- Volume 59 , Number 1
- Page: 12–9
Indeterminate leprosy: histopathologic and histochemical predictive parameters involved in its possible change to paucibacillary or multibacillary leprosy
ABSTRACT
In an attempt to find clinical, bacteriological, histopathological, and immunohistochemical parameters to predict the progress of indeterminate leprosy patients to either paucibacillary (PB) or multibacillary (MB) leprosy, skin biopsies from 51 patients with indeterminate leprosy were retrieved from the files of the São Paulo Health Institute (Brazil). All of these patients had progressed to either PB or MB leprosy over a period of time which varied from 2 months to 24 years. Clinical records were examined, and new sections were cut from the paraffin blocks and stained by hematoxylin-eosin and Fite-Faraco stains; the avidin-biotin peroxidase technique was used with primary antibodies to detect bacillary antigens (anti-BCG serum) and nerve branches (antiS-100 protein anti-serum). A moderate (++) or strongly positive (+ + +) Mitsuda skin test was observed in some patients progressing to PB leprosy. Noteworthy is that even patients initially Mitsuda negative may evolve to PB leprosy, a) A 2+ bacterial index and /or the presence of bacilli, even though few in number, in various dermal structures; b) multiple positive antigen sites as detected by anti-BCG anti-serum; and c) dermal nerve involvement, when evaluated as single parameters, correlated with a progression of indeterminate to MB leprosy. An index resulting from the summation of the above three parameters identified 13 (72%) of 18 of these cases which progressed to MB leprosy.RÉSUMÉ
Dans une tentative de trouver des paramètres cliniques, bactériologiques, histopathologiques et immunohistochimiques pour prédire l'évolution de patients présentant une lèpre indéterminée vers une lèpre soit paucibacillaire (PB), soit multibacillairc (MB), les biopsies cutanées de 51 patients présentant une lèpre indéterminée ont été recherchées dans les dossiers de l'Institut de la Santé de São Paulo (Brésil). Tous ces patients avaient évolué vers une lèpre soit PB soit MB au cours d'une période variant de 2 mois à 24 ans. Les dossiers cliniques furent examinés, et de nouvelles coupes furent faites à partir des blocs de paraffine et colorées à l'hématoxyline-éosine et au Fite-Faraco; la technique de l'avidine-biotine peroxydase fut utilisée avec des anticorps primaires pour détecter les antigènes bacillaires (sérum anti-BCG) et les filets nerveux (antiserum vis-à-vis des protéines anti-S-100). Un test cutané de Mitsuda modéré (++) à fortement (+ + +) positif a été observé chez certains patients évoluant vers la lèpre PB. Il faut noter que même des patients avec un Mitsuda initialement négatif peuvente évoluer vers une lèpre PB. Un index bactérien de 2+ et/ou la présence de bacilles, même en nombre réduit, dans différentes structures dermiques (a); des sites antigéniques positifs multiples comme détectés par l'anti-sérum anti-BCG (b); et un envahissement des nerfs du derme (c), évalués comme paramètres isolés, étaient córreles à une progression de la lèpre indéterminée vers la lèpre MB. Un index résultant de la somme des 3 paramètres ci-dessus identifia 13 (72%) des 18 cas qui ont évolué vers la lèpre MB.RESUMEN
Se reexaminaron las biopsias de piel de 51 pacientes con lepra indeterminada mantenidas en los archivos del Instituto de Salud del estado de Sao Paulo (Brasil), con la intención de encontrar parámetros clínicos, bacteriológicos, histopatológicos, e inmunoquimicos, que pudieran ayudar a predecir el progreso de la enfermedad indeterminada hacia las formas paucibacilar (PB) o multibacilar (MB) de la lepra. Todos estos pacientes habían progresado a la forma PB o MB de la lepra en un periodo que osciló de 2 meses a 24 años. Se examinaron los registros clínicos y se prepararon nuevas secciones de los bloques de parafina las cuales se tiñeron por las técnicas de hematoxilina-eosina y de Fite-Faraco. La técnica de la avidina-biotina-peroxidasa se usó con anticuerpos primarios para detectar antigenos bacilares (con un suero anti-BCG) y ramificaciones nerviosas (con un suero anti-proteína S-100). En algunos de los pacientes que progresaron a la forma PB de la lepra se observó una reacción de Mitsuda moderada (++) o fuertemente positiva (+ + +). Un hallazgo notable fue que aún los pacientes inicialmente Mitsuda negativos pueden evolucionar a la forma PB de la lepra. Los siguientes parámetros: (a) un indice bacteriano de 2 + y/o la presencia de bacilos (aun cuand o sean escasos) en varias estructuras dérmicas, (b) múltiples sitios positivos para antígeno, y (c) afección de nervios, correlacionaron con la progresión de la lepra intermedia a la forma MB de la misma. La suma de los 3 parámetros permitió identificar a 13 (72%) de 18 estos casos que evolucionaron a la forma MB de la lepra.Indeterminate leprosy is a valuable and valid clinical concept which should be considered in conjunction with histopathologic and bacteriologic data (2,17). It is an early and unstable form of leprosy and, though it sometimes persists unchanged, it usually either regresses spontaneously or changes to one of the definitive forms in the leprosy spectrum. It occurs particularly among close contacts of open cases of leprosy and is the form of the disease more frequently seen in patients 15 years old or younger in countries where leprosy is endemic (13). Clinically, it usually manifests as a single or multiple area of persistent disturbance of pigmentation and /or erythema with impairment of sensation but without palpable thickening of the main peripheral nerves (4). Indeterminate leprosy may be histopathologically defined as a chronic dermatitis in which the striking feature is a neurovascular inflammation (3,7,12 ,15) with secondary involvement of dermal adnexa. In about 20% of the cases, scanty acid-fast bacilli may be present in the perineural infiltrate, arrec tores pilorum muscles, and in the dermal nerves themselves.
The search for clinical, histopathological, and immunological data which might foresee the probable polarity of the indeterminate lesion has been the aim of early research in leprosy. A positive Mitsuda skin test indicates, when the disease progresses, a change to the tuberculoid pole (3,21). However, the absence of Mitsuda sensitivity is not indicative of an inability of the patient to deal with the paucibacillary infection (21).
The present study was undertaken to investigate possible clinical, histopathologic, and immunohistochemical predictive parameters which might be indicative of a possible change of indeterminate leprosy toward either the paucibacillary or the multibacillary form of the disease.
MATERIALS AND METHODS
Paraffin blocks and histological sections of 10% formalin-fixed, full-thickness biopsies taken from the skin lesion(s) of 51 cases of indeterminate leprosy were retrieved from the files of the São Paulo Health Institute, Brazil. All of the patients had changed, over a time period which varied from 2 months to 24 years, either to paucibacillary (BT and TT) or multibacillary leprosy (LL, BL and BB). Diagnosis was confirmed by routine histopathological examination according to Ridley and Jopling (20) and Ridley (15) in hematoxylin-eosin (H&E)- and Fite-Faraco-stained sections for acid-fast bacilli (AFB).
Clinical records were examined and data, such as the number and morphological characteristics of the lesion(s), the reported length of time for the lesions to appear, the Mitsuda skin-test results at the first clinical examination and /or during the subsequent progress of the disease and treatment, were registered.
New sections from the paraffin blocks were prepared and routinely stained with H&E and Fite-Faraco stains. The search for bacilli in the skin biopsies was done in multiple sections, using the Ridley and Hilson (19) logarithmic index. The results were compared with the first registered search of bacilli, which was done usually in two or three histologic sections. The following histological variables were analyzed in each H&E-stained section of the first biopsy and semiquantitatively graded: 0 when absent; 1 when slight; 2 when moderate; and 3 when marked: a) epidermal changes; b) perivascular inflammation; c) dermal-nerve involvement score was defined by the summation of semiquantitative values of the intensity of the perineural inflammation, the presence of the inflammatory infiltrate within the nerve branches, and the Schwanncell proliferative response; and d) inflammatory involvement of dermal adnexa (hair follicles, arrectores pilorum muscles, sudoriparous and sebaceous glands).
