- Volume 59 , Number 1
- Page: 5–11
Persistent reduced solubilization of immune complexes in lepromatous leprosy patients with reactions
ABSTRACT
It is held that immune complexes (IC) play a vital role in the pathogenesis of some of the reactions in leprosy. The complement system is known to solubilize and render IC innocuous. We have previously shown that patients undergoing lepra reactions had lowered complement-mediated IC solubilization (CMS). We, therefore, undertook a prospective study of untreated multibacillary leprosy patients and monitored their CMS levels sequentially while on therapy. In addition, the concentrations of the complement component C3d, immunoglobulins G, A and M, and circulating immune complexes (CIC) were also estimated. A total of 26 patients were included in the study and were investigated at 3-month intervals for 3 years. Thirteen of the 14 patients who did not develop reactions at all had normal CMS values, although all of them showed elevated CIC. From the inception of treatment, 10 of the 12 patients who developed lepra reactions had low CM S values which remained below normal levels even after evidence of complement activation disappeared and long after the subsidence of reaction. It is suggested that this defective CMS acts as a predisposing cause of lepra reactions.RÉSUMÉ
Il est admis que les complexes immuns (CI) jouent un rôle vital dans la pathogénèse de certaines réactions observées dan la lèpre. Le système du complément est connu pour rendre soluble et inoffensif les complexes immuns. Nous avons montré précédemment que les patients présentant des réactions lépreuses avaient une diminution de la solubilisation des CI sous la médiation du complément (SMC). De ce fait, nous avons entrepris une étude prospective de lépreux multibacillaires non traités et suivi leurs taux de SMC dans le temps au cours du traitement. De plus, les concentrations du composant C3d du complément, les immunoglobulines G, A et M, et les complexes immuns circulants (CIC) ont été également mesurés. Un total de 26 malades ont été inclus dans l'étude et furent examinés à intervalles de 3 mois pendant 3 ans. Treize des 14 patients qui n'ont pas développé de réaction avaient des valeurs de SMC normales, bien que tous montraient des CIC élevés. Depuis le début du traitement, 10 des 12 patients qui ont développé des réactions lépreuses avaient des valeurs basses de SMC, et celles-ci restèrent inférieures à la normale même après que les signes d'activation du complément aient disparu et longtemps après la fin de la réaction. Ceci suggère que cette SMC déficiente agit comme un facteur prédisposant aux réactions lépreuses.RESUMEN
Se acepta que los complejos inmunes (CI) juegan un papel importante en la patogénesis de algunas de las reacciones de la lepra. Se sabe que el complemento solubiliza y hace inocuos a los CI. Previamente, se ha demostrado que los pacientes que presentan reacciones leprosas tienen disminuida su capacidad de solubilización de CI mediada por complemento (CSMC). Con esta base, empezamos un estudio prospectivo de pacientes con lepra multibacilar sin tratamiento previo en los cuales cuantificamos su CSMC en función del tratamiento. También se cuantificaron sus niveles de C3d, de inmunoglobulinas G, A y M, y de complejos inmunes circulantes (CIC). Se incluyeron 26 patientes que fueron investigados a intervalos de 3 meses durante 3 años. Trece de los 14 pacientes que no desarrollaron reacciones tuvieron valores normales de CSMC, aunque todos mostraron niveles elevados de CIC. A partir del inicio del tratamiento, 10 de los 12 pacientes que desarrollaron reacciones leprosas tuvieron una baja CSMC, que permaneció por debajo de lo normal aún después de que desaparecieron las evidencias de activación del complemento y mucho después de la remisión de la reacción. Se sugiere que esta defectuosa CSMC actúa como causa predisponente de las reacciones leprosas.Reactions that occur in leprosy are acute episodes superimposed on the underlying chronic inflammation. These are most commonly seen after starting treatment, and can lead to serious sequelae arising from severe neuritis, uveitis, nephritis or arthritis. It is held that immune complexes (IC) actively participate in the production of lepromatous reactions (10,19). We had earlier shown that while circulating immune complexes (CIC) were elevated in all lepromatous leprosy (LL) patients, reactions occurred predominantly in those whose complement system was unable to solubilizc IC in vitro 12). Precipitated IC are solubilized and released by the complement system, and such complexes have been shown to lose their pathogenic potential (7,17). We, therefore, considered it important to define the temporal relationship between the levels of complement-mediated solubilization (CMS) and the occurrence of reactions clinically. We also measured sequentially the levels of CIC, immunoglobulins, and the complement catabolic fragment, C3d, in these patients.
