- Volume 59 , Number 2
- Page: 278–91
Differential handling of bacterial antigens in macrophages infected with Mycobacterium leprae as studied by immunogold labeling of ultrathin sections
ABSTRACT
Mycobacterium leprae were purified f rom the livers of experimentally infected armadillos, and the purity of the bacterial preparation was established by electron microscopy, immunoelectrophoresis of purified bacilli with rabbit serum raised against liver tissues f rom a noninfected armadillo, and gas chromatography. Such purified and intact bacilli were fixed and embedded by a gelatin-Lowicryl method for electron microscopy which preserved the mycobacterial antigens. Ultrathin sections were labeled with antisera raised in rabbits against the total antigens of the following species of mycobacteria: M. leprae, M. bovis BCG, M. avium, and a rapid-growing, nonpathogenic species, M. fallax. Bacteria were also labeled using serum raised against 2,3-diacyl-tre-halose-2'-sulfate (sulfolipid-IV or SLIV) isolated and purified f rom M. tuberculosis. The immunolabeling was visualized under the electron microscope (EM) by using a secondary probe (goat-antirabbit IgG, H + L, coupled to 5 nm gold particles; GAR-5). EM results showed that M. leprae bacilli were highly labeled with all of the antisera used except SLIV, which was present only in discrete amounts. All of the antisera used labeled the bacterial ";capsule,"; showing that this structure was not an artifact since it contained mycobacterial antigens. In parallel experiments, the murine J-774 macrophage cell line was infected with purified M. leprae, and fixed for EM at various time intervals for 1 week. Although the phago-cytized bacteria did not multiply during the 1-week experiment, macrophages were unable to lyse them. Immunogold labeling of bacterial antigens in ultrathin sections of infected macrophages helped us to conclude: a) bacterial death and/or lysis is not a prerequisite for processing of antigens by infected macrophages; b) there was conclusive evidence for a differential antigen handling, i.e., some antigens were rapidly released (within 2 days, mostly capsular antigens) inside infected macrophages and transported to the macrophage surface, whereas others (the majority of them located in the cell-wall skeleton and in deeper bacterial structures) remained unreleascd even after 4 to 7 days of infection; c) although relatively fewer epitopes reacting with anti-SL.v antibodies were found, they were rapidly released (within 2 days) inside macrophages, and exocytized to the macrophage surface. These novel findings are discussed in relation to leprosy and the current knowledge about the processing of bacterial antigens.RÉSUMÉ
Du Mycobacterium leprae a été purifié des foies dc tatous infectés expérimentalement, et la pureté dc la préparation bactérienne a été vérifiée par microscopic électronique, immunoélectrophorèse de bacilles purifiés avec du sérum de lapin active contre les tissus hépatiques à partir d'un tatou non infecté, et par chromatographic gazeuse. Dc tels bacilles purifiés et intacts ont été fixés et inclus dans dc la gélatine-Lowicryl, méthode utilisée pour la microscopic électronique et qui préserve les antigènes mycobacteriens. Des coupes ultra-minces ont été marquées avec des anti-sérums activés chez des lapins contre des antigènes totaux des espèces suivantes dc mycobactéries: M. leprae, M. bovis B.C.G., M. avium, et une espèce non pathogène à croissance rapide, M. fallax. Les bactéries ont aussi été marquées à l'aide de sérum activé contre le 2,3-diacyl-tréhalose-2'-sulfatc (sulfolipide IV ou SLIV) isolé et purifié à partir de AI. tuberculosis. Le marquage immu-nologique a été visualisé par microscopie électronique (ME) en utilisant une sonde secondaire (IgG dc chèvre anti-lapin, H + L, couplé à des particules d'or de 5 nm; GAR-5). Les résultats de ME ont montré que les bacilles de AI. leprae étaient fortement marqués par tous les antiserums utilisés, à l'exception du SLIV, qui n'était présent qu'en quantités discrètes. Tous les antiserums utilisés ont marque la ";capsule"; bactérienne, montrant que cette structure n'était pas un artefact puisqu'elle contenait des antigènes mycobactériens. Dans des études entreprises en parallèle, la lignée cellulaire macropha-gique J-774 d'origine murine a été infectée avec du M. leprae infecté, et fixé pour ME à différents intervalles de temps pour une semaine. Bien que les bactéries phagocytées ne se sont pas multipliées durant l'expérimentation d'une semaine, les macrophages n'étaient pas capables dc les détruire. Le marquage immuno-logique des antigènes bactériens avec de l'or dans les coupes ultra-minces des macrophages infectés nous ont aidés à conclure que: a) la mort bactérienne et/ou la lysc n'est pas une condition préalable à la prise en charge des antigènes par les macrophages infectés; b) on a eu la preuve décisive d'une prise en charge différenciée des antigènes, c'est-à-dire que certains antigènes étaient libérés rapidement (dans les deux jours, surtout les antigènes capsulaires) à l'intérieur des macrophages infectés et transportés à la surface des macrophages, alors que d'autres (la majorité dc ceux localisés dans le squelette de la paroi cellulaire et dans les structures bactériennes plus profondes) n'étaient pas libérés même après 4 à 7 jours d'infection; c) bien qu'on a trouvé relativement peu d'épitopes réagissant avec les anticorps anti-SLIV, ils furent rapidement libérés (endéans les 2 jours) à l'intérieur des macrophages et expulsés à la surface du macrophage par exocytose. Ces nouvelles découvertes sont discutées dans la perspective de la lèpre et des connaissance actuelles de la prise en charge des antigènes bactériens.RESUMEN