For immunohistochemical procedures, sections from paraffin blocks of skin were used. All antisera used were purchased from DAKO, Copenhagen, Denmark. Rabbit anti-BCG serum (diluted 1:15,000) and rabbit anti-S-100 protein (diluted 1:2000) were used as primary antibodies overnight. Secondary antibodies included biotinylated goat anti-rabbit IgG 1:1500 (Vector Laboratories, Inc., Burlingame, California, U.S.A.). Avidin-biotin-peroxidase complex (ABC) 1:1000 (Vector) was used at the amplification third-step of the reaction. ABC was applied as described by Hsu, et al. (6) after proteolytic digestion with trypsin (14).
Negative controls included: 1) omission of the primary antibody; 2) normal rabbit serum used as the primary antibody layer; 3) absorption of the BCG antiserum with excess BCG antigen; 4) absorption of the protein S-100 antiserum either with excess of BCG antigen or with sonicated Mitsuda antigen, with consequent maintenance of dermal nerve branches staining and total suppression of Mycobacterium leprae staining properties (24).
Positive controls included histologic sections containing either structures rich in M. leprae antigen (positive control for anti-BCG serum) or nerve branches (positive control for anti-S-100 protein serum).
Evaluation of the antigenic deposits was 0 when absent and + when present, and referred to as BCG antigenic index; the number of positive antigen sites in different structures of the skin and cells of the inflammatory exudate were also evaluated (The Table).
Statistical analysis. Age and other quantitative parameters were analyzed by the Student / test after detection of similar variance between groups. Correlation between events were analyzed by the Spearman rank correlation (C) (8-24). Graphic representation used was the histogram, made up of rectangles in which the base is the amplitude and the height the number of patients in each class. Qualitative variables were represented in contingency tables. In tables 2 x 2, Fischer's exact test was used; in contingency tables m x n, with levels in ordinal scale, Spearman's correlation coefficient was applied.
RESULTS
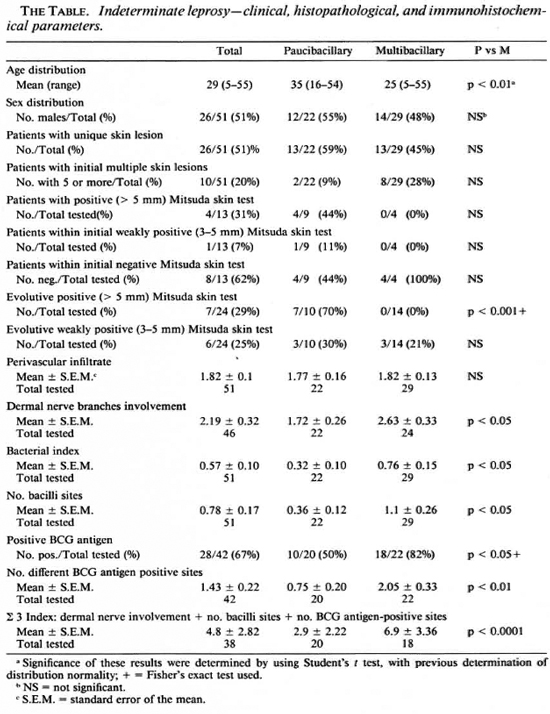
The main findings are shown in The Table and The Figure. The average age of indeterminate leprosy patients who, on subsequent evolution, developed multibacillary leprosy was significantly lower when compared to the patients changing to paucibacillary leprosy (The Table).
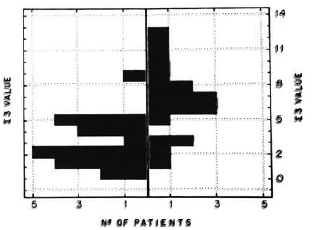
The figure. Histogram showing, to the right of the central boundary line, patients progressing to multibacillary leprosy and, to the left, patients evolving to paucibacillary leprosy as evaluated by the ∑ 3 index.