MATERIALS AND METHODS
Subjects. Twenty-six, untreated, adult borderline and lepromatous leprosy patients were diagnosed according to the criteria of Ridley and Jopling (u), and were put on multidrug regimens as per the schedule prescribed by the National Leprosy Association program of India (4). Blood was collected from them at the inception of treatment and at 2-month intervals up to 20 months. It was also taken when they presented with features of reaction.
The 24 control subjects consisted of healthy laboratory volunteers and hospital staff of Central JALMA Institute for Leprosy, Agra, India. Sera from 3-5 of these individuals were run along with those of the patients in all of the tests. The control subjects were bled from time to time, and serum pooled from any five of the controls (designated as normal human serum or NHS) was used as the stand ard.
Serum. Blood was collected by venipuncture into centrifuge tubes which were incubated at 37șC for 30 min and 4șC for 2 hr. After clot retraction had occurred, the tubes were centrifuged at 1250 x g x 15 min at 4șC, and the separated serum was aliquoted to be stored at -70șC. Each aliquot was used only once after thawing.
Materials. Bovine conglutinin was prepared as described (5) from normal bovine serum which was kindly supplied by Dr. V. D. Padmanabhan, Madras Veterinary College, Madras, India. Conglutinin and bovine serum albumin (BSA) were labeled with 125I (Bhabha Atomic Research Centre, Bombay, India), using iodogen (3). Antiserum against BSA was raised in rabbits. Antisera against C3d (Dakopatts, Glostrup, Denmark) and immunoglobulins G, A, and M (Hocchst India, Ltd.) were purchased.
Methods. Circulating immune complexes were estimated using 125I-labeled conglutinin by a PEG precipitation method (10). Serum C3d was measured using an electroimmunodiffusion procedure in agarose containing anti-C3d antibody (10).
Complement-mediated IC solubilization was performed using125I-labeled BSA-anti-BSA complexes prepared at an antibody: antigen ratio of2.9 (l2). Briefly, 1 ng ofcomplexed antibody was added to 0.2 ml of serum diluted 1 in 2 in PBS. After incubation at 37șC for 1 hr, the reaction was stopped by the addition of ice-cold PBS EDTA containing 0.05% sheep erythrocytes. The mixture was centrifuged at 1500 x g x 10 min, and the radioactivity in the supernatant and that in the pellet were counted in a gamma counter. From this, the percentage IC solubilized was calculated.
Immunoglobulins G, A, and M were quantitated using an immunodiffusion technique in agarose incorporated with groupspecific antisera (8).
Analysis of results. The results were analyzed at the end of the study period after completing all ofthe laboratory tests to avoid bias. Furthermore, the patients were divided into two groups: a) patients who remained free of reactions (LL), and b) those who suffered from reactions (LR). Student's two-tailed unpaired-? test was used for intcrgroup comparisons. The paired-/ test was used for comparisons within a group at different periods relative to a fixed time point.
RESULTS
Clinical features of reactions. Of the 26 patients, 12 developed reactions. The remaining 14 were free ofreactions during the study period of 24 months and also during the subsequent clinical follow up until 48 months. Features of severe reaction manifested themselves at varying time periods in LL patients after starting chemotherapy. The clinical features of these reactions are summarized in The Table. Circulating immune complexes. The amount ofl25I conglutinin (K) bound to the IC was quantitated. In each day's assay, the following samples were routinely included in addition to the patients' sera: pooled NHS, NHS treated with 10 mM EDTA (since binding of conglutinin to IC is a Ca2+-dependent process), NHS incubated at 37șC for 60 min with BSA-antiBSA complexes at 5 times antibody excess. Binding ofl25I-K to EDTA-treated serum was less than 5%; while NHS bound between 5% and 14%, and BSA-antiBSA complexes between 45% and 55%. The results of the assay in the controls and patients were expressed as an index of the NHS value:

As seen in Figure 1, the levels were significantly elevated in all of the patients, even when they were untreated. The levels started to increase after initiation of treatment, reaching a peak by 5-8 months. In the nonreactional patients, the levels started to decrease gradually, although at the end of the study period they were still elevated significantly. The reactional LL patients also continued to exhibit high levels of CIC.