Se purifico Mycobacterium leprae del higado de armadillos infectados experimentalmente y la pureza de la preparación bacteriana se estableció por microscopía electrónica, por inmunoelectroforésis contra un suero de conejo anti-tejido hepático dc un armadillo sano, y por cromatografía dc gases. Los bacilos purificados intactos dc lijaron y embebieron para microscopía electrónica en gclatina-Lowicryl, un método que preserva los antigenos micobacterianos. Las secciones ultradcl-gadas se hicieron reaccionar con antisucros dc conejo dirigidos contra los antígenos totales de M. leprae, M. bovis BCG, M. avium, y M. fallax, una especie no patógena dc crecimiento rápido. Las bacterias también se hicieron reaccionar con un suero contra el 2, 3-dia-cil-trehalosa-2-sulfato (o sulfolípido IV, SLIV) del M. tuberculosis. La reactividad se visualizó al microscopio electrónico (ME) usando un anticuerpo de cabra anti-IgG dc conejo, H + L, acoplado a partículas dc oro de 5 nm; GAR-5. Los resultados al ME mostraron que los bacilos de la lepra reaccionaron intensamente con todos los antisueros, excepto con el anti-SLIV, el cual sólo estuvo presente en cantidades discretas. Todos los antisueros usados marcaron la cápsula de la bacteria, indicando que esta estructura contiene antígenos micobacterianos. En experimentos paralelos, se infectaron macrófagos murinosde la linca J-774 con M. leprae purificados y se fijaron para ME a varios intervalos de tiempo hasta completar una semana. Aunque las bacterias fagocitadas no se multiplicaron durante el experimento de 1 semana, los macrófagos fueron incapaces de lisarlas. El inmunomarcaje con oro dc los antígenos bacterianos en las secciones ultradelgadas de los macrófagos infectados, nos ayudó a concluir: (a) que ni la muerte bacteriana ni su lisis es un pre-re-quisito para el procesamiento dc antigeno por los macrófagos infectados; (b) que hay un manejo diferencial dc los antígenos, i.e., algunos antígenos, principalmente capsulares, fueron rapidamente liberados (en dos días) dentro de los macrófagos infectados y transportados a la superficie de los macrófagos, mientras que otros (la mayoría de ellos localizados en el esqueleto de la pared celular y en las estructuras bacterianas más profundas) permanecieron sin ser liberados aún después de 4 a 7 días de infección, y (c) que aunque hubieron relativamente menos epitopes reactivos con los anticuerpos anti-SLIV, ellos fueron rápidamente liberados (en dos días) dentro de los macrófagos y exo-citados a la superficie celular. Estos hallazgos novedosos se discuten en relación a la lepra y al conocimiento actual sobre el procesamiento de los antígenos bacterianos.One of the current challenges to the scientific community is the designing of a new vaccine to combat mycobacterial diseases, including leprosy which afflicts about 15 to 20 million people in the world (35). Instrumental in the designing of a ";subunit"; vaccine is the comprehension of those species-specific antigens which may be successfully processed and presented by host macrophages to initiate specific clonal expansion of protective T lymphocytes. One of the dogmas linked to this domain has been the notion that bacterial killing and antigen processing inside infected macrophages are somehow linked, which does not fit well in the case of mycobacterial pathogens, many of which may remain intact for days, or even months inside macrophages (30). It has been suggested recently that killing and antigen presentation are two independent events in Mycobacterium leprae-intected macrophages (4), with T-cell anergy being the primary defect since unresponsiveness to M. leprae could be restored by the exogenous addition of lymphokines only in a fraction of patients. The limited reactivity was suggested to be linked to the expansion of a few clones essentially crossreactive to environmental mycobacteria (4).
Crossreactive epitopes among mycobacteria (13,25,36,42) and various actinomycetes (24,36) have been reported, and some of the human T-cell-activating, recombinant mycobacterial antigens were found to be shared antigens (18). Prior exposure to environmental mycobacteria has been reported to affect the later host response to M. leprae (13,22,44) Based on these lines, it has been shown that: 1) immunization of mice with M. vaccae and M. bovis BCG provided protection against infection by M. leprae (40); b) immunotherapy with M. vaccae as an adjunct to chemotherapy in the treatment of pulmonary tuberculosis gave promising results (43) and c) the coating of M. leprae surface antigens by common mycobacterial antibodies reversed the inhibition of phago-some-lysosome fusion (PLF) in infected macrophages (7,31).
In the present study, we decided to study the distribution of M. leprae antigens on ultrathin sections using various antisera to determine crossreactivity of antigens located in ultrastructurally defined bacterial structures. Bacteria were also labeled with antisulfolipid-IV (SLIV) antibodies in light of recent observations that both leprosy and tuberculosis patients contained antibodies to SL1V antigens (2). In macrophages experimentally infected with M. leprae, the processing of the above-mentioned antigens was subsequently followed during 1 week of infection. The results obtained gave novel information about the mechanisms of pathogenicity of M. leprae.
MATERIALS AND METHODS
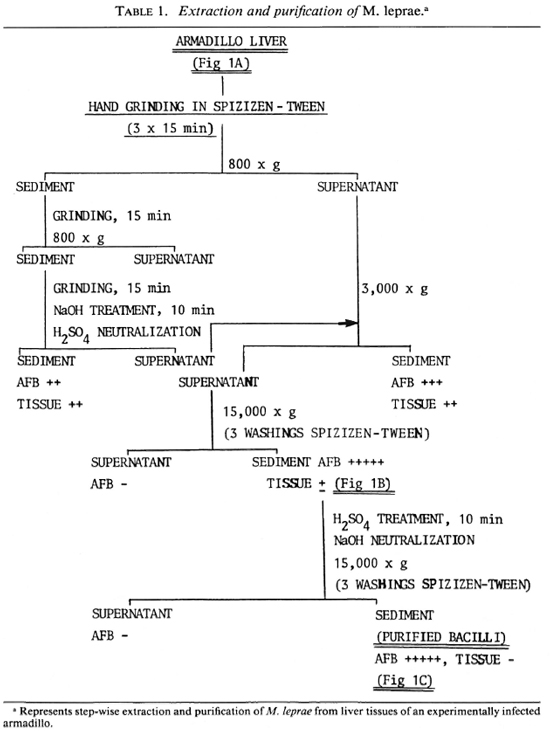
Extraction of bacteria. M. leprae were freshly isolated from the liver of an experimentally infected armadillo. The detailed scheme is summarized step-wise in Table 1, and typical electron-micrographic observations are represented in Figure 1. Briefly, nonirradiated, infected liver from an armadillo (Fig. 1A) was manually ground in Spizizen salt solution (7,31) containing 0.05% (v/v) Twecn-80 to avoid clumping (3 x 15 min), and centrifuged at 800 x g at 4°C to remove the tissue debris. The tissue debris was treated two to three times similarly to further extract the bacilli. The pooled supernatants were centrifuged at 800 x gand 3000 x g, successively, to remove larger bacterial clumps. The bacilli from the 3000 x g supernatant were recovered by centrifugation at 15,000 x g to obtain a sediment which was washed three times with 250 volumes of Spizizen-Tween to remove a maximum of tissue debris (partially purified bacterial preparation; Fig. 1B). The remaining host tissues from such partially purified bacteria were digested by H2SO4 treatment (4%, 10 min), neutralized using 6% NaOH, and centrifuged at 15,000 x g (with three consecutive washings with 250 volumes of Spizizen-Tween) to give a sediment of purified bacilli [Fig. 1C; acid-fast bacilli (AFB) observed by Ziehl-Neelsen staining = 5 +, absence of host tissues]. The relative purity of all the preparations so obtained was tested by running them against anti-armadillo serum (raised in rabbits by inoculating liver tissues of a healthy, female armadillo) by immunoelectrophoresis (Fig. 2), and by comparing the gas chromatographic profiles of their fatty acids for the presence of tuber-culostearic acid (Fig. 3).