At the first clinical examination, skin lesions were described as single or multiple areas of hypopigmentation and /or erythema, with altered sensation both in the patients with multibacillary or paucibacillary evolution. No correlation was observed between the number of lesions as detected in the first clinical examination and the further evolutive form of the disease (The Table).
Twenty-two patients progressed to paucibacillary leprosy and 29 patients evolved to multibacillary leprosy. The length of time between the diagnosis of indeterminate leprosy and the subsequent change to paucibacillary or multibacillary leprosy varied: 4 patients changed after 6 months, 12 after 1 to 2 years, 12 after 2 to 5 years, 9 after 6 to 10 years, and 14 after 11 to 24 years. No correlation was observed between these lengths of time and the further evolutive form of the disease.
Most of the patients were treated with sulfones, and statistical analysis showed no significant influence of treatment on the subsequent evolution of the disease (p > 0.05).
Histological examination showed involvement of the epidermis by the inflammatory infiltrate in three cases only. In all other patients, the epidermis appeared normal. The striking histological feature in indeterminate leprosy was a neurovascular inflammation, with both histiocytes and lymphocytes present in the inflammatory infiltrate. To a lesser degree the inflammatory exudate also encircled hair follicles, sweat gland s and arrectores pilorum muscles. The localization of dermal nerves and their involvement by the inflammatory infiltrate was much facilitated after S-100 protein immunohistochemical staining (5).
Bacilli were found in 34 cases (45%) of indeterminate leprosy in almost similar frequencies in the perivascular (12 times), periadenexial inflammatory infiltrate (11 times), and in the arrectores pilorum muscles (11 times). They were detected in the dermal nerves in six instances.
Immunohistochemical detection of bacillary antigens using the anti-BCG serum was carried out in 42 patients of indeterminate leprosy, and were positive in 67% of the cases. They were detected as particulate antigen, and seen more frequently in the cytoplasm of isolated cells of the perivascular inflammatory infiltrate (22 instances) followed by the arrectores pilorum muscles (15 instances) and, finally, by the small nerve branches (12 instances). In nine instances antigen was detected in the periadenexial inflammatory infiltrate, and in two instances it was present at the endothelial lining of the small dermal vessels. Control sections stained with normal rabbit serum or antiserum absorbed with BCG showed no antigen deposits.
Some of the histopathological and immunopathological events may be grouped by the high correlation to evolutive forms, as determined by the Spearman rank test, in an attempt to better differentiate the two groups of patients. A ∑ 3 index can be estimated by the summation of the dermal nervous involvement score, number of bacilli sites, and number of BCG antigen sites. This index, performed with our patients' biopsies, excluding those where one of the above values cannot be unequivocally determined, is shown in The Figure.
DISCUSSION
At first sight, none of clinical parameters analyzed proved to be significant to predict further clinico-pathological change of indeterminate leprosy to either the paucibacillary or the multibacillary form of the disease. Noteworthy, however, was the fact that the age median of patients changing to paucibacillary leprosy (34.95 years) was significantly higher (p < 0.05) when compared to patients evolving to multibacillary leprosy (25.24 years), an apparent contrast to previous studies showing a benign evolution of leprosy in infancy (13-20). The present study, however, did not comprise indeterminate patients who stayed indeterminate or those in whom the disease resolved. Therefore, the differences of age observed, in spite of being statistically significant, cannot be taken into account.
Ridley (15) reported a paucibacillary change of early leprosy cases with single skin lesions. In our study, no significant correlation was observed between the number of skin lesions and the patient's disease progress. However, a positive and significant (p < 0.05) Spearman rank correlation was noted between the number of skin lesions and dermal nerve damage (C = 0.3206), bacterial index (C = 0.3611), and number of dermal sites where isolated bacilli (C = 0.3672) or antigen deposits (C = 0.3342) were found. These parameters proved to be important to the evaluation of further progress of the indeterminate leprosy patients. Statistical analysis showed no influence of the treatment on the subsequent evolution of the disease. From the 8 patients following a regular treatment, 2 progressed to paucibacillary and 6 to multibacillary leprosy. Two possible explanations for this might be: a) resistance to sulfoncs or inadequate absorption of the drug; b) the patient did not follow the prescribed regular treatment. Early clinical discharge was possibly the reason why from 12 other patients, 7 evolved to multibacillary and 5 to paucibacillary leprosy.
