acid composition between multibacillary patients and paucibacillary patients. We conclude that dietary supplementation with essential fatty acids may be indicated in patients with leprosy, particularly in those with a long treatment duration.RÉSUMÉ
Nous avons étudié la composition en acides gras des phospholipides plasmatiques de 61 patients présentant une lèpre de divers types cliniques et soumis à un traitement de courte ou de longue durée. Tous les patients avaient des niveaux diminués d'acide linoléique et al-pha-linoléique, les acides gras ";pères"; respectivement des familles n-6 et n-3. Les patients ayant suivi un traitement de plus de 6 mois avaient des niveaux si-gnificativement bas d'acide arachidonique et d'acide eicosapentanoique, comparés aux témoins ou aux patients ayant suivi un traitement de moins de 6 mois.RESUMEN
Investigamos la composición en ácidos grasos de los fosfolípidos del plasma en 61 pacientes con diferentes tipos clínicos de lepra y diferentes tiempos de tratamiento. Todos los pacientes tuvieron niveles significativamente disminuidos de los ácidos linoleico y alfa-linoleico, los ácidos grasos progenitores de las familias n-6 y n-3, respectivamente. Comparados con los controles o con los pacientes con menos de 6 meses de tratamiento, aquellos pacientes tratados por más de 6 meses tuvieron niveles significativamente bajos de los ácidos araquidónico y cicosapentacnoico. No hubieron diferencias en la composición de ácidos grasos entre los pacientes multibacilares y los paucibacilares. Concluímos que es conveniente administrar una dicta suplementada con ácidos grasos esenciales a los pacientes con lepra, particularmente a aquellos con tiempos prolongados de tratamiento.Among the factors which determine host resistance and response to the leprosy bacillus, the cellular immune response is of paramount importance (17). Levels of cellular immunity in any individual are the result of a poorly understood interplay between heredity and environment. As yet, no hereditary factors have been shown conclusively to account for the susceptibility of cither an individual or a community to leprosy or to various expressions of the disease, nor to the response to therapy (16,20).
Of the environmental factors which determine cellular immune responses, dietary influences may be among the most important, and yet the leprosy literature over the last few decades has been virtually bereft of investigative work relating diet to leprosy (5). The essential fatty acids (EFAs) are of particular importance to the cellular immune response, both because of their role as precursors of immunoregulatory prostanoids (4) and because of their structural contribution to the maintenance of normal cell membranes (15). We have measured EFAs in the phospholipid fraction of the plasma of patients with various forms of leprosy at various stages of their disease.
WHAT ARE ESSENTIAL FATTY ACIDS?
The essential fatty acids (EFAs) were discovered by George and Mildred Burr (University of Minnesota, U.S.A.) who noted the symptoms of fat deficiency in laboratory animals fed on a fat-free diet (3). These symptoms are: a) poor growth, b) flexural erythema with scaling, c) hair loss, d) itching, e) poor wound healing, f) tendency to cutaneous infection, and g) increased transepi-dermal water loss. Subsequently, it became clear that EFA-deficient humans showed exactly the same signs. If a dietary fat relieves these signs, then it is an essential fatty acid. Hence, the principal criterion for determining the essentiality of a fatty acid is nutritional.
Biochemistry of essential fatty acids. There are two families of essential fatty acids, the n-6 and the n-3 family. The metabolism of each family proceeds by a series of desaturation and elongation steps, with the molecule becoming progressively longer and more polyunsaturated (8) (Fig. 1). The long-chain polyunsaturates (LCPs) containing 20 carbon atoms act as substrates for cyclo-oxygenase and lipoxygenase enzymes with the consequent production of prostaglandins, thromboxanes and leukotrienes.

Fig. 1. Pathways of metabolism of the n-6 and n-3 series of essential fatty acids. Both the n-6 and n-3 series of fatty acids are metabolized by the same series of enzyme reactions.