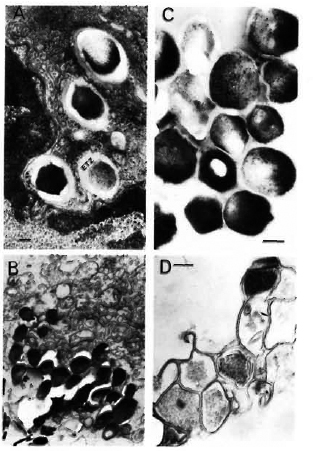
Fig. 1. Transmission-electron microscopy using standard epon embedding and uranyl acetate-lead citrate staining of Mycobacterium leprae fixed during various steps of its extraction and purification. A = M. leprae liver of an experimentally infected armadillo showing the presence of many intact bacilli surrounded by an electron-transparent zone (ETZ) inside the host tissues; B = partially purified bacilli still containing many host tissues; C = a purified bacterial preparation showing structural integrity of bacilli with many ri-bosomal structures and electron-dense cytoplasm. It should be noted that processed by the standard epon-embedding method, the bacterial capsule (corresponding to the ETZ observed inside host tissues) is not visible; D = although sonication of partially purified bacilli as shown in B and consequent washings with Spizizen salt solution resulted in the removal of host tissues, the method could not be used since it also resulted in massively broken bacterial cells (bar marker represents 100 nm).
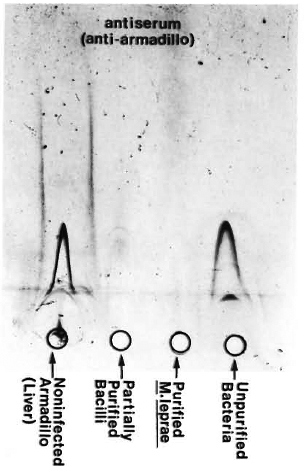
Fig. 2. Immunoelectrophoresis of various bacterial fractions run against anti-armadillo serum (raised in rabbits by inoculating liver homogenate from a non-infected, female armadillo). Lack of precipitation lines in the purified bacterial preparation as compared to positive controls confirmed the immunological purity of our bacteria.
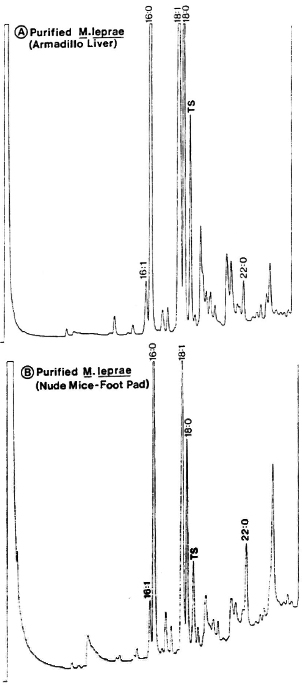
Fig. 3. Gas-chromatography of methyl esters of fatty acids in chloroform-methanol extracts of M. leprae purified from the liver of an experimentally infected armadillo (A) or from the foot pads of experimentally infected nude mice (B). Peaks represent fatty acid esters: numbers indicate, respectively, carbon atoms, double bonds and alcoholic functions; TS (tuberculostearic acid or 10-methyloctadecanoic) peak represents a selective marker for mycobacteria.
Our efforts to remove the remaining host tissues in partially purified bacteria (Fig. 1B) by ultrasonic treatment were fruitful. However, the method was not used in practice since electron-microscopic examination showed that this resulted in broken cells (Fig. 1D).
Our extraction method thus resulted in bacteria which were solid-staining, preserved the normal ultrastructure of M. leprae, and contained about 400 pg of ATP per 106 organisms (7,31).
Fatty acid analysis. Among mycobacterial fatty acids, the 10-methyloctadeca-noic acid (tuberculostearic acid, TS) is considered a reliable chemical marker for indicating the presence of mycobacteria. Consequently, we also tested the M. leprae extracted and purified by our method for their fatty-acid analysis by gas-liquid chromatography (GLC) as reported earlier (33).
Briefly, the bacilli harvested from either experimentally infected armadillo liver or foot pads of nude mice were extracted overnight with 2:1 (v/v) chloroform-methanol. The chloroform layer was evaporated to dryness, and the native lipids were saponified using 5% (w/v) KOH in methylcello-solve. The saponification mixtures were acidified, fatty acids were extracted in diethyl ether, and the extracts were evaporated to dryness and methylated using diazo-methane. The methylated fatty acids were analyzed by GLC using a Delsi 330 apparatus equipped with flame ionization detector (33).
Macrophage culture and infection. The J-774 mouse cell line is derived from a tumor which arose in a female BALB/c/NIH mouse (26). These cells have a number of properties characteristic of macrophages (26,41), e.g., the cells are readily adherent, phagocytic, able to bind antibody-coated erythrocytes, able to function as effector cells in antibody-dependent cellular toxicity, show similar chemotactic responsiveness in vitro as resting peritoneal macrophages, and contain significant adenosine deaminase activity (an enzyme important for normal immune function and maturation of macrophages). The J-774 macrophages have recently been used to propagate a variety of pathogenic mycobacteria (27,29,34).
The method used for culturing J-774 cells has been explained in detail earlier (29). Briefly, macrophage cultures were prepared by seeding 5 x 104 cells per 35-mm Falcon tissue-culture dish in the presence of 1.5 ml of RPMI 1640 medium supplemented with 10% (v/v) fetal calf serum (FCS), and 2 mM L-glutamine. Three days later, each culture dish contained about 106 cells (the optimal division time of J-774 cells in the medium used was about 16 hr). At this step, the culture medium was replaced with new medium containing only 1% (v/v) FCS and 2 mM L-glutamine, which stopped the further macrophage division but maintained the cell viability during a 7-day period as verified by trypan blue staining (29). Such nondividing, adherent, and viable macrophages were used for all experiments with M. leprae.
The macrophages in individual culture dishes were infected with freshly isolated M. leprae (5 x 107 bacilli in a volume of 0.1 ml of RPMI 1640 medium per culture dish), incubated for 4 hr at 37°C, and washed three times with Hanks' balanced salt solution to remove all extracellular bacteria. Both the absence of extracellular bacilli as well as the mean number of M. leprae per macrophage were established by Ziehl-Neelsen staining of two dishes in parallel. About 50% to 60% of the macrophages were found to be infected at this step, and they harbored from 5 to 50 bacilli per cell. A majority of the infected macrophages (about 70%) contained around 20 to 25 bacilli per cell. The infected macrophages were maintained for a total of 7 days in the presence of 5% CO2 in a humidified atmosphere at 37°C, with one change of medium at day 4. Infected cells were fixed for electron microscopy at time 0, 2 days, 4 days, and 7 days. All cell culture reagents were purchased from Flow Laboratories, Rockville, Maryland, U.S.A.
Electron microscopy (EM). The EM processing of bacteria as well as infected macrophages was performed using low temperature Lowicryl embedding which preserved the bacterial antigens in ultrathin sections (32). Briefly, infected macrophages were scraped with a rubber policeman, fixed for 1 hr at 4°C using 1.25% (w/v) glutaralde-hyde and 1.25% (w/v) paraformaldehyde in cacodylate buffer (0.1 M, pH 7.2) containing 0.1 M sucrose, rinsed once in the same buffer, treated for 30 min with 50 mM NH4C1 in cacodylate buffer to remove excess aldehydes, and kept overnight at 4°C in the buffer alone. The samples were then pre-embedded in 2% (w/v) agar, postfixed for 30 min at 4°C with 0.5% (w/v) uranyl acetate in veronal buffer, dehydrated in a graded ethanol series and embedded using HM20 grade Lowicryl at -25°C (46).