Histopathology confirmed that indeterminate leprosy is a form of chronic dermatitis in which neurovascular features are prominent (3,7,2). As was expected, careful examination of multiple histological sections stained for acid-fast microorganisms improved the positive diagnosis of leprosy. Routine examination of the retrieved sections from the files gave an 18% positivity; after multiple section analysis, the confirmed diagnosis rate rose to 45%. However, even a complete examination of multiple sections may not reveal the presence of microorganisms. On these occasions, immunologically based techniques should be of value since they combine both specificity and, chiefly, are not dependent on the presence of viable organisms (1,9,10).
Antigenic analysis indicates that cell antigens detected in M. leprae sonicates are widely crossreactive among mycobacterial species, and especially so with M. bovis (BCG) (9). It was on this basis that rabbit anti-BCG was used as the primary antibody to demonstrate both the bacilli and its antigenic) in indeterminate leprosy and their evolutive forms. Monoclonal antibodies to demonstrate specific M. leprae antigens were used by Narayanan, et al. (11). Mshana, et al. (9), using polyclonal antibodies, reported the detection of mycobacterial antigens in 8 out of 10 (80%) indeterminate patients, although in only half of them were the antigens related to the host tissue response. In our study, mycobacterial antigen(s) were detected in 67% of indeterminate patients, a result superior than that obtained using acidfast stains (45%). Therefore, the immunohistochemical techniques may enable the more precise categorization of suspected early leprosy lesions. However, there still remain a number of cases where acid-fast bacilli and /or their antigen(s) are not identifiable, and the histologic diagnosis is then of a non-specific dermatitis (2). In these instances, the finding of an aggregation of lymphocytes, either cuffing or within one or more dermal nerve branches, better identified by anti-S-100 protein serum staining, can be of further help to differentiate indeterminate leprosy from a nonspecific chronic dermatitis. It should be noted that commercially available anti-S-100 protein serum is raised in rabbits using complete Freund's adjuvant and, consequently, it stains M. leprae similarly to anti-BCG antiserum. Therefore, before its use, a previous adsorption of the S-100 protein anti-serum with lepromin or BCG is necessary. This procedure, done in the present study, removes most of the serum antibodies to mycobacteria but preserves the specific staining of Schwann cells and antigen-presenting cells (23).
In indeterminate leprosy, the bacilli and / or their antigens seem to be able to lodge in all parts of the skin. They do not appear to have, at first, a predilection for dermal nerves alone. In our cases, bacilli and /or their antigens were more frequently detected in the perivascular infiltrate than in the dermal nerves. Furthermore, no correlation was observed between a specific locus of the bacilli or their antigen(s) and the further evolutive form of the disease. This contrasts with a substantial number of patients at the tuberculoid end of the leprosy spectrum who show no morphologically definable bacilli within their tissues, except nerves. These patients show a strong cell-mediated immune response with consequent clearance of bacilli and /or their antigen(s). Thus, the nerve is probably the main protected tissue for M. leprae in human skin (16). Since M. leprae is characteristically nontoxic, nerve damage appears as a result of an immune reaction to the bacillary antigens.
Early attempts to predict, on immunological grounds, the possible progress of indeterminate leprosy patients relied on the Mitsuda skin test (3). Our study confirms that a moderate or strongly positive Mitsuda skin test identifies, if the disease progresses, a change to paucibacillary leprosy; whereas a weakly positive or negative Mitsuda skin test is not of predictive value.
Data from conventional histopathology, such as epidermal alterations, small blood vessel damage, the extent and intensity of the perivascular and periadnexial inflammation, the cell types in the inflammatory exudate, proved not to be significant so far as the prediction of the possible changes in indeterminate leprosy was concerned. However, a significant correlation (p = 0.0001; C = 0.5458) was observed between the perivascular inflammatory infiltrate and dermal neuritis. Dermal nerve involvement was more accentuated (p < 0.05) in indeterminate leprosy patients evolving to multibacillary leprosy. This finding contrasts with previous observations which show nerve involvement in patients with strong immune reactions against bacillary antigens (10,16,18).