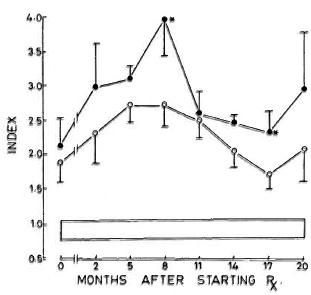
Fig. 1. Levels (mean ± S.E.M.) of circulating immune complexes in patients and subjects expressed as an index of values obtained with pooled normal human serum. Values in both groups of leprosy patients were significantly elevated compared to control values at all time periods tested. * = Values of LR patients were significantly elevated compared to nonreactional patients at 8 and 17 months; 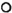 = LL patients who did not suffer from reactions;
= LL patients who did not suffer from reactions;  = LL patients who suffered from reactions;
= LL patients who suffered from reactions;  = 95% confidence interval values of control subjects.
= 95% confidence interval values of control subjects.
Complement C3d. The levels of C3d which were elevated in the beginning decreased by the sixth month in the nonreactional LL patients; whereas in the LR group wide fluctuations in the levels were observed during the study period (Fig. 2).
Fig. 2. Levels of scrum C3d (mean ± S.E.M.) expressed as arbitrary units (AU).  = Nonreactional LL patients;
= Nonreactional LL patients;  = 95% confidence interval values of control subjects.
= 95% confidence interval values of control subjects.
Complement-mediated IC solubilization.
The most striking feature of the study was that the nonreactional LL patients had CMS levels comparable to those of NHS; whereas the LR patients had significantly lower levels throughout the period of the study (Fig. 3). When the CMS levels of the individual patients of the two groups were compared with NH S values, it was found that one LL patient and 10 LR patients had abnormal values on more than four of the eight occasions they were tested. Patients whose CMS levels were less than 64.4% (mean - 2 S.E. of control values) were considered to be abnormally low.
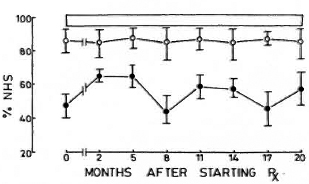
Fig. 3. Levels of complement-mediated IC solubilization (mean ± S.E.M.) expressed as percentage of values obtained with pooled normal human serum. Values of LL patients were comparable with those of controls; those of LR patients were significantly depressed at all time points tested.  = Nonreactional LL patients;
= Nonreactional LL patients;  = reactional LL patients;
= reactional LL patients;  = 95% confidence interval values of control subjects.
= 95% confidence interval values of control subjects.
Immunoglobulins. All three immunoglobulins were significantly elevated in the patients at the inception of treatment. In the nonreactional patients, IgM came down to normal levels by the 12th month, and IgG followed at the 17th month; IgA remained high at 20 months. In the LR patients, IgA and IgM levels returned to normal by the 20th month; while IgG was significantly elevated even at 20 months (Figs. 4-6).
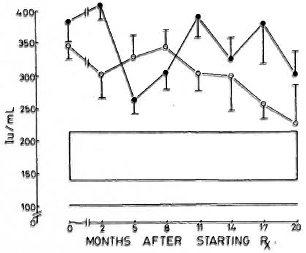
Fig. 4. Levels of immunoglobulin G (mean ± S.E.M.) expressed as international units (IU).  = Non-reactional LL patients;
= Non-reactional LL patients;  = reactional LL patients;
= reactional LL patients;  = 95% confidence interval values of control subjects.
= 95% confidence interval values of control subjects.
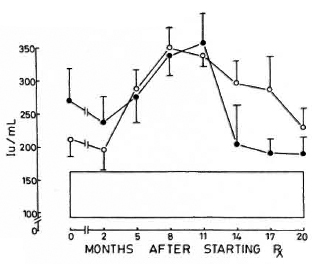
Fig. 5. Levels of immunoglobulin A (mean ± S.E.M.) expressed as international units (IU). Key issame as for Fig. 4.
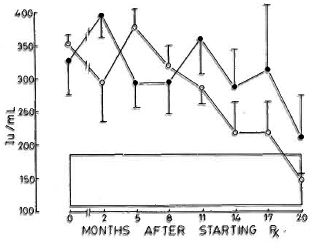
Fig. 6. Levels of immunoglobulin M (mean ± S.E.M.) expressed as international units (IU). Key is same as for Fig. 4.