The above agar-Lowicryl method resulted in good ultrastructural preservation of infected macrophages. However, in samples containing mycobacteria alone, the EM resolution of the bacterial cell envelope (particularly the cell surface and the capsular structure around bacterial cells) could be considerably enhanced by slightly modifying the method and using a gelatin pre-embedding of the bacterial cells instead of agar pre-embedding (32). Consequently, in the case of M. leprae alone, the samples were treated in a similar way except that the cacodylate buffer was devoid of sucrose, and the bacteria were pre-embedded in 10% (w/ v) gelatin instead of 2% (w/v) agar (32).
Immunolabeling of ultrathin sections. The ultrathin sections of the bacteria alone or infected macrophages were mounted on formvar-carbon-coated, 200-mesh nickel grids. The grids were first labeled using specific antibodies raised in rabbits, and the labeling was then visualized using a secondary probe (goat anti-rabbit IgG, H + L, coupled to 5 nm gold particles; GAR-5; Janssen Laboratories, Belgium). Bovine serum albumin (BSA) at 0.5% or 1% (w/v) in phosphate buffered saline (PBS) was used at all the steps to avoid nonspecific labeling. For immunolabeling, grids were floated at room temperature on drops of, respectively, PBS-BSA 1%, 30 min; antiserum 1/100 diluted in PBS-BSA 1%, lhr; 3 x PBS-BSA 0.5%; GAR-5 1/200 diluted in PBS-BSA 1%, 1 hr; 3 x PBS-BSA 0.5%; 3 x double-distilled, deionized water. The grids were examined under a Philips CM-12 electron microscope after mild staining with uranyl acetate and lead citrate.
Lack of labeling in parallel controls in which either the primary antiserum was omitted or was replaced with nonimmune rabbit serum showed the specificity of the reaction. The maximum, nonspecific, background labeling was estimated to be not more than 3-5 gold particles per frame (observed at x 40,000) in less than 5% of the bacteria observed.
Antisera. Specific antisera were raised in rabbits against whole sonicated antigens of M. leprae (pathogenic, noncultivable species); M. avium (serotype 2, opportunistic pathogen, slow grower); M. bovis BCG (slow grower) and M. fallax (nonpathogenic, fast grower). The antisera were raised and characterized as reported earlier (24,31). Specific antiserum against 2,3-diacyl-trehalose-2'-sulfate (SLIV antigen) extracted and purified from M. tuberculosis (23), and characterized as reported recently (2,23) was also used to label the ultrathin sections. The M. avium antiserum was a gift from M.-F. Thorel, Laboratoire Central de Recherches Vétérinaire, 94700-Maisons Alfort, France. All other antisera used in this study were kindly provided by F. Papa, Institut Pasteur, Paris.
RESULTS
Preliminary observations. M. leprae still remains a noncultivable species, and the bacteria for all studies have to be obtained from infected host tissues. When such bacilli have to be used for studying their antigenicity, chemical markers, and molecular interactions between the bacteria and host cells, it is important to obtain freshly isolated bacteria in such a way that the bacilli will be devoid of contaminating host tissue but still remain intact and fairly viable. The method of extraction and purification as applied by us (Table 1) resulted in intact bacilli (Fig. 1) which were devoid of host tissues (Fig. 2), gave a reliable GLC profile with a characteristic TS peak (Fig. 3), and contained about 400 pg of ATP per 106 bacilli. Only M. leprae so characterized were used for all further investigations for studying bacteria-macrophage interactions and antigen processing.
Immunolabeling of bacteria. Mycobacterial antigens can be classified in four broad groups (42-44): a) common mycobacterial antigens (group I); b) slow-grower associated (group II); c) fast-grower associated (group III); and d) species-specific (group IV). We therefore used both homologous and heterologous mycobacterial sera covering all of the spectrum of the mycobacterial antigen groups in M. leprae-infected macrophages in our study to investigate whether there exists a differential handling of various antigens during the M. leprae processing by macrophages.
When ultrathin sections of bacteria embedded alone by the gelatin-Lowicryl method were labeled using various antisera and the labeling was revealed using a GAR-5 probe (Fig. 4), the following significant findings were observed: a) Use of gelatin instead of agar for bacterial samples resulted in its reaction with uranyl acetate to give a darker background to the plastic, permitting the observation of a slightly more electron-transparent ";capsule"; around the bacterium; b) Use of a less-polar HM20 grade Low-icryl instead of K4M and pretreatment of bacteria with uranyl acetate before embedding allowed better cross-linking with the lipidic components of the mycobacterial outer layer and capsule; c) A 30-nm to 180-nm thick ";capsule"; was preserved in more than 50% of the bacteria which totally surrounded them; this ";capsule"; was not an artifact but a true bacterial structure as revealed by its specific labeling by mycobacterial antisera (Fig. 4); d) All of the antisera raised against total mycobacterial antigens (irrespective of slow or rapid growers, pathogenic or nonpathogenic species) highly labeled the M. leprae. Indeed, 100% of the bacteria were effectively labeled, showing the presence of common antigens inside the bacteria as well as in the capsule (Fig. 4). However, it was interesting to note in EM preparations that bacilli which had undergone massive plasmolysis (Fig. 4B) lost a majority of their common antigens (results not shown), but retained some of the peripheral and cell-wall-skeleton antigens reacting abundantly with the anti-M. leprae antiserum (Fig. 4B).
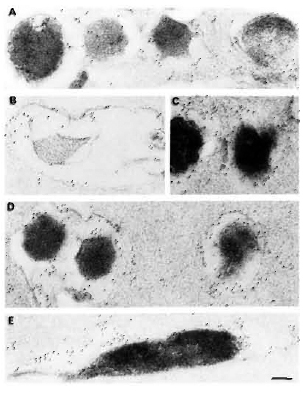
Fig. 4. Immunogoldlabeling of ultrathin sections of M. leprae embedded by a novel gelatin-Lowicryl method using: A and B = anti-M. leprae serum; C = anti-BCG serum; D = anti-M. avium serum; E = anti-M. fallax serum. Secondary probe was GAR-5. All antibodies heavily labeled M. leprae as well as its capsule, showing the presence of crossreactive epitopes. In some plasmolyzed bacilli (b), most of the crossreactive antigens were lost (results not shown). However, M. leprae-specific labeling still remained at periphery of bacterial capsule and in cell-wall skeleton (bar marker represents 100 nm).
Discrete immunolabeling with the anti-SLIV serum was observed in about 30% of the M. leprae (Fig. 7A). In this case, 15 to 25 gold particles per bacterial section in patches of 3 to 5 particles could be observed. This finding was interesting, since the SLIV antiserum used in the present study reacted specifically with the antigen purified from M. tuberculosis and crude lipid extracts of M. tuberculosis and M. africanum but not with the antigens purified from the other 39 mycobacterial species tested (23). On the contrary, rabbit serum inoculated with total antigens of M. leprae recognized native SLIV antigen obtained from M. tuberculosis (23).