The n-6 family of EPAs are much more effective at relieving the symptoms of EFA deficiency and, thus, may be regarded as being more essential. In fact, there are very few consequences of deficiency of n-3 EFAs, and there has been much debate about whether they are essential at all. In mammalian systems, members of one family of EFAs cannot be converted to members of another series. The biochemistry of the EFAs has been reviewed at greater length elsewhere (24).
PATIENTS AND METHODS
All patients examined were part of the leprosy control program in Zimbabwe. Many of them were seen in the leprosy unit at Harare Hospital, Harare, Zimbabwe, during the course of regular follow-up clinics, while others were seen at ";outreach"; clinics instituted as part of the comprehensive post-independence leprosy surveillance program. Control samples were taken from healthy blood donors attending the national blood transfusion service blood donor sessions.
The basic diet of patients and controls was similar, although a formal dietary history was not taken. The staple Zimbabwean diet comprises ground maize made into a stiff porridge (sadza) and eaten twice daily with a gravy made from vegetables (relish). Stewed meat, usually chicken or beef, accompanies this sadza and relish from once weekly to twice daily.
Blood was taken from patients into bottles containing ethylenediamine tetraacetic acid (EDTA). This calcium-chelating agent prevents platelet activation and clotting. It was separated within 3 hr of collection by centrifugation. The plasma was stored at - 25°C until analysis. Plasma lipids were extracted into chloroform : methanol (2:1, v/v), filtered through sodium sulfate, and evaporated to dryness under nitrogen before being taken up into 0.5 ml chloroform. This lipid extract was applied in a band 1.5 cm wide, 2 cm from the bottom of a 20 cm x 20 cm thin-layer chromatography (TLC) plate bearing a 0.5 mm uniform layer of silica gel G (Whatman, England). The chromatography plates were developed in hex-ane : diethyl ether (80:20) in which solvent mixture the phospholipid fraction remains at the origin. This fraction was identified by visualization under ultraviolet (UV) light after spraying with 0.05% dichlorofluores-cein in 95% methanol, and scraped off into glass tubes before being methylated.
The phospholipid fraction was methylated according to the method of Morrison and Smith (13). Briefly, the phospholipid band identified on the TLC plate and scraped off was added to 2 ml of 7% boron trifluoride in methanol. The tubes were flushed with nitrogen, sealed, and placed in a boiling water bath for 10 min. After cooling on ice, methyl esters were extracted into 4 ml hex-ane followed by 2 ml water. The upper phase was removed and the lower phase re-extracted with 2 ml hexane. The combined extracts were evaporated to dryness under nitrogen, and taken up into 100 μ1 of hexane for gas chromatography. Methyl esters of prepared phospholipids were separated by gas chromatography on a Hewlett-Packard 5880 gas chromatograph on a six-foot column packed with 10% Silar 10C on Chro-masorb WAW 106/30. The carrier gas was helium (30 ml/min). Oven temperature was programmed to rise from 165°C to 190°C at 2°C per min. Injector temperature was 200°C and detector temperature was 220°C. Fatty-acid methyl esters were identified by comparing the chromatography peaks with a set of standards (Nuchek Prep Inc., Ely-sian, Minnesota, U.S.A.) and automatically quantitated by a Hewlett Packard Level 4 Integrator.
For purpose of analysis, the patients were considered to have multibacillary leprosy if they were classified as lepromatous (L) or borderline lepromatous (BL) according to Ridley (21), and to have paucibacillary leprosy if they were classified as having borderline tuberculoid (BT) or tuberculoid (T) disease. They were further divided according to duration of treatment into those who had had treatment for less than 6 months and those who had been treated for more than 6 months. The results for the various groups were compared using a two-tailed Student's t test, adopting the null hypothesis that there was unlikely to be any difference between groups. Differences were considered to be significant when the possibility of their occurring by chance was 5% or less.