A two plus (+ +) bacterial index by Ridley and Hilson's scale (19), and /or the identification of few acid-fast bacilli in different sites, and /or multiple antigenic sites in different skin structures and in macrophages of the inflammatory infiltrate, were found more frequently in patients changing to multibacillary leprosy (p < 0.05), reflecting patients without an adequate response to bacillary antigens.
Dermal nerve involvement, the number of sites where bacilli were found, and the number of antigenic sites marked by the anti-BCG serum could be evaluated as a whole in 38 patients. The summation of these parameters (∑ 3) could identify 72% of 18 patients progressing to multibacillary leprosy. The ∑ 3 index  6 detected patients evolving to multibacillary leprosy (The Figure). Other summation indexes using different clinical and histopathological parameters were tested, but proved to be less useful in differentiating patients evolving either to multibacillary or paucibacillary leprosy. The marked dermal nerve involvement, in conjunction with bacteriological and immunohistochemical markers of low resistance to the infection, are indicative of a probable early downgrading progress of the disease.
6 detected patients evolving to multibacillary leprosy (The Figure). Other summation indexes using different clinical and histopathological parameters were tested, but proved to be less useful in differentiating patients evolving either to multibacillary or paucibacillary leprosy. The marked dermal nerve involvement, in conjunction with bacteriological and immunohistochemical markers of low resistance to the infection, are indicative of a probable early downgrading progress of the disease.
In conclusion, the use of the ∑ 3 index, in association with the Mitsuda skin test, which identifies from the beginning those patients evolving to paucibacillary leprosy, might be of help in public health services for predicting the potential evolution of cases of early leprosy. Control and treatment might, then, be accordingly provided.
REFERENCES
1. BARROS, U.,LADIWALA, U.,BIRDI, T.J. and ANTIA, N. H. Localisation and retention of mycobacterial antigen in lymph nodes of leprosy patients. Br.J. Exp. Pathol. 68(1987)733-741.
2. BROWNE, S. G. Indeterminate leprosy-review. Int.J. Dermatol. 24(1985)555-559.
3. BUNGELER, W. Die pathologische Anatomie der lepra. Virchow Arch. [A] 310(1943)493-565.
4. COMMITTEE ON CLASSIFICATION. Technical resolutions: classification. Sixth International Congress of Leprosy, Madrid, 1953. Int.J. Lepr. 21(1953)504-516.
5. FLEURY, R.N. and BACCHI, C. E. S-100 protein and immunoperoxidase technique as an aid in the histopathologic diagnosis of leprosy. Int.J. Lepr. 55(1987)338-344.
6. HSU, S. M., RAINE, L. and FANGER, H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures.J. Histochcm. Cytochem. 29(1981)577-580.
7. Liu, T.-C,YEN, L.-Z.,YE, G.-Y. and DUNG, G.-J. Histology of indeterminate leprosy. Int.J. Lepr. 50(1982)178-176.
8. MILLER, S. Experimental Designand Statistics. London: Methuen & Co. Ltd., 1975.
9. MSHANA, R.N., BELEHU, A.,STONER, G. L., HARBOE, M. and HAREGEWOIN, A. Demonstration of mycobacterial antigens in leprosy tissues. Int.J. Lepr. 50(1982)1-10.
10. MSHANA, R.N., HUMBER, D. P.,HARBOE, M. and BELEHU, A. Demonstration of mycobacterial antigens in nerve biopsies from leprosy patients using peroxidasc-antiperoxidase immunoenzyme technique. Clin. Immunol. Immunopathol. 29(1983)359-368.
11. NARAYANAN, R. S.,RAMU, G., SINHA, S.,SENGUPTA, U.,MALAVIYA, G. N. and DESIKAN, K. V. Demonstration of Mycobacterium leprae specific antigens in leprosy lesions using monoclonal antibodies. IndianJ. Lepr. 57(1985)258-264.