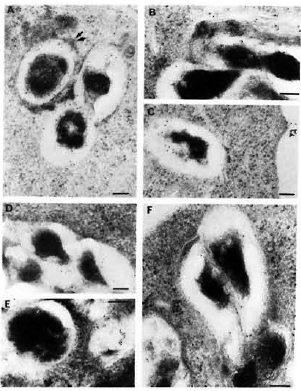
Fig. 5. Immunolabelingof M. leprae-infected macrophages observed after 2, 4, and 7 days of infection, respectively, using anti-M. leprae (A, B, C) or anti-BCG serum (D, E, F). Secondary probe used was GAR-5. Double arrows in A = positive PLF event with transfer of some of bacterial antigens to periphery of fusing lysosomal body; single arrow in C = transfer of bacterial antigens to macrophage surface. After 7 days of infection, the bacterial capsule still had many preserved antigens recognized by anti-M. leprae (homologous) antibodies (C). On the other hand, capsular antigens crossreacting with anti-BCG antibodies were released inside macrophages within 4 days, and at day 7 only a few crossreactive epitopes remained inside the bacterial capsule (F) (bar marker represents 100 nm).
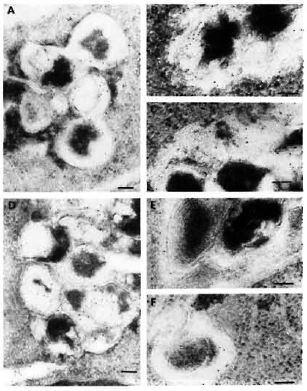
Fig. 6. Immunolabelingof M. leprae-infected macrophages observed after 2, 4, and 7 days of infection, respectively, using: M. avium (A, b, C) or M. fallax (d, E, F) antisera. A = a positive PLF event with subsequent transfer of crossrcactive epitopes to fusion bodies. Although some M. avium crossreactive epitopes were effectively transferred to various macrophage compartments, the bacterial capsule still retained many of them embedded inside its capsule at day 4 (b) and day 7 (C). On the contrary, epitopes crossrcactivc with the rapid-growing, nonpathogenic species M. fallax were most rapidly released and efficiently handled by infected macrophages within 2 days of infection (d). Most crossreactive epitopes were absent in the bacterial capsule at day 4 (E) and day 7 (F). Secondary probe used was GAR-5 (bar marker represents 100 nm).
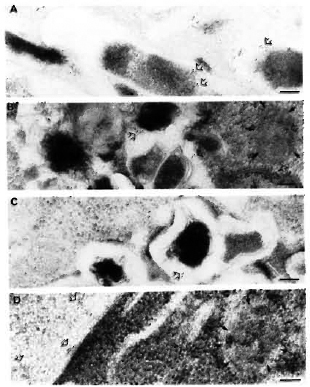
Fig. 7. Immunogoldlabeling of M. leprae bacilli and infected macrophages using SLIV specific antibodies. A = Only 30% of the purified M. leprae bacilli were discretely positive for SLIV epitopes (open arrows) mostly located in the bacterial capsule. A few epitopes were also localized in the bacterial cell wall, and more rarely inside the cytoplasm. When macrophages were immunolabeled after 2, 4, and 7 days of infection (b, C, d, respectively), it was found that this particular antigen was rapidly released and found within 2 days of infection in macrophage compartments other than phagosomes (C). d and E show the transfer of these antigens to various macrophage compartments and to the macrophage surface, respectively. Open arrows = gold labeled epitopes; solid arrows delimit the periphery of macrophage compartments. Secondary probe used was GAR-5 (bar marker represents 100 nm).
Similarly, IgG and IgM antibodies in the sera of leprosy patients reacting with SLIV antigens have been reported (2,12). All of the above findings suggest the possibility that intracellular M. leprae may contain trace amounts of the SLIV antigen which is successfully presented at the macrophage surface, triggering the specific clonal expansion of lymphocytes, and also being instrumental in the reported inhibition of phagosomc-lysosome fusion (PLF) in M. leprae-infected macrophages (7,31). The role of sulfolipid antigens as a primary mycobacterial virulence factor (10) and in altering normal phagocyte functions (10,11,21,48) has been strongly suggested.
Immunolabeling of infected macrophages. M. leprae were rapidly phagocy-tized by J-774 macrophages, their index of phagocytosis being close to that observed earlier for other pathogenic mycobacteria (29,34). Observed for 7 days, the morphology of infected J-774 macrophages was well preserved, and no significant bacterial lysis inside phagosomes or phagolysosomes was observed. Despite PLF inhibition (as observed using acid phosphatase cytochemistry 7,31; results not shown), many positive fusion events could be observed in infected macrophages, permitting the comparison of antigen processing in bacteria residing inside phagosomes and phagolysosomes. The typical EM observations are illustrated in Figures 5-7, and major conclusions based on the observation of about 100 frames/section and 2-3 sections/sample (a total observation of about 300 infected macrophages and about 1000 intracellular bacilli per point) are summarized in Table 2.
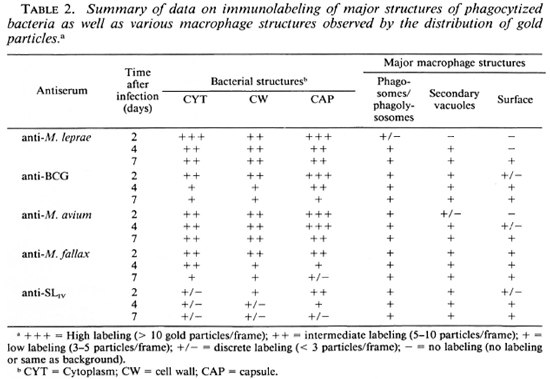
Labeling with homologous serum. In infected macrophages immunolabeled with anti-M. leprae serum, most of the bacteria containing phagosomes did not fuse with lysosomes at day 2. In the relatively rare cases (about 25% of the phagosomes) where PLF did exist, the bacterial capsule prevented contact between the lysosomal material and the bacterial cell wall (Fig. 5A). In the latter case, some of the capsular antigens were transferred to the periphery of the fused lysosomal compartment but not in other macrophage compartments since only rarely could 2 to 3 gold particles be observed in less than 5% of infected macrophages (the same as background). Observed at day 4 (Fig. 5B), about 50% of the bacteria containing phagosomes effectively fused with lysosomes and contained bacterial antigens. Labeling was also found in macrophage compartments other than phagosomes or phagolysosomes. Although the PLF did not change considerably after 7 days of infection, bacterial antigens were transported to the macrophage surface (Fig. 5C, arrow). However, antigens specifically reacting with anti-M. leprae antibodies were still abundant both inside the bacterial cell and in the capsule after 7 days of infection (Table 2).
Labeling with heterologous sera. In contrast to the above observations, it was remarkable that within 2 days of infection (Fig. 5D) antigens in the M. leprae capsule which were common to BCG were rapidly handled (Fig. 5E) and transferred to secondary vacuoles and to the macrophage surface within 4 days (Table 2). At day 7, the bacterial capsule had lost most of the antigens reacting with anti-BCG antibodies (Fig. 5F).