RESULTS
There were 14 patients with multibacillary leprosy of treatment duration less than 6 months (Group A). There were 13 patients with paucibacillary leprosy and a treatment duration of less than 6 months (Group B). Thirteen patients with multibacillary leprosy were treated for more than 6 months (Group C), and 12 patients with paucibacillary leprosy were treated for more than 6 months (Group D). There were nine patients with leprosy of either type who were in reaction, either erythema nodosum lep-rosum (ENL) or reversal reaction (Group E). The results for each group are shown in Table 1 for the principal fatty acids.
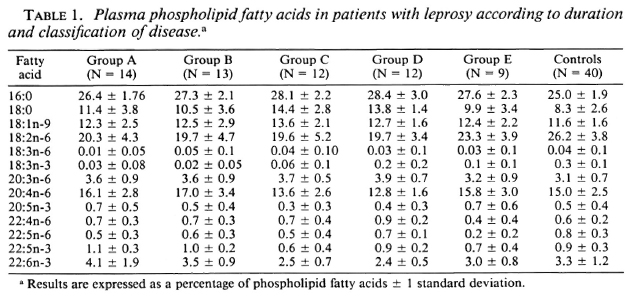
If the two groups of patients with multibacillary disease (Groups A and C) are compared to the two groups with paucibacillary disease (Groups B and D), there are no significant differences. Major differences emerge when patients with a short duration of treatment are compared to those with a long duration of treatment, regardless of disease classification. Table 2 compares all patients with a treatment duration of less than 6 months to those with a treatment duration of more than 6 months. (For purposes of convenience, the former group will be called ";untreated,"; and the latter group ";treated.";)

Stearic acid (18:0) was present in all patients at significantly greater amounts than in the control group (Fig. 2), but treated patients had significantly higher levels than untreated patients. Linoleic acid (18:2n-6) was present in significantly decreased amounts in both treated and untreated groups compared to controls (p < 0.01), as was its desaturated and elongated derivative dihomogamma linolenic acid (20:3n-6). The two groups diverged in the proportions of arachidonic acid (20:4n-6) found in their plasma phospholipids. Untreated patients had significantly increased levels compared to controls (p < 0.05); treated patients had significantly decreased levels (p < 0.05). The equivalent fatty acid to arachidonic acid of the n-3 family is eicosapentaenoic acid (20: 5n-3) (Fig. 1). This followed a similar pattern, being significantly elevated in the untreated group (p < 0.05) and decreased in the treated group (p < 0.01). These results are expressed graphically in Figure 2, where EFA levels in patients arc expressed as a percentage of control values. When compared to each other, untreated patients had significantly higher levels of arachidonic acid and eicosapentaenoic acid than did treated patients, even though levels of the parent fatty acid of the n-6 series, linoleic acid, were similar in both groups.
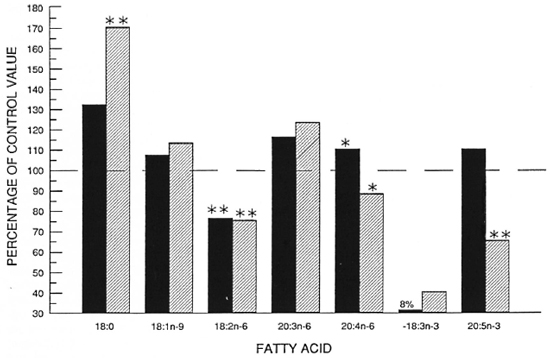
Fig. 2. Principal fatty acids in plasma phospholipids of patients with leprosy expressed as a percentage of normal.  = treated < 6 months (N = 27);
= treated < 6 months (N = 27);  = treated > 6 months (N = 24); * = p < 0.01 compared to control value; ** = p < 0.001 compared to control value.
= treated > 6 months (N = 24); * = p < 0.01 compared to control value; ** = p < 0.001 compared to control value.