Relationship between reactional episodes and laboratory parameters. Since the main objective of the study was to determine the temporal relationship between each of the parameters and the occurrence of reactions, the data were analyzed in relation to the reaction. It was found that a maximal elevation of CIC and C3d levels occurred at the time of reactions. Although evidence of complement activation disappeared by 3 6 months after the clinical subsidence of reactions, the CIC levels were still significantly elevated when compared to control values. The levels of CMS were consistently low during the entire period (Fig. 7). There were no correlations between the levels of C3d and CMS or CIC. Similarly, there were no associations between the levels of CMS and the severity, number of episodes, duration or manifestations of reactions. The immunoglobulin levels were also not significantly related to the occurrence of reactions (Fig. 8).
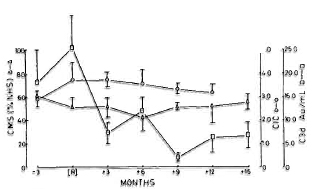
Fig. 7. Levels (mean ± S.E.M.) of CIC ( ); C3d (
); C3d ( ); and CMS (Δ) in reactional patients at 3 months prior to onset of reactions, at time of reactions (R), and at subsequent months. Levels of CIC were not estimated at 15 months postrcaction. Levels of C3d were significantly lower from 3 months postrcaction compared to values at time of reaction; CIC and CMS did not alter subsequently.
); and CMS (Δ) in reactional patients at 3 months prior to onset of reactions, at time of reactions (R), and at subsequent months. Levels of CIC were not estimated at 15 months postrcaction. Levels of C3d were significantly lower from 3 months postrcaction compared to values at time of reaction; CIC and CMS did not alter subsequently.
Fig. 8. Mean levels of immunoglobulins G (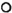 ), A (
), A ( ), and M (Δ) measured at same time points as those given in Fig. 7.
), and M (Δ) measured at same time points as those given in Fig. 7.
DISCUSSION
The range of clinical manifestations observed in LL patients with reactions, collectively termed erythema nodosum leprosum (ENL) syndrome, was seen in 46% of the patients studied. The remaining patients were free of reactions for 4 years of clinical follow up. This finding is in agreement with the earlier reported observations that reactions occur only in a proportion of the leprosy patients, and that these are generally seen in the first 18 months after initiation of treatment (13). It is widely believed that mycobacterial antigens are released during chemotherapy and that they complex with antibody which precipitates at various sites. These precipitated complexes activate the various mediator systems producing inflammation which results in damage at the site of precipitation.
It was interesting to note that the levels of CIC, which were elevated initially, increased further, reaching a peak between the fifth and the 11th months after starting chemotherapy, and falling slowly thereafter.
However, these were still significantly high er when compared to the control values at 18 months (Fig. 1). This probably is a reflection of the antigenic load in these patients.
The levels of CIC were paralleled by increases in the concentrations of immunoglobulins G and M which were elevated in both groups of LL patients and returned to normal levels by the 20th month of treatment. Immunoglobulin A, on the other hand, showed a marked elevation by the eighth month, falling slowly to normal levels from the 11th month onward (Figs. 4-6).
Although CIC were elevated in all of the patients, reactions were seen predominantly in those who had markedly low CMS levels. There was no association between the levels of CMS and C3d in both groups of patients. The disappearance of C3d in the nonreactional LL patients after initiating treatment probably indicates a substantial lowering of the complement activators of mycobacterial origin11) as a response to chemotherapy (Fig. 3). When the data for the LR patients with reactional episodes as their focal point were analyzed, it was observed that there was a maximal activation of the complement system at this point, indirectly confirming the participation of this system in the genesis of reactive states. A similar association of C3d levels with clinical reaction has been found in other sequential studies also (15,18).
In this sequential study, it was found that the reaction-prone patients had reduced CMS well before the onset of reactions, and this persisted for a long time after the subsidence of reactions. Chakrabarty and his colleagues have reported a similar reduced IC solubilization in leprosy patients (2). The reasons for this persistent reduced CMS arc not clear. This is seen even in the absence of complement activation and after stopping antireaction treatment. Similarly, this is unlikely to be due to the antigenic load because the nonreactional LL patients have similar CIC levels. Nor is this due to administration of clofazimine, a known inhibitor of the complement system, since patients in both of the groups received this drug. We had previously shown that the CMS was reduced in reactional LL patients although their hemolytic complement levels were found to be comparable with those of nonreactional LL patients or individuals without leprosy (12). Similarly, significant changes in C4 levels were also not found in earlier studies (1,15). The reasons for this apparent dissociation between hemolytic complement and CMS are not known. However, it has been shown that the polymorphic variants of C4, C4A and C4B have different hemolytic and protein binding activities (6). Thus, future studies should concentrate on measuring CMS levels and relating them to the genetic variants of C4.