Labeling with anti-M. avium antibodies remained essentially limited to the capsule of M. leprae. However, some common antigens were transferred to the phagolysosomal compartments (Fig. 6A). At day 4 (Fig. 6B) and day 7 (Fig. 6C), various macrophage compartments were immunola-beled. However, contrary to the labeling with anti-BCG serum at day 7, the bacterial cell as well as the capsule still retained most of their common antigens (Fig. 6C, Table 2).
When infected macrophages wero im-munolabeled with antibodies raised against the nonpathogenic, rapid-growing species M. fallax, we found that the common antigens recognized by this serum were the ones which were most efficiently handled and transferred to various macrophage compartments and the surface from day 2 (Fig. 6D) onward. Within 4 days (Fig. 6E), most of the common antigens were handled by the macrophages and at day 7 (Fig. 6F), only discrete labeling of the bacteria inside phagosomes and phagolysosomes could be observed (Table 2).
Labeling with anti-SLIV serum. As described above, only about 30% of the bacterial population was effectively labeled using the anti-SLIv antibodies and, in this case, 15 to 25 gold particles per bacterial section in patches of 3 to 5 particles mostly limited to the cell wall and the capsule could be observed (Fig. 7A). In infected macrophages, the antigens reacting with this specific antibody were released within 2 days of infection, and were found captured inside other macrophage compartments (Fig. 7B). At day 4, intracellular bacilli retained only a few epitopes (Fig. 7C), most of them being localized in macrophage compartments other than bacteria-containing phagosomes or phagolysosomes and transported to the macrophage surface within 7 days of infection (Fig. 7D, Table 2). In all of the EM illustrations, single arrows delimit the periphery of macrophage compartments; double arrows indicate the gold particles.
DISCUSSION
Pathogenic mycobacteria are efficient in a variety of ways in circumventing normal microbicidal mechanisms of the host macrophages (6,30) as well as other immune pathways (l7). In some, the possible roles of various chemically defined substances present in the mycobacterial cell envelope have recently been proposed, e.g., a) inhibition of human lymphoproliferative responses by mycobacterial phenol glycolipids (including PGL-I of M. leprae) in a concentration-dependent manner (5); b) scavenging of reactive oxygen species in activated phagocytes by purified PGL-I (20); c) lack of activation by gamma-interfcron (IFN-7) of M. leprae-infected macrophages (38), probably due to induction of prostaglandin-E2 (PGE2) levels (39) by lipoarabinomannan (LAM) in the M. leprae cell envelope (37); and d) total reversal of enhanced functions in IFN-7 or lipopo-lysaccharide (LPS)-primed macrophages by sulfolipid from M. tuberculosis (21).
Mycobacteria contain various antigens, some of which play a protective role by stimulating the macrophages and T lymphocytes, while others may inhibit or even suppress normal macrophage functions and lymphoproliferation. Unlike M. leprae, which contain large quantities of immunosuppressive complex fatty acids and carbohydrates and cannot be satisfactorily used as a good vaccine, their delipidated cell-wall preparations, containing low molecular weight proteins in avid association with peptidoglycan (PG), have been reported to confer unexpectedly high protection to BALB/c mice against leprosy (8). Similar results have been reported by other workers using various cell-wall-related subcellular fractions of M. leprae (1,9,14-16,19).
The observed reduction in clinical leprosy, although variable, among M. bovis BCG-vaccinated persons supports the notion that species crossreactive determinants are likely to be important in mediating the T-cell-protective responses (45) and should be cloned to prepare a possible broad-spectrum vaccine against mycobacterial diseases, including tuberculosis and leprosy. In the cloning and screening of such future vaccines, one of the most important factors which has as yet not been investigated is the way in which M. leprae antigens are handled by infected macrophages, a step which is crucial in the establishment of cell-mediated immunity. This is the first report dealing with this topic, and shows that there exists a differential handling of various M. leprae antigens in infected J-774 macrophages.
Our results may be summarized as follows. First of all, while doing any study with the as yet uncultivable pathogen M. leprae, it is important to establish an extraction and purification protocol giving good quality bacilli. We have established that bacteria obtained in our case were devoid of host-antigenic contaminants (Fig. 2), but still remained intact (Fig. 1C) and preserved their antigens (Fig. 4). Using a novel gelatin-Low-icryl embedding of the bacteria, we showed that M. leprae effectively contained a capsule-like structure around the bacteria which was not an artifact since it contained many of the specific as well as crossreactive epitopes (Fig. 4). Antisera raised against total bacterial antigens of M. leprae, M. bovis BCG, M. avium, and a rapid-growing, nonpathogenic species, M. fallax, resulted in heavy labeling of bacterial sections (Fig. 4). On the other hand, anti-SLIV serum localized to only a few sites on the leprosy bacillus, suggesting that this antigen or epitopes crossreacting with it were only present as traces (Fig. 7A).
Although the phagocytized bacteria did not multiply during 1 week of experimentation, the macrophages were unable to lyse them and the handling of various bacterial antigens was not done in the same way (Table 2). We observed that bacterial death and/ or lysis was not a prerequisite for the processing of antigens by infected macrophages, and that there was evidence for a differential handling of antigens: Some antigens were released within 2 days (mostly capsular antigens crossreactive with M. fallax; a nonpathogenic, rapid-growing species which contained the antigens of groups I and III in Stanford's classification 42-44) and were transported to the macrophage surface. An opposite situation existed in immuno-labeling with anti-M. leprae antibodies (groups I and IV-positive; group II-?), since it took nearly 7 days for antigens to be transported to the macrophage surface and, even at day 7, much labeling remained restricted to the bacterial capsule inside the phagosomes or phagolysosomes (Table 2). Somewhat intermediate situations were found in the immunolabeling of M. leprae-infected macrophages using antisera raised against M. bovis BCG and M. avium (containing groups I and II antigens in Stanford's classification). On the other hand, epitopes reacting with anti-SLIv antibodies were rapidly released (within 2 days) inside macrophages, and were transported to the macrophage surface (Table 2). It was remarkable that even after 7 days of infection, most of the bacterial cell-wall-skeleton antigens reacting specifically with the anti-M. leprae antibodies still remained located inside the cell wall structure, suggesting that they were only slowly released inside infected macrophages.