DISCUSSION
This study has demonstrated significant abnormalities of fatty-acid composition in plasma phospholipids of patients with leprosy according to the duration of treatment. A number of questions arise. Perhaps most pertinent is the question of whether these changes reflect disease activity or treatment, or whether disease activity may in any way be affected by these abnormalities in fatty-acid composition.
Linoleic acid (18:2n-6) is the principal dietary essential fatty acid, and is found particularly in seed oils. The staple diet eaten by Zimbabwean people provides a plentiful supply of linoleic acid, since approximately 70% of the lipid content of maize is linoleic acid (19). In dietary essential fatty-acid (EFA) deficiency, the low intake of linoleic acid is reflected not only in low levels of its 6-de-saturated long-chain polyunsaturated metabolites, but also by the appearance in the blood of the ";Mead acid,"; the triene 20: 3n-9 (23). In patients with leprosy, not only were the levels of the 6-desaturated derivatives high but the ";Mead acid,"; indicative of EFA deficiency, was not detected. Thus, although we did not conduct a formal study of diet in these patients, it seems highly unlikely that they are EFA deficient.
Low levels of linoleic acid may have several consequences, both biochemical and physiological. There is a compensatory rise in other 18 carbon fatty acids, and in this study both the saturated fatty acid, stearic acid (18:0), and the mono-unsaturate, oleic acid (18:ln-9), were elevated, significantly in the former. This finding helps to validate the results. Cutaneous barrier function depends predominantly on the incorporation of linoleic acid into epidermal lipids for its integrity (1). Replacement of linoleic acid by other 18 carbon fatty acids results in increased transepidermal water loss and dry skin (11). Thus, the low levels of linoleic acid found in patients with leprosy may account for the dry skin which is a feature of the disease. Confirmation of this would require specific study of epidermal stratum cor-neum barrier lipids in patients with leprosy, but it is possible that a parallel may exist in atopic dermatitis, where deficient incorporation of linoleic acid into epidermal sphingolipids may be responsible for the increased transepidermal water loss and dry skin typical of that condition (11).
In a European population, low levels of linoleic acid are a powerful and independent risk factor for coronary artery disease (18,22). Perhaps because leprosy during the latter half of this century has been predominantly a disease of the developing world, there is very little information concerning coronary artery disease in patients with leprosy. Mou-lopoulos, et al. (14) found the prevalence of coronary artery disease to be substantially higher in 475 patients with leprosy than in reported epidemiological studies of patients without leprosy, and they were unable to relate this apparently high prevalence to blood cholesterol levels. They proposed ";some other risk factor that contributes to the high prevalence of CHD (coronary heart disease).";
The metabolism of linoleic acid involves a series of desaturation and elongation steps (Fig. 1). The initial desaturation of linoleic acid occurs at the [6;-6 position and is the rate-limiting step of the pathway. It is a part of the cytochrome P450 enzyme system, its election flux being supplied by NADH cytochrome b5 reductase (9). With low levels of linoleic acid but increased levels of its 6-desaturated metabolites, dihomogamma linolenic acid (20:3n-6) and arachidonic acid (20:4n-6) found in the group with a short treatment duration, it is possible that this desaturation process may be enhanced. Our high levels of eicosapentaenoic acid (20:5n-3), the equivalent of arachidonic acid in the n-3 series, is further evidence of this, since the same enzyme systems are probably responsible for the metabolism of both the n-6 and n-3 series of fatty acids (23). It may be that leprosy chemotherapy may be responsible for increasing the rate of the [6;-6-desaturation of linoleic acid by inducing enzyme activity, although there are no laboratory data to support this hypothesis.
