- Volume 59 , Number 2
- Page: 320–2
Reactions to antigens f rom actinomycetes including Mycobacterium leprae in leprosy patients
To the Editor:
Several investigators have discussed the possibility that contact with bacteria cross-reacting with Mycobacterium leprae is of importance for the development of leprosy (5). It has also been claimed that exposure to such organisms influences the clinical type of leprosy which develops (2). Not only mycobacteria, but also many other actinomycetes share antigens with the leprosy organism (4).
The present communication gives a brief account of a study in which the humoral and cellular immune responses in leprosy patients and healthy controls to various actinomycetes antigens were investigated. The effect of M. leprae antigens on the cellular response to some of these antigens was also studied.
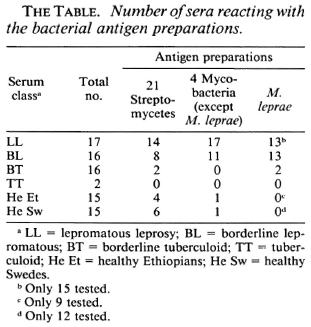
Analyses of the humoral immune response. Sera from 51 leprosy patients (clinical types are given in The Table) and 30 healthy controls from Sweden and Ethiopia were analyzed, using antigen preparations from 21 strains of Nocardia, Nocardiopsis, Streptomyces, Streptoverticillium (collectively referred to as streptomycetes), from four strains of Mycobacterium, and from M. leprae. The serological method used was immunodiffusion and the results are given in The Table. The analyses demonstrated that antibodies against antigens from streptomycetes are common in leprosy patients as well as in healthy controls, while antibodies against mycobacteria only are common in lepromatous patients, but not in tuberculoid or healthy controls.
Analyses of the cellular immune response. The responses to the above-mentioned antigen preparations were analyzed by a lym-phoproliferation assay (1) using peripheral blood mononuclear cells from six borderline tuberculoid patients and nine lepromatous patients (BL or LL). Most of the streptomycetes antigens tested did not induce proliferative cellular response in either tuberculoid or lepromatous leprosy patients, while most patients responded to the antigens from mycobacteria (apart from M. leprae in lepromatous patients). However, a limited number of streptomycetes antigens were recognized by the cells, but the responders were randomly distributed between the two patient groups (Fig. 1). A similar response was obtained when the antigen preparation from the environmental strain of M. phlei was tested (Fig. 1). The investigations thus indicate that the presence of streptomycetes and certain mycobacteria in the environment is not of importance for the development of leprosy in spite of the fact that these organisms share antigens with M. leprae and humans produce antibodies against them.
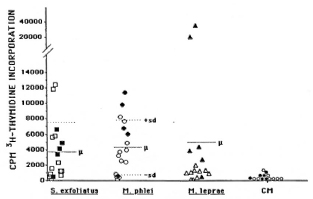
Fig. 1. Lymphoproliferative responses to S. exfoliatus  , M. phlei
, M. phlei  , M. leprae Δ, and culture medium (CM,
, M. leprae Δ, and culture medium (CM,  ) in lepromatous (open symbols) and tuberculoid (solid symbols) leprosy patients. Statistically significant differences in responsiveness between the two groups were only for responses to M. leprae (p < 0.025). Cellular responses to other Streptomycetes antigens were only observed sporadically. Three healthy Ethiopian controls responded only to mycobacterial antigens (not shown).
) in lepromatous (open symbols) and tuberculoid (solid symbols) leprosy patients. Statistically significant differences in responsiveness between the two groups were only for responses to M. leprae (p < 0.025). Cellular responses to other Streptomycetes antigens were only observed sporadically. Three healthy Ethiopian controls responded only to mycobacterial antigens (not shown).
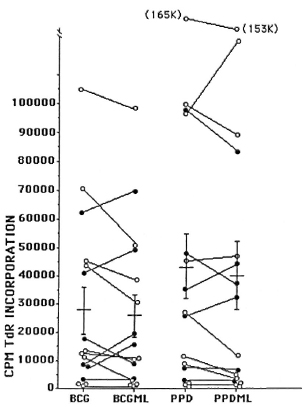
Fig. 2. Selective reduction of responsiveness to BCG but not PPD in cells from lepromatous but not tuberculoid leprosy patients in the presence of M. leprae. Peripheral blood mononuclear cells of leprosy patients were incubated with mycobacterial antigens in the presence (i.e., BCGML or PPDML) or absence (i.e., BCG or PPD) of M. leprae. Solid circles represent tuberculoid patients and open circles, lepromatous leprosy patients.
The effect of the presence of M. leprae on the cellular immune response to strepto-mycetal and mycobacterial antigens was also investigated. Significant reduction of responses to BCG and Streptomyces exfolia-tus was observed, but not to the other organisms tested, nor to PPD or PHA, and only in lepromatous not in tuberculoid patients (Fig. 2). These findings make the hypothesis that such depressions are due to endotoxin (3) less likely. The results further suggest a specific interaction between BCG, M. leprae, and lymphocytes from lepro-matous patients. In view of the use of the mixture of BCG and M. leprae as a vaccine, the interaction of these two antigens merits further investigation.
- Ronny Ohman, M.D.
Department of Medical Microbiology and Immunology
University of Göteborg
Götborg, Sweden
- Charlotte Walford
Department of Biology
Bogazici University
Istanbul, Turkey
- Paul Converse, Ph.D.
Department of Immunology and Infectious Diseases
The Johns Hopkins University
Baltimore, Maryland, U.S.A.
- Malin Ridell, Ph.D.
Department of Medical Microbiology and Immunology
University of Göteborg
Guldhedagatan 10
S-413 46 Göteborg, Sweden
Acknowledgment. Most of the studies were performed at the Armauer Hansen Research Institute (AHRI), Addis Ababa, Ethiopia. AHRI is supported by the Norwegian and Swedish Save the Children Funds and by the Norwegian (NORAD) and Swedish (SAR-EC) international development agencies. AHRI is affiliated with the All Africa Leprosy Rehabilitation and Training Center (ALERT). We are particularly grateful to Dr. Martin Dietz (ALERT), who identified patient material for inclusion in the study. Financial support for this study from SAREC and King Oscar II's Jubilee Fund, Sweden, is gratefully acknowledged.
REFERENCES
1. Converse, P. J., Ottenhoff, T. H. M., Gebre, N., Ehrenberg, J. P. and Kiessling, R. Cellular, humoral and gamma interferon responses to M. leprae and BCG antigens in healthy individuals exposed to leprosy. Scand. J. Immunol. 27 {1988) 515-525.
2. Lyons, N. F. and Naafs, B. Influence of environmental mycobacteria on the prevalence of leprosy clinical type. Int. J. Lepr. 55 (1987) 637-645.
3. Molloy, A., Gaudernack, G., Levis, W. R., Cohn, Z. A. and Kaplan, G. Suppression of T-ccll proliferation by M. leprae and its products: the role of lipopolysaccharide. Proc. Natl. Acad. Sci. U.S.A. 87 (1990) 973-977.
4. Ridell, M. Cross-reactivity between M. leprae and various actinomycetes and related organisms. Int. J. Lepr. 51 (1983) 185-190.
5. Shield, M. J. The importance of immunological effective contact with cvironmcntal mycobacteria. In: The Biology of Mycobacteria. Vol. 2. Immunological and Environmental Aspects. Ratledge, C. and Stanford, J., eds. London: Academic Press, 1983, pp. 343-415.
Reprints requests to Dr. Ridell.