The above results indicate that differential handling of the M. leprae antigens by macrophages leads to the release of capsular antigens in the first instance, and that this event is independent of bacterial degradation by host macrophages. Knowing that the mycobacterial capsule (32) and outer layer (OL) (3) mostly contain various amphipathic substances (phenol glycolipids, lipopolysac-charides, lipo-oligosaccharides, sulfolipids, cord factor, etc.), most of which are known to inhibit normal macrophage functions (8,21,30,48) the rapid handling of M. leprae capsular antigens may be partly responsible for the anergy observed in lepromatous leprosy patients. The data obtained also suggest that handling of M. leprae antigens may be broadly classified in the following order by host macrophages: capsular antigens > crossreactive with rapid-growing, nonpathogenic mycobacteria > crossreactive with slow-growing, pathogenic mycobacteria > M. leprae-specific capsular antigens > M. leprae-specific cell-wall-skeleton antigens. Although this order of handling can be convincingly established only after similar studies are performed using monoclon-als raised against different antigenic fractions of M. leprae (47), our previous data on the coating of M. leprae surface antigens with the same antisera as used in this study gave concordant data (7,31). Indeed, we observed previously that coating M. leprae prior to phagocytosis with the various antisera used in the present study reverted the usual PLF inhibition caused by ingested bacteria except when anti-M. fallax antibodies were used (31)- In the case of anti-M. fallax antibodies, although the bacteria were efficiently coated due to abundant labeling of surface antigens crossreacting with M. fallax, the coated antigens were too rapidly released (within 2 hr to 4 hr of ingestion) inside the macrophages to be able to inhibit the PLF event (31).
This investigation, therefore, suggests a differential handling of M. leprae antigens by infected macrophages. It would be interesting to perform similar studies with genetically resistant and susceptible animals using a full range of monoclonals to know whether the differences observed in antigen processing may be linked to the wide spectrum of leprosy observed in man. Last, but not least, our immunolabcling data support the notion that intracellular M. leprae may contain traces of SLIV, or some other closely related substance with common epitopes to SLIV, which is rapidly handled by infected macrophages. Whether this substance may play a role similar to native sulfolipid antigens implicated in M. tuberculosis virulence still remains a matter of debate and merits further investigation.
Acknowledgment. We are grateful to M.-F. Thorel (Laboratoire Central de Recherche Vétérinaire, Maisons Alfort, France) and F. Papa (Institut Pasteur, Paris) for providing various antisera used in this study, and to H. L. David for helpful discussions. We also acknowledge the kind help of A. Varnerot and V. Lévy-Frébault (Institut Pasteur, Paris) for performing the gas-chromatography of M. leprae fatty acids.
REFERENCES
1. Britton, W. J., Hellqvist, L. and Basten, A. Separate antigenic determinants on cell wall associated carbohydrate antigens of Mycobacterium leprae defined with monoclonal antibodies. Int. J. Lepr. 54(1986)545-554.
2. Cruaud, P., Yamashita, J. T., Casabona, N. M., Papa, F. and David, H. L. Evaluation of a novel 2,3-diacyl-trehalose-2'-sulfate (SLIV) antigen for case finding and diagnosis of leprosy and tuberculosis. Res. Microbiol. 141(1990)679-694.
3. David, H. L., Rastogi, N., Clavel-Sérès, S., Clément F. and Thorel, M.-F. Structure of the cell envelope of Mycobacterium avium. Zcntralbl. Bakteriol. Mikrobiol. Hyg. A 264(1987)49-66.
4. Desai, S. D., Birdi, t. j. and Antia, n. H. Correlation between macrophage activation and bactericidal function and Mycobacterium leprae antigen presentation in macrophages of leprosy patients and normal individuals. Infect. Immun. 57(1989)1311-1317.
5. Fournie, J.-J., Adams, E., Mullins, R. J. and Basten, A. Inhibition of human lymphoprolifer-ative responses by mycobacterial phenolic glyco-lipids. Infect. Immun. 57(1989)3653-3659.
6. Fréhel, C, de Chastellier, C, Lang, T. and Rastogi, N. Evidence for inhibition of fusion of lysosomal and prelysosomal compartments with phagosomes in macrophages infected with pathogenic Mycobacterium avium. Infect. Immun. 52(1986)252-262.
7. Fréhel, C. and Rastogi, N. Mycobacterium leprae surface components intervene in the early phagosome-lysosomc inhibition event. Infect. Immun. 55(1987)2916-2917.
8. Gelber, R. H., Brennan, P. J., Hunter, S. W., Munn, M. W., Monson, J. M., Murray, L. P., Siu, P., Tsang, M., Engleman, E. G. and Mohagheghpour, N. Effective vaccination of mice against leprosy bacilli with subunits of Mycobacterium leprae. Infect. Immun. 58(1990)711-718.
9. Gillis, T. P., Miller, R. A., Young, D. B., Khanolkar, S. R. and Buchanan, T. M. Immunochemical characterization of a protein associated with Mycobacterium leprae cell wall. Infect. Immun. 49(1985)371-377.
10. Goren, M. B., Brokl, O. and Schaefer, W. B. Lipids of putative relevance to virulence in Mycobacterium tuberculosis: correlation of virulence with elaboration of sulfatidcs and strongly acidic lipids. Infect. Immun. 9(1974)142-149.
11. Goren, M. B., d'Arcy Hart, P., Young, M. R. and Armstrong, J. A. Prevention of phagosome-lysosomc fusion in cultured macrophages by sulfatidcs of Mycobacterium tuberculosis. Proc. Nat. Acad. Sei. U.S.A. 73(1976)2510-2514.
12. Khuller, G. K., Malik, U. and Kumar, B. Antibodies to sulfolipids in human leprosy sera. Int. J. Lepr. 50(1982)319-321.
13. Lyons, N. F. and Naafs, B. Influence of environmental mycobacteria on the prevalence of leprosy clinical type. Int. J. Lepr. 55(1987)637-645.
14. Marolia, J., Robinson, P. and Mahadevan, P. R. Complex component modulating immune-dc-ficicnt cells in leprosy patients leading to loss of viability of Mycobacterium leprae-a. possible vaccine. Clin. Exp. Immunol. 79(1990)7-14.
15. Mehra, V., Bloom, B. R., Torigian, V. K., Mandich, D., Reichel, M., Young, S. M. M., Salgame, P., Convit, J., Hunter, S. W., McNeil, M., Brennan, P. J., Rea, T. H. and Modlin, R. L. Characterization of Mycobacterium leprae cell wall-associated proteins with the use of T lymphocyte clones. J. Immunol. 142(1989)2873-2878.
16. Mohagheghpour, N., Munn, M. W., Gelber, R. H. and Engleman, E. G. Indentification of an immunostimulating protein from Mycobacterium leprae. Infect. Immun. 58(1990)703-710.
17. Mshana, R. N., Hastings, R. C. and Krahen-buhl, J. L. Infection with live mycobacteria inhibits in vitro detection of la antigen on macrophages. Immunobiology 177(1988)40-54.
18. Mustafa, A. S. Identification of T-cell-activating recombinant antigens shared among three candidate antileprosy vaccines, killed M. leprae, M. bo-vis BCG, and Mycobacterium w. Int. J. Lepr. 56(1988)256-273.
19. Mutis, T., van Schooten, C. A. and de Vries, R. R. P. A peptidoglycan protein complex purified from M. leprae cell walls contains most or all immunodominant M. leprae T-cell antigens. Int. J. Lepr. 57(1989)788-793.
20. Neill, M. A. and Klebanoff, S. J. The effect of phenolic glycolipid-1 from Mycobacterium leprae on the antimicrobial activity of human macrophages. J. Exp. Med. 167(1988)30-42.