Even were this the case, we would still have to account for the finding of low levels of arachidonic acid and eicosapentaenoic acid in the patients with a long treatment duration. If we accept that the dietary intake of linoleic acid is likely to be adequate, then we only account for low levels of its metabolite, cither by increased consumption or by decreased formation. Decreased formation of arachidonic acid would have the consequence of an increased level of its precursor, dihomogamma linolenic acid (DGLA), because of a reduction in the activity of the [6;-5-desaturase enzyme. These results do indeed show DGLA levels to be increased compared to controls. Again, there are very few data to suggest that antileprosy chemotherapy may affect this enzyme although several antibiotics may provoke an increase in the rate of synthesis of prostaglandins from their fatty-acid precursors (6), raising the possibility that drugs may decrease ar-achidonic-acid levels either by reducing the activity of [6;-5-desaturase, or more likely by increasing the rate of conversion of arachidonic acid to prostaglandins. Arachidonic acid is the substrate for cyclo-oxygenase and lipoxygenase enzymes, with the consequent synthesis of pro-inflammatory prostaglandins and leukotrienes. Low levels of arachidonic acid in cell membranes may also result in profound alterations in the behavior of the cell. This may be especially true of the nervous system, which is particularly rich in long-chain polyunsaturates of both the n-6 and n-3 series (10), but is also true of the immune system, where n-6 fatty acids may have a profound influence (12).
The finding of fatty-acid abnormalities in leprosy is not new. Anderson and Anderson reported in 1935 (2) that patients with leprosy had abnormal fatty acids which returned toward normal in those who responded to treatment with chaulmoogra oil (a mono-unsaturated fatty acid itself). These early data received some support from a recent study using the same methods as those used in the investigation reported here (25). Our data add to these previous reports of fatty-acid abnormalities in leprosy, and although we cannot account for the variable immune response to Mycobacterium leprae by changes in fatty-acid composition, we do conclude that further study of this relationship between leprosy and EFA composition is likely to be rewarding. In particular, the finding of low levels of linoleic acid in leprosy patients overall suggests that the effect of dietary supplementation with linoleic acid in patients with leprosy should be studied.
Il n'y avait aucune difference entre patients multiba-cillaires et paucibacillaires dans la composition en acides gras. Nous en concluons qu'un régime alimentaire enrichi en acides gras essentiels peut être indiqué chez les malades de la lèpre, et particulièrement chez ceux soumis à un traitement de longue durée.
Acknowledgment. We would like to thank Dr. Bernard Naafs for helpful advice, both in this country and in Zimbabwe. The leprosy program in Zimbabwe was greatly aided by Amici di Raoul Follereau, Bologna, Italy. Dr. Wright was supported by a research grant from the Leprosy Relief Association (LEPRA) and a ";Quinoderm Travelling Scholarship"; from the British Association of Dermatologists. We are grateful to Ms. Wendy Straughair for preparing the manuscript.
REFERENCES
1. Abraham, W., Wertz, P. W. and Downing, D. T. Linoleate-rich acylglucosylceramides of pig epidermis: structure determination by proton magnetic resonance. J. Lipid Res. 26(1985)761-766.
2. Anderson, H. H. and Anderson, H. V. D. Iodine values and total lipids of leprous human blood sera. Proc. Soc. Exp. Biol. Med. 32(1935)1470-1476.
3. Burr, G. O. and Burr, M. M. A new deficiency disease produced by the rigid exclusion of fat from the diet. J. Biol. Chem. 82(1929)345-348.
4. Crawford, M. A. Essential fatty acids and their prostanoid derivatives. Br. Med. Bull. 39(1983)210-213.
5. Foster, R. L., Sanchez, A. L., Stuyvesant, W., Foster, F. N., Small, C. and Lau, B. H. S. Nutrition in leprosy: a review. (Editorial) Int. J. Lepr. 56(1988)66-81.
6. Garraffo, R., Jambou, D. and Lapalus, P. Effects de quelques antibiotiques sur la production de prostaglandins par les macrophages in vitro. Pathol. Biol. 37(1989)643-648.
7. Holman, R. T., Smythe, L. and Johnson, S. Effect of sex and age on fatty acid composition of human serum lipids. Am. J. Clin. Nutr. 32(1979)2390-2399.