12. NAYAR, A.,NARAYANAN, J. S. and JOB, C.K. Histopathological study of early skin lesions in leprosy. Arch. Pathol. 94(1972)199-204.
13. NOUSSITOU, F. M. School surveys in Burma. (Abstract) Int.J. Lepr. 31(1963)565-566.
14. PINKUS, G. S.,O'CONNOR, E. M.,ETHERIDGE, C. L. and CORSON, J. M. Optial immunoreactivity of keratin proteins in formalin-fixed, paraffin embedded tissue requires preliminary trypsinization-an immunoperoxidase study of various tumors using polyclonal and monoclonal antibodies. J. Histochem. Cytochem. 33(1985)465-472.
15. RIDLEY, D. S. Pathology and bacteriology of early lesions in leprosy. Int.J. Lepr.39(1971)216-224.
16. RIDLEY, D. S. The pathogenesis of the early skin lesions in leprosy.J. Pathol. 111(1972)191-206.
17. RIDLEY, D. S. Indeterminate leprosy. (Editorial) Lepr. Rev. 45(1974)95-97.
18. RIDLEY, D. S. Histopathological classification and the immunological spectrum of leprosy. Bull. WHO 51(1974)451-465.
19. RIDLEY, D. S. and HILSON, G. R.F. A logarithmic index of bacilli in biopsies. I. Method. Int.J. Lepr. 35(1967)184-186.
20. RIDLEY, D. S. and JOPLING, W. H. Classification of leprosy according to immunity; a five-group system. Int.J. Lepr. 34(1966)255-273.
21. ROTBERG, A. Valor prognóstico da leprominoreação de Mitsuda: observação de 445 casos durante 5-6anos. Rev. Bras. Leprol. 12(1944)367-377.
22. SEHGAL, V. N. and SRIVASTAVA, G. Leprosy in children. Int. J. Lepr. 26(1987)557-566.
23. SIGUEIRA, S. A. C,WAKAMATSU, A., ALVES, V. A. F. and DE BRITO, T. Commercially available anti- S-100 protein serum stains M. leprae in leprosy tissues by immunohistochemical procedures. (Letter) Rev. Inst. Med. Trop. São Paulo 32(1990)70-71.
24. SNEDECOR, G . W . and COCHRANE, W. G. Statistical Methods. 7th cd. Ames, Iowa: Iowa State University Press, 1980, p. 192.
1. M.D.; Health Institute and Institute Adolfo Lutz, Pathology Division, São Paulo Health Service, Dematologic Clinic (Hospital das Clinicas), and Institute of Tropical Medicine, São Paulo Medical School, University of São Paulo, São Paulo, Brazil.
2. M.D.; Health Institute and Institute Adolfo Lutz, Pathology Division, São Paulo Health Service, Dematologic Clinic (Hospital das Clinicas), and Institute of Tropical Medicine, São Paulo Medical School, University of São Paulo, São Paulo, Brazil.
3. Ph.D.; Health Institute and Institute Adolfo Lutz, Pathology Division, São Paulo Health Service, Dematologic Clinic (Hospital das Clinicas), and Institute of Tropical Medicine, São Paulo Medical School, University of São Paulo, São Paulo, Brazil.
4. M.D., Health Institute and Institute Adolfo Lutz, Pathology Division, São Paulo Health Service, Dematologic Clinic (Hospital das Clinicas), and Institute of Tropical Medicine, São Paulo Medical School, University of São Paulo, São Paulo, Brazil.
5. M.D., Health Institute and Institute Adolfo Lutz, Pathology Division, São Paulo Health Service, Dematologic Clinic (Hospital das Clinicas), and Institute of Tropical Medicine, São Paulo Medical School, University of São Paulo, São Paulo, Brazil.
Request for reprints to: Drs. T. De Brito and M. D. Takahashi, Faculdade de Medicina da USP, Departamento de Patologia, Av. Dr. Arnaldo 455,01246 São Paulo, Brasil.
Received for publication on 5 March 1989.
Accepted for publication in revised form on 23 August 1990.