21. Pabst, M. J., Gross, J. M., Bronza, J. P. and Goren, M. B. Inhibition of macrophage priming by sulfatide from Mycobacterium tuberculosis. J. Immunol. 140(1988)634-640.
22. Palmer, C. E. and Long, M. W. Effects of vaccination with atypical mycobacteria on BCG vaccination and tuberculosis. Amcr. Rev. Respir. Dis. 94(1966)553-568.
23. Papa, F., Cruaud, P. and David, H. L. Antigenicity and specificity of selected glycolipid fractions from Mycobacterium tuberculosis. Res. Microbiol. 140(1989)569-578.
24. Papa, F., Rauzier, J. Y. and David, H. L. Occurrence of antigen BCG-60 in leprosy-derived coryneforms. Acta Leprol. (Geneve) 2(1984)351-358.
25. Price, J. E. BCG vaccination in leprosy. Int. J. Lepr. 50(1982)205-212.
26. Ralph, P., Prichard, J. and Cohen, M. Reticulum cell sarcoma: an effector cell in antibody-dependent cell mediated immunity. J. Immunol. 114(1975)898-905.
27. Rastogi, N. Killing intracellular mycobacteria in in vitro macrophage systems: what may be the role of known host microbicidal mechanisms? In: 5th Forum in Microbiology, ";Killing intracellular mycobacteria: dogmas and realities."; (Rastogi, N., ed.) Res. Microbiol. 141(1990)217-230.
28. Rastogi, N. and Blom-Potar, M.-C. A comparative study on the activation of J-774 macro-phagc-like cells by gamma-interferon, 1,25-dihy-droxyvitamin D, and lipo peptide RP-56142: ability to kill intraccllularly multiplying Mycobacterium tuberculosis and Mycobacterium avium. Zentralbl. Baktcriol. Mikrobiol. Hyg. 273(1990)344-361.
29. Rastogi, N., Blom-Potar, M.-C. and David, H. L. Comparative intracellular growth ofdifficult-to-grow and other mycobacteria in a macrophage cell line. Acta Leprol. (Geneve) 7Suppl. 1(1989)156-159.
30. Rastogi, N. and David, H. L. Mechanisms of pathogenicity in mycobacteria. Biochimie 70(1988)1101-1120.
31. Rastogi, N. and Frehel, C. Evidence that coating of Mycobacterium leprae surface antigens reduces its ability to hinder host microbicidal functions. Zentralbl. Baktcriol. Mikrobiol. Hyg. 272(1990)337-346.
32. Rastogi, N. and Hellio, R. Evidence that the capsule around mycobacteria grown in axenic media contains mycobacterial antigens: implications at the level of cell envelope architecture. FEMS Microbiol. Lett. 70(1990)161-166.
33. Rastogi, N., Levy-Frebault, V., Blom-Potar, M.-C. and David, H. L. Ability of smooth and rough variants of Mycobacterium avium and M. intracellular to multiply and survive intraccllularly: role of C-mycosidcs. Zentralbl. Baktcriol. Mikrobiol. Hyg. [A] 278(1989)345-360.
34. Rastogi, N., Potar, M.-C. and David, H. L. Intracellular growth of pathogenic mycobacteria in the continuous murine macrophage cell line J-774: ultrastructure and drug-susceptibility studies. Curr. Microbiol. 16(1987)79-92.
35. Reich, C. V. Leprosy: cause, transmission, and a new theory of pathogenesis. Rev. Infect. Dis. 9(1987)590-594.
36. Ridell, M. Crossrcactivity between Mycobacterium leprae and various actinomycetes and related organisms. Int. J. Lepr. 51(1983)185-190.
37. Sibley, L. D., Hunter, S. W., Brennan, P. J. and Krahenbuhl, J. L. Mycobacterial lipoarabino-mannan inhibits gamma interferon-mediated activation of macrophages. Infect. Immun. 56(1988)1232-1236.
38. Sibley, L. D. and Krahenbuhl, J. L. Mycobacterium leprae-burdened macrophages are refractory to activation by gamma interferon. Infect. Immun. 55(1987)446-450.
39. Sibley, L. D. and Krahenbuhl, J. L. Induction of unresponsiveness to gamma interferon in macrophages infected with Mycobacterium leprae. Infect. Immun. 56(1988)1912-1919.
40. Singh, N. B., Lowe, A. C. R. E., Rees, R. J. W. and Colston, M. J. Vaccination of mice against Mycobacterium leprae infection. Infect. Immun. 57(1989)653-655.
41. Snyderman, R., Pike, M. C, Fischer, D. G. and Koren, H. S. Biologic and biochemical activities of continuous macrophage cell lines P388D1 and J-774.1. J. Immunol. 119(1977)2060-2066.
42. Stanford, J. L. Skin testing with mycobacterial antigens in leprosy. Tubercle 65(1984)63-74.
43. Stanford, J. L., Bahr, G. M., Rook, G. A. W., Shaaban, M. A., Chugh, T. D., Gabriel, M., Al-Shimali, B., Siddiqui, Z., Ghardani, F., Sha-hin, A. and Behbehani, K. Immunotherapy with Mycobacterium vaccae as an adjunct to chemotherapy in the treatment of pulmonary tuberculosis. Tubercle 71(1990)87-93.
44. Stanford, J. L., Shield, M. J. and Rook, G. A. W. How environmental mycobacteria may predetermine the protective efficacy of BCG -hypothesis. Tubercle 62(1981)55-62.
45. Stanley, S. J., Howland, C, Stone, M. M. and Sutherland, I. BCG vaccination of chidrcn against leprosy in Uganda: final results. J. Hyg. 87(1981)233-248.
46. Whitehouse, R. S., Benichou, J.-C, Couture-Tosi, E., Schenckman, S. and Ryter, A. Immunolabelling of bacteriophage X receptor protein (lam B) on thin sections of Escherichia coli embedded in Lowicryl. Biol. Cell. 51(1984)389-394.
47. Wright, E. P., Kolk, A. H. J. and Rastogi, N. Monoclonal antibodies and mycobacteria. In: Nucleic Acid and Monoclonal Antibody Probes: Applications in Diagnostic Microbiology. Swamina-than, B. and Prakash, G., cds. New York: Marcel Dckkcr, Inc., 1989, pp. 517-556.
48. Zhang, L., Goren, M. B., Holzer, T. J. and Andersen, B. R. Effect of Mycobacterium tuberculosis-derived sulfolipid I on human phagocytic cells. Infect. Immun. 56(1988)2876-2883.
1. M.Sc, Ph.D., D.Sc, Chargé de Recherche, Unité de la Tuberculose et des Mycobactérics; Unité de Biologie des Membranes, Institut Pasteur, 75724 Paris 15, France.
2. Technicienne; Unité de Biologie des Membranes, Institut Pasteur, 75724 Paris 15, France.
3. M.Sc., Ingénieur de Recherche en Microscopic Electronique, Unité de Biologie des Membranes, Institut Pasteur, 75724 Paris 15, France.
Received for publication on 31 October 1990.
Accepted for publication in revised form on 7 January 1991.