8. Horrobin, D. F. Gamma linolenic acid: an intermediate in essential fatty acid metabolism with potential as an ethical pharmaceutical and as a food. Rev. Contemp. Pharmacother. 1(1990)145.
9. Horrobin, D. F. The regulation of prostaglandin biosynthesis by the manipulation of essential fatty acid metabolism. Rev. Pure Appl. Pharmacol. Sci. 4(1983)339-383.
10. Jamal, G. A., Carmichael, H. and Weir, A. L. Gamma linolenic acid in diabetic neuropathy. Lancet 1(1986)1098-1099.
11. Melnik, B., Hollman, J. and Plewig, G. Decreased stratum corneum ceramides in atopic individuals-a pathobiochemical factor in xerosis. Br. J. Dermatol. 120(1988)547-548.
12. Mertin, J. and Mertin, L. A. Modulation of in vivo immune responses following changes in the intake of essential fatty acids. Prog. Allergy 44(1988)176-206.
13. Morrison, W. R. and Smith, L. M. Preparation of fatty acid methyl esters and dimethyl acetáis from lipids with boron trifluoride-methanol. J. Lipid Res. 5(1964)600-609.
14. Moulopoulos, S. D., Diamantopoulos, E. J., Adamopoulos, P. N. and Anthopoulos, L. P. Epidemiology of coronary artery disease among Hansen's patients. Angiology 31(1980)82-90.
15. Murphy, M. G. Dietary fatty acids and membrane protein function. J. Nutr. Biochem. 1(1990)68-79.
16. Nakajima, S., Kobayashi, S., Nohara, M. and Sato, S. HLA antigen and susceptibility to leprosy. Int. J. Lepr. 45(1977)273-277.
17. Nath, I. Immunology of human leprosy-current status. Lepr. Rev. Special Issue (1983)31S-45S.
18. Oliver, M. F., Riemersma, R. A., Thomson, M., Fulton, M., Abraham, R. A. and Wood, D. A. Linoleic acid and coronary heart disease. Br. J. Hosp. Med. 42(1989)301-302 (23 rel).
19. Paul, A. A., Southgate, D. A. T. and Russell, J. First Supplement to McCance and Widdow-son's The Composition of Foods: Amino Acids and Fatty Acids. London: H.M.S.O., 1980, p. 105.
20. Rea, T. H., Levan, N. E. and Terasaki, P. I. Histocompatibility antigens in patients with leprosy. J. Infect. Dis. 134(1976)615-618.
21. Ridley, D. S. Histological classification and the immunological spectrum of leprosy. Bull. WHO 51(1974)451-465.
22. Riemersma, R. A., Wood, D. A., Butler, S., Elton, R. A., Oliver, M., Salo, M., Nikkari, T., Vartiainen, E., Puska, P., Gey, F., Rubba, P., Mancini, M. and Fidanza, F. Linoleic acid content in adipose tissue and coronary heart disease. Br. Med. J. 292(1986)1423-1427.
23. Rivers, J. P. W. and Frankel, T. L. Essential fatty acid deficiency. Br. Med. Bull. 37(1981)59-64.
24. Wright, S. Essential fatty acids and the skin. Prostaglandins Leukot. Essent. Fatty Acids 38(1989)229-236.
25. Wright, S. Essential fatty acids in the plasma phospholipids of patients with leprosy. Br. J. Dermatol. 112(1985)673-677.
1. M.A., M.D., M.R.C.P., Department of Dermatology, Royal Free Hospital and School of Medicine, London NW3 2QG, England.
2. Ph.D., Efamol Research Institute, Kentville, Nova Scotia, Canada.
3. Ph.D., Efamol Research Institute, Kentville, Nova Scotia, Canada.
Received for publication on 7 November 1990.
Accepted for publication in revised form on 14 January 1991.