- Volume 59 , Number 1
- Page: 25–31
Detection of phenolic glycolipid-I antigen and antibody in sera from new and relapsed lepromatous patients treated with various drug regimens
ABSTRACT
Since phenolic glycolipid-I (PGL-I) is an unequivocal marker of Mycobacterium leprae, the antigen has been a good cand idate for the serodiagnosis and monitoring the effectiveness of leprosy chemotherapy. As an effort to define the kinetics of the PGL-I antigen and its antibodies in leprosy patients, this study was initiated to examine the serum specimens obtained serially from lepromatous patients under chemotherapy trials. PGL-I was detectable in 64 (94.1%) of 68 new lepromatous (bacterial index, BI = 3.2 to 5.8) and in 26 (78.8%) of 33 relapsed lepromatous patients (BI = 3.0 to 5.3). Meanwhile, virtually all of the new and relapsed patients were strongly seropositive to PGL-I. PGL-I was not detectable in any of the patients about 18 months after chemotherapy was initiated; however, anti-PGL-I reactivity declined by 50% at 2 years and by about 70% at 5 years after chemotherapy regardless of the drug regimens under study. Considering the rapid disappearance of the PGL-I antigen and steady decrease in anti-PGL-I IgM antibodies following chemotherapy, the PGL-I-bascd serology may be useful for monitoring the effectiveness of treatment, at both the early and late stages, in leprosy patients whose initial sera contain a significant level of PGL-I antigen or antibodies.RÉSUMÉ
Puisque le glycolipide phénolique-I (PGL-I) est un marqueur spécifique du Mycobacterium leprae, cet antigène a été un cand idat valable pour le diagnostic sérologique et la surveillance de l'efficacité de la chimiothérapie de la lèpre. Dans un effort pour définir la cinétique de l'antigène PGL-I et de ses anticorps chez les malades de le lèpre, cette étude a été entreprise pour examiner les échantillons de sérum obtenus en série chez des patients lépromatcux participant à des essais thérapeutiques. PGL-I a été détecte parmi 64 (94, 1%) de 68 nouveaux lépromatcux (indice bactériologique IB de 3, 2 à 5, 8) et 26 (78, 8%) de 33 cas de rechute lépromatcusc (IB de 3,0 à 5, 3). De même, pratiquement tous les nouveaux cas et les rechutes étaient fortement séropositifs vis-à-vis du PGL-I. Le PGL-I ne put être détecté chez aucun des malades environ 18 mois après le début de la chimiothérapie; cependant la réactivité vis-à-vis du PGL-I a diminué de 50% après 2 ans et d'environ 70% après 5 ans de chimiothérapie, quel que soit le régime thérapeutique étudié. Considérant la disparition rapide de l'antigène PGL-I et la diminution régulière des anticorps IgM anti-PGL-I après la mise sous traitement, la sérolgic vis-à-vis du PGL-I pourrait être utile pour suivre l'efficacité du traitement, aussi bien au début que par la suite, chez des malades de la lèpre dont le sérum initial contient des taux significatifs d'antigènes PGL-I ou d'anticorps.RESUMEN
Puesto que el glicolipido fenólico-I (PGL-I) es un marcador inequívoco del Mycobacterium leprae, este antigeno ha sido un material adecuado para el scrodiagnóstico de la lepra y para el seguimiento de la efectividad de la quimioterapia antileprosa. Con al intención de definir la cinética del PGL-I y de sus anticuerpos en los pacientes con lepra, se inició un estudio en el cual se examinaron las muestras de suero obtenidas en forma seriada de pacientes lepromatosos bajo tratamiento. El PGL-I fue detectable en 64 (94.1%) de 68 casos lepromatosos nuevos (Índice bacteriano, IB, de 3.2-5.8) y en 26 (78.8%) de 33 pacientes lepromatosos recurrentes (IB = 3.0-5.3). Prácticamente todos los casos nuevos y recurrentes fueron fuertemente seropositivos para PGL-I. El PGL-I no fue detectable en ninguno de los pacientes a los 18 meses de iniciado el tratamiento; sin embargo, la reactividad anti-PGL-I disminuyó un 50% a los 2 años y un 70% a los 5 años después de la quimioterapia, y ésto fue independiente del esquema de tratamiento. Considerand o la rápida desaparición del antigeno PGL-I y la disminución gradual de los anticuerpos IgM anti-PGL-I consecuentes a la quimioterapia, la serologia basada en el PGL-I puede ser útil en el seguimiento de la efectividad del tratamiento tanto en las etapas tempranas como en las tardias, sobre todo en aquellos pacientes cuyos sueros iniciales contienen niveles significantes de antigeno PGL-I o de anticuerpos.Since phenolic glycolipid (PGL-I), a Mycobacterium leprae-specific antigen, was isolated and characterized (13-14), it has been widely used for serological testing in leprosy. Various studies suggest that antibodies to PGL-I are mainly of the IgM class and are more likely detectable in sera from lepromatous patients (2,9,19,20). There also have been efforts to detect the antigen itself, in serum (7,21,22) and in urine (15) for the diagnosis of leprosy.