from the foregoing, it is clear that reduced CMS is one feature which distinguishes reactional from nonreactional LL patients. Thus, although CIC are formed in all LL patients in situ, the complement system solubilizes and renders the complexes innocuous in the nonreactional patients. It should, however, be noted that two of the LR patients had normal CMS levels and one LL patient without reaction had abnormal CMS values. It is probable that reactions were independent of the CMS levels in these patients. Since the CMS levels were low throughout the period of the study and this was the only feature which distinguished the LR from the nonreactional patient, it is suggested that the defective CM S acts as a predisposing cause of reactions. However, factors whose nature is yet to be properly understood are probably responsible for precipitating reactive phenomena in these patients.
Acknowledgments. This work was supportcd by a grant from the Indian Council of Medical Research. We wish to thank Dr. V. D. Padmanabhan, Madras Veterinary Collège, Madras, India, for the gift of normal bovine serum; Mrs. K. Saroja and Mr. Anil Kumar Chopra, for secretarial assistance; and Mr. Hari Om for preparing the iconography.
REFERENCES
1. BJORVATN, B., B ARNETSON, R. ST.C, K RONVALL, G., Z UBLER, R. H. and L AMBERT, P. H. Immunc complexes and complément hypercatabolism in patients with leprosy. Clin. Exp. Immunol.26(1976)388-396.
2. CHAKRABARTY, A. K., KASHYAP, A., SEHGAL, V. N. and SAHA, K. Solubilization of preformed immune complexes in sera of patients with type1 and type 2 lepra reactions. Int.J. Lepr. 56(1988)559-565.
3. HUDSON, L. and HAY, F. C. Practical Immunology. Oxford: Blackwell Scientific Publications,1980, p. 243.
4. INDIAN ASSOCIATION OF LEPROLOGISTS. Summary of the seminar on Consensus of treatment regimens in leprosy and problems of drug delivery. Indian J. Lepr.56(1984)158-159.
5. LACHMANN, P.J. and HOBART, M.J. Complement technology. In: Hand book of Experimental Immunochemistry. Weir,D. M., ed. Oxford: Blackwell Scientific Publications,1978, p.5A-7.
6. LAW, S. K. A.,DODDS, A. W. and PORTER, R. R. A comparison of the properties of two classes, C4A and C4B, of the human complement C4. EMBOJ. 3(1984)1819-1823.
7. MILLER, G. W. and NUSSENZWEIG, V. A. A new complement function: solubilization of antibody antigen aggregates. Proc. Natl. Acad. Sci. U.S.A. 72(1972)418-422.
8. OUCHTERLONY, O. and NILSSON, L.-A. Immunodiffusion and immunoelectrophoresis. In: Hand book of Experimental Immunochemistry. Weir,D. M., ed. Oxford: Blackwell Scientific Publications, 1978, pp. 19.10-19.12.
9. RAMANATHAN, V.D., CURTIS, J. and TURK, J. L. Activation of the alternative pathway of complement by mycobacteria and cord factor. Infect. Immun. 29(1984)30-35.
1. M.B.B.S., Ph.D.; Central JALMA Institute for Leprosy, Taj Ganj, P. O. Box 31, Agra 282 001, India.
2. M.Sc; Central JALMA Institute for Leprosy, Taj Ganj, P. O. Box 31, Agra 282 001, India.
3. M.B.B.S., D.P.M.; Central JALMA Institute for Leprosy, Taj Ganj, P. O. Box 31, Agra 282 001, India.
4. M.D.; Central JALMA Institute for Leprosy, Taj Ganj, P. O. Box 31, Agra 282 001, India.
5. M.V.Sc, Ph.D., Central JALMA Institute for Leprosy, Taj Ganj, P. O. Box 31, Agra 282 001, India.
6. M.D., Central JALMA Institute for Leprosy, Taj Ganj, P. O. Box 31, Agra 282 001, India.
Reprint requests to; Dr. V. D. Ramanathan, Tuberculosis Research Centre, Spur Tank Road, Chetput, Madras 600 031, India.
Present address for Dr. Ramu: 8β32, Door No. 3E, Pioneer Complex, Avinasi Road, Coimbatore 641 018, India.
Received for publication on 18 January 1990.
Accepted for publication in revised form on 23 August 1990.