- Volume 59 , Number 2
- Page: 334–6
Circulating antispermatozoal antibodies in leprosy
To the Editor:
The presence of autoantibodies in leprosy, especially in lepromatous leprosy, has been widely investigated. Autoantibodies have been found against almost all body tissues, cellular and nuclear material, and immunoglobulins (8).
Autoantibodies reacting with testicular germinal cells and spermatozoa have been reported in both tuberculoid and lepromatous patients (7,9). Antibodies may be formed because of antigenic similarity between Mycobacterium leprae and testicular tissue or due to an adjuvant-like action of M. leprae. Various studies have given widely varying figures, probably because of the various techniques employed to check the presence of antispermatozoal antibodies (ASA).
The present study was undertaken keeping in mind the widely variable results and tests employed not permitting a scientific comparison.
Subjects and serum samples. Sera from 30 healthy men of proven fertility and 68 male leprosy (bacillary-positive BL, LL) patients were obtained. The duration of disease varied from 1 to 14 years. None of the patients had or gave a history of erythema nodosum leprosum in the recent past, reasonably confirmed by taking a relevant history and asking leading questions.
Blood samples were collected in vacuum tubes, centrifuged at 1500 x g and the sera were separated and frozen at - 70°C within 3 hr of collection. Decomplementation of the sera was done before performing the sperm agglutination test (SAT4) and the sperm immobilization test (SIT3). A titer of > 1:8 was considered positive for the SAT. A sperm immobilization value of > 2.0 constituted a positive SIT result.
Sperm preparation. A pool of normal spermatozoa obtained from a group of healthy fertile men served as antigen. The pooled specimen was diluted 1:10 with P13S-EGTA. The suspension was centrifuged at 500 x g for 25 min for sedimentation of the sperm. The sediment was resuspended in PBS-EGTA and was warmed to 37°C. The washing procedure was repeated three times and finally resuspended to a final concentration of 6 x 106 sperm/ml.
ELISA. The ASAs were assessed by the method of Witkin (10) using an enzyme-linked immunosorbent assay (ELISA). The class of antibody bound to spermatozoa was determined by the use of 0.2 ml of heavy-chain specific, alkaline-phosphatase conjugated, goat anti-human IgG, IgA, or IgM (Sigma Chemical Co., St. Louis, Missouri, U.S.A.) diluted 1:1000 in the Tween wash.
Results. The percentage positivity ASAs detected in leprosy sera was highest with the ELISA (23.5%) compared to the SAT and SIT methods. The figures obtained from controls were 6.6% by the same method (Table 1).
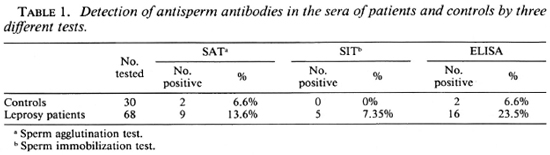
Table 2 summarizes the distribution of IgG, IgA, and IgM antibodies in serum. Evidently the IgA and IgM classes of antibodies reacted more often with the sperm antigen as compared to IgG immunoglobulins.

Discussion. In leprosy both the sper-matogenic and androgenic function of the testes are affected, although the former usually precedes the later (1). The concept of immunofertility has been widely accepted since the antigenicity of spermatozoa was first demonstrated by Landsteiner (5). Disruption of the blood testes barrier is probably a requisite for antisperm antibody formation. In leprosy patients they may either be produced as a result of a generalized autoimmune mechanism, more so in leproma-tous disease, or due to direct damage to testicular tissue in general or germinal epithelium by leprosy bacilli due to blockage of efferent ducts. Immunological injury may thus play a significant role in the pathogenesis of testicular involvement. Testicular biopsies, even in tuberculoid patients with positive antisperm antibodies, showed changes suggestive of immune damage (2).
Antispermatozoal antibodies may affect reproduction by mechanisms such as agglutination or immobilization of spermatozoa, serum cytotoxicity, impairment of sperm penetration of ova membranes, and enhanced phagocytosis of sperm in the genital tract by macrophages (6).
The different antigenic determinants on human spermatozoa might produce different types of antibodies which could be detected by sperm agglutination, sperm immobilization, sperm hemagglutination, or ELISA. False-positive or -negative results by the ELISA are extremely rare. A proper correlation of the various figures in different studies is possible only if a uniform standard method is employed for the detection of ASA, otherwise all comparison would be unfair and unrewarding. Although all three classes of antibodies were elevated in mul-tibacillary patients, IgM showed greater elevation which may be due to the unmasking of specific antigenic determinants by the disease process.
Wall and Wright (9) found antisperma-tozoal antibodies in 44 out of 50 patients (74.6%) with lepromatous disease and in 4 out of 10 patients (40%) with tuberculoid disease. Gupta, et al. (2) reported incidences of 39.4% and 33.3% in lepromatous and tuberculoid patients, respectively, by SIT which they thought was the best test compared to SAT and the sperm hemagglutination test.
In the present study, the low degree of positivity (23.3%) in lepromatous patients obtained by a sensitive ELISA does not correspond with the high percentage positivity obtained by Wall and Wright and Gupta, et al. So, in addition to the prolonged disease, there must be other unidentified causes responsible for the reported testicular dysfunction and histopathological abnormalities.
- Bhushan Kumar, M.D., M.N.A.M.S.
Additional Professor
Department of Dermatology
- S. Majumdar, M.D., Ph.D.
Additional Professor
Department of Experimental Medicine
Postgraduate Institute of Medical Education and Research
Chandigarh 160012, India
REFERENCES
1. El-Shiemy, S., El-Hefnawi, H., Abdel-Fattaii, A., El-Okdi, M. and Farid, A. Testicular and cpididymal involvement in leprosy patients, with special reference to gynecomastia. Int. J. Dermatol. 15(1976)52-28.
2. Gupta, S. C, Bajaj, A. K. and Singh, P. A. Testicular biopsy in antispermatozoal antibody positive tuberculoid leprosy patients. (Letter) Int. J. Lepr. 52(1984)255.
3. Isojima, S. and Tsuzuku, O. Problem of ABO blood group incompatibility and sterility: the effect of blood group antibody on spermatozoa. Am. J. Obstet. Gynecol. 102(1968)304-306.
4. Kibrick, S., Belding, D. L. and Merrill, B. Methods for the detection of antibodies against mammalian spermatozoa. II. A gelatin agglutination test. Fertil. Steril. 3(1952)430-438.
5. Landsteiner, K. Sur kenntnis dcr spezifisch auf blutkorperchen wirkenden sera. Centralbl. Bakteriol. Parasitol. Infekt. 25(1899)546-549.
6. McShane, P. M. Immunologic aspects of male infertility. Semin. Urol. 2(1984)107-114.
7. Saha, K. and Gupta, I. Immunologic aspects of leprosy with special reference to the circulating antispcrmatozoal antibodies. Int. J. Lepr. 45(1975)28-37.
8. Sehgal, S. and Kumar, B. Circulating and tissue immune complexes in leprosy. Int. J. Lepr. 49(1981)294-391.
9. Wall, J. R. and Wright, D. J. M. Antibodies against testicular germinal cells in lepromatous leprosy. Clin. Exp. Immunol. 17(1974)51-59.
10. Witkin, S. S. Enzyme linked immunosorbent assay (ELISA) for detection of antibodies to spermatozoa. Res. Reprod. 15(1983)1-4.