Besides disease diagnosis, PGL-I-based serology has been considered useful for monitoring the response of patients to chemotherapy. During the early stage of development, Cho, et al. (9) showed significantly reduced anti-PGL-I reactivity among lepromatous patients treated more than 2 years, and demonstrated elevated antibody levels among relapsed patients (6), pointing to PGL-I as a useful tool for monitoring relapsed patients. Since then, Douglas, et al. (10) and Levis, (16) also have reported reduced anti-PGL-I reactivity in patients following chemotherapy.
Recognition of the value of the PGL-I assay for the antigen in monitoring the effectiveness of chemotherapy arose from reports that the levels of the antigen in sera declined rapidly after the onset of chemotherapy and were no longer detectable after 2 to 6 months of effective treatment (7,22). However, most of the information thus far has been obtained from cross-sectional studies and not from the same patients followed sequentially after the initiation of chemotherapy. There was also no information on the proportion of new or relapsed patients with circulating PGL-I antigen or the effect of various drug regimens on the levels of circulating antigen and antibodies in new and relapsed patients.
In an effort to resolve questions concerning the relationship between circulating antigens and antibodies and its clinical significance, a sequential study was conducted with leprosy patients during the course of drug trials at the Leonard Wood Memorial Center for Leprosy Research in Cebu, The Philippines.
MATERIALS AND METHODS
Serum specimens. Serum and blood specimens were taken serially from patients on drug regimens at the time of clinical observation and bacteriological examination during and after chemotherapy. Specimens were obtained from patients who were participants in two major drug trials: one was the Rifampin Trial conducted in 1971 - 1976 in which new lepromatous patients were divided into three groups and given dapsone, rifampin, or both. The other one was the Joint Chemotherapy Trial conducted in 1979-1986 in which new or relapsed lepromatous patients were given combination therapy. Only patients whose first serum sample was obtained within 2 months of initiating chemotherapy were chosen for this study because PGL-I antigen may disappear rapidly from the circulation after treatment (7,22). The results obtained from the patients in the two trials were analyzed separately to permit comparison of each drug regimen.
Detection of antibodies to PGL-I. An enzyme-linked immunosorbent assay (ELISA) described by Voller, et al. (17) was employed with minor modification as reported previously (8-9). However, instead of the native glycolipid, the semi-synthetic neoglycoprotein O-(3,6-di-O-metriyl-β-D-glucopyranosyl)-(1→4)-(2,3-di-O-methyl-β-L-rhamnopyranosyl)-(1→9) - oxynonanoyl - bovine serum albumin (natural disaccharide-octyl-BSA; ND-O-BSA) (5) was used. Briefly, 50 μl of diluted ND-O-BSA (20 ng sugar/ml) in carbonate buffer, pH 9.6, was added to the wells of U-bottom microtitcr plates (Dynatech Laboratories, Inc., Alexand ria, Virginia, U.S.A.), and incubated overnight at 37ºC in a moist chamber. The wells were then washed with phosphate buffered saline (PBS) solution, pH 7.4, containing 0.05% Tween 20 (PBST), and blocked by the addition of 100 μl of PBST-0.05% bovine serum albumin (BSA) at 37ºC for 1 hr. After emptying the wells, 50 μl of serum diluted 1:300 in PBST-5% normal goat serum (NGS) (Gibco Laboratories, Grand Island, New York, U.S.A.) was added to the wells which were incubated at 37ºC for 90 min. After washing the wells, 50 μl affinity-purified peroxidase-conjugated goat anti-human IgM (Behring Diagnostics, Inc., San Diego, California, U.S.A.) diluted 1:5000 in PBST-5% NGS was added, and incubation was continued at 37ºC for 1 hr. After another washing, 50 μl of substrate solution, H2O2-o- phenylenediamine, was added to the wells which were incubated at room temperature for about 15 min. The reaction was then stopped with 50 μl of 2.5 N H2SO4 and the absorbance was read at 490 nm.
Each test was performed in triplicate, and the mean absorbance of wells with BSA only was subtracted from those of wells with ND-O-BSA. The criteria for the seropositivity was determined by adding 2 S.D. to the mean absorbance of controls, and the absorbance > 0.200 was considered seropositive as reported previously (8).
Detection of PGL-I antigen in serum specimens. Our procedure for detection of PGL-I antigen in the serum specimens is a modification of that described previously (7) and is faster (8). In order to facilitate extraction of total lipids from serum specimens, a single 100 μl specimen was added to a filter paper disc (½ inch in diameter) (Schleicher & Schuell, Inc., Keenc, New Hampshire, U.S.A.) and dried completely. Lipids were then extracted using 2-3 ml of CHCl3:CH3OH (2:1) solution and dried under N2. Serum lipids were dissolved in CHC13 and applied to a Pasteur pipette packed with florisil, 60-100 mesh (Sigma Chemical Co., St. Louis, Missouri, U.S.A.) and eluted with CHCI3, followed by 5% CH3OH in CHCI3. The lipid fraction eluted with 5% CH,OH was saved and dried under N2 and examined for the presence of PGL-I.
The dot-ELISA described by Hawkes, et al. (12) was employed with minor modification as reported previously (7,8). The purified lipid was dissolved in 100 μl of hexane and a.5 μl portion was applied to a Tuffryn (polysulfone) membrane (HT-200) (Gelman Sciences, Inc., Ann Arbor, Michigan, U.S.A.) as originally used by Young, et al. (21). High-titer rabbit anti-PGL-I antiserum, prepared as described (7), was used as the primary antibody, and peroxidaseconjugated goat anti-rabbit IgG (Cooper Biomedical, Inc., Malvin, Pennsylvania, U.S.A.) was used as the secondary antibody. For color development, 4-chloro-l-naphthol (Biorad Laboratories, Inc., Richmond, California, U.S.A.) was used, and the results were read visually.
Statistics. The chi-squared test was used to compare the prevalence of anti-PGL-I IgM antibodies and PGL-I antigen between groups of leprosy patients.
RESULTS
Serum specimens obtained from groups of lepromatous leprosy patients, either before or within 2 months of the initiation of chemotherapy, were examined for the presence of PGL-I antigen and IgM antibodies to PGL-I. As shown in Table 1, PGL-I was detected in 41 (93.2%) of 44 new lepromatous patients in the first trial and in 23 (96.0%) of 24 new lepromatous patients in the second trial, respectively. Overall, 64 (94.1 %) of 68 new lepromatous patients, untreated or treated less than 2 months, had circulating PGL-I detectable by the assay used in this study. The average bacterial index (BI) ± S.D. were 4.8 ± 0.4 (range 3.5-5.8) for the patients in the first trial and 4.6 ± 0.5 (range 3.2-5.3) in the second trial.
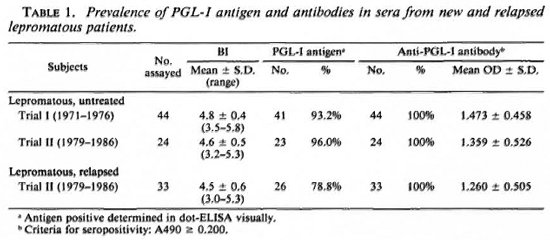
Among relapsed lepromatous patients, PGL-I was detectable in 26 (78.8%) of 33 patients in the second trial and the mean BI ± S.D. was 4.5 ± 0.6 (range 3.0-5.3) for the patients. Although a significant number of relapsed patients had circulating PGL-I, the antigen was detected more frequently in new patients than in the relapsed lepromatous patients (p < 0.05, chi-squared test). On the other hand, virtually all of the new and relapsed patients showed a high IgM antibody response to PGL-I, and there was no significant difference in the average absorbance between the two patient groups.
When the serial serum specimens from the lepromatous patients given various drug regimens were examined for the presence of PGL-I antigen and anti-PGL-I IgM antibodies, there was a steady decrease in anti-PGL-I reactivity following chemotherapy from the initial mean absorbance of about 1.400 to about 0.700 in 2 years and to about 0.450 in 5 years (Table 2). The BI also declined gradually from the value of 4.6 to 2.8 in 2 years and to 0.8 in 5 years. There were no significant differences in mean absorbances among patients treated with different drug regimens (Table 2). Figure 1 shows the average percentage of residual absorbance compared to the initial level following chemotherapy with various drug regimens. In general, anti-PGL-I reactivity declined by about 50% at 2 years and by about 70% at 5 years after chemotherapy was initiated, regardless of the drug regimen. None of the serum specimens obtained from the patients treated for more than 18 months had detectable PGL-I antigen in serum by the assay employed in this study, indicating again that the PGL-I antigen disappears much more rapidly than anti-PGL-I IgM antibodies (7).
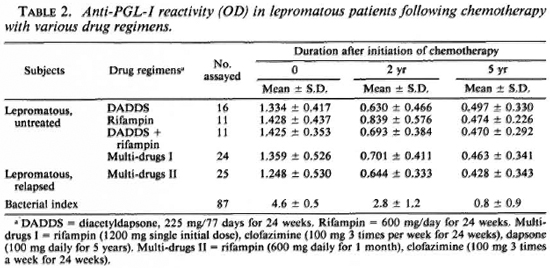
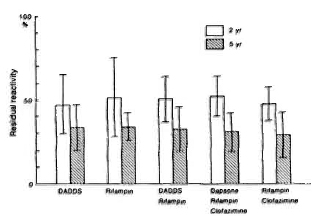
Fig. 1. Percent (mean ± S.D.) residual anti-PGL-I antibody reactivity in ELISA among leprosy patients 2 and 5 years after initiation of chemotherapy with various drug regimens compared to initial antibody reactivity. DADDS = discetyldapsone.
When anti-PGL-I reactivity was compared to the BI in patients following chemotherapy, both parameters declined as the observation period increased. However, there were a few aberrant cases in which the BI and anti-PGL-I reactivity remained high 5 years after chemotherapy was initiated (Fig. 2). In the other patients, anti-PGL-I IgM antibodies declined significantly but theBI was stable, at about 2.0, 5 years afterstarting chemotherapy.
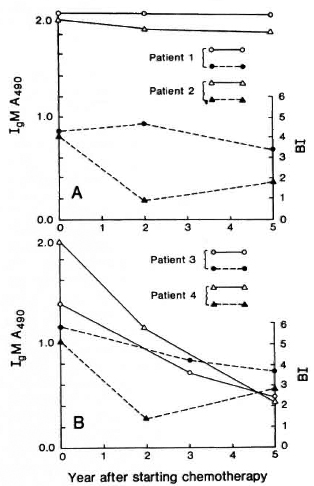
Fig. 2. Changes in anti-PGL-I seroreactivity and bacterial index (BI) in leprosy patients following chemotherapy. ( ) and (Δ) = anti-PGL-I IgM antibody levels; (
) and (Δ) = anti-PGL-I IgM antibody levels; ( ) and (
) and ( ) = bacterial indices.
) = bacterial indices.
DISCUSSION
Serodiagnostic tools have long been sought for the effective control of leprosy. However, the lack of a specific antigen was a major obstacle to developing specific serodiagnostic tests for this disease. The iso-lation of PGL-I, an M. leprae-specific an-tigen (13,14), has thus provided an unprec-edented opportunity for developing serodiagnostic tools and for understanding the pathogenesis of leprosy. Soon after the isolation of PGL-I, methods for the detection of antibodies to the antigen were developed and used extensively for the evaluation of the antigen in serodiagnosis andin monitoring the efficacy of treatment (1-4, 9, 10, 16, 19). Recent detection of PGL-I
antigen from serum and urine has also provided valuable information for under- standing the disease process (3, 7, 8, 15, 22). Continuing our efforts to understand the dynamics of PGL-I antigen and antibody levels during chemotherapy, this study was initiated to examine the sera from lepromatous patients under various chemotherapy trials.
As expected, most lepromatous patients untreated or treated for less than 2 months had detectable PGL-I antigen in their sera.This was consistent with the previous reports by Young, et al. (22) and Cho, et al. (7). Of particular interest was that more than 75% of the relapsed lepromatous patients with dapsone-resistant M. leprae had detectable PGL-I antigen in their sera. It should be mentioned, however, that most of the lepromatous patients in this study had high Bis, averaging 4.5 to 4.8 (range 3.0-5.8). Therefore, the use of PGL-I antigen detection for serodiagnosis remains to be evaluated in studies involving patients with a broader range of bacterial indices.
Monitoring the response of leprosy patients to chemotherapy has been one of the important applications of the PGL-I-based serological test. Although multiple drug therapy (MDT) has reduced the risk of clinical relapse, there is ample reason for monitoring patients after chemotherapy because of the the uncertainty of patient compliance during MDT (11) and partly because of the possible emergence of multiple-drug resistance C8). From cross-sectional studies, it was known that inactive lepromatous patients treated for long periods had lower anti-PGL-I IgM antibodies compared to untreated patients or to those treated for relatively short periods (6,10). However, in the present study, the same patients were followed for up to 5 years, and the serial samples were examined for the anti-PGL-I IgM antibodies.
This study shows that anti-PGL-I reactivity declined by about 50% in 2 years regardless of drug regimen, and approximately 30% of the initial reactivity remained 5 years after chemotherapy was initiated. From this study, therefore, one can expect a reduced but significant IgM antibody level in patients for a period under chemotherapy. Since a decrease in anti-PGL-I reactivity correlates well with the lowering of the BI, the PGL-I-based serological test can also be used as an alternate tool for monitoring the effectiveness of chemotherapy.
In this study, none of the serum specimens obtained more than 18 months after the start of chemotherapy had detectable PGL-I; thus, the detection of PGL-I may be useful for monitoring patients only in the early stage of chemotherapy. This finding was consistent with reports that PGL-I rapidly disappeared from the circulation as early as 2 months after chemotherapy was started (7-22). However, the significance of the early disappearance of PGL-I from circulation as it relates to the pathogenesis of M.leprae infection and the viability of the organisms has yet to be determined.
For clues to understand ing the relationship between the PGL-I antigenemia and the viability of M. leprae, it was of interest to note in this study that there were no differences among the various regimens in the clearance of PGL-I antigen and in the decrease of anti-PGL-I IgM antibodies. In another study, clofazimine was also reported to be effective in reducing anti-PGL-I reactivity (10). Considering that dapsone and clofazimine are bacteriostatic and rifampin is bactericidal, any one of these drugs or drug combinations can successfully halt the metabolic process of M. leprae and hence the production of PGL-I antigen. Therefore, one can envision the reduction of existing PGL-I in circulation and in tissues, and with less antigen, a slow reduction of anti-PGL-I IgM antibodies. Although it is not yet possible to determine whether the early disappearance of PGL-I antigen reflects the rapid killing of M. leprae, one can at least be sure that drug(s) given a patient halt(s) the metabolism as early as 2 weeks after chemotherapy (7).
In summary, lepromatous patients who have relapsed because of drug-resistant M. leprae had PGL-I antigen and abundant anti-PGL-I IgM antibodies in their sera at levels comparable to new lepromatous cases. Considering the rapid disappearance of the PGL-I antigen and the steady decrease in anti-PGL-I reactivity following chemotherapy, the PGL-I-based serology may be useful for monitoring the effectiveness of chemotherapy, both at the early and late stages, in leprosy patients whose initial sera contain a significant level of PGL-I antigen or antibodies. Further prospective studies, however, are desirable to evaluate fully whether or not the PGL-I-based serology will provide useful information for the early detection of new and relapsed lepromatous leprosy cases.
Acknowledgments. This work was supported in part by the Leonard Wood Memorial (American Leprosy Foundation), Rockville, Maryland ; a research grant (1988) from the Heiser Program for Research in Leprosy, New York, N.Y.; and by the Sasakawa Memorial Health Foundation, Tokyo, Japan. The authors thank The Philippine Government Department of Health for their cooperation. We also thank E. O. Shin for technical assistance and M. Hcin for preparing the manuscript.
REFERENCES
1. ANDREOLI, A., BRETT, S. J. D. RAPER, P., PAYNE, S. N. and ROOK, G. A. W. Changes in circulating antibody levels to the major phenolic glycolipid during erythema nodosum leprosum in leprosy patients. Int. J. Lepr. 53(1985)211-217.
2. BRETT, S. J., DRAPER, P., PAYNE, S. N. and REES, R. J. W. Serological activity of a characteristic phenolic glycolipid from Mycobacterium leprae in sera from patients with leprosy and tuberculosis. Clin. Exp. Immunol. 52(1983)271-279.
3. CHANTEAU, S., CARTEL, J.-L., CELERIER, P., PLICHART, R., D ESFORGES, S. and Roux, J. PGL-I antigen and antibody detection in leprosy patients: evolution under chemotherapy. Int. J. Lepr. 57(1989)735-743.
4. CHANTEAU, S., CARTEL, J.-L., ROUX, J., PLICHART, R. and BACH, M.-A. Comparison of synthetic an tigens for detecting antibodies to phenolic glycolipid I in patients with leprosy and their household contacts.J. Infect. Dis. 157(1988)770-776.
5. CHATTERJEE, D.,CHO, S .-N., BRENNAN, P.J. and ASPINALL, G. O. Chemical synthesis and scrorcactivity of (9-(3, 6-di-O-methyl-β-D-glucopyranosyl)-(1→4)-O-(2,3-di-O-methyl-β-L-rhamnopyranosyl)-(1→9)-oxynonanoyl-bovine serum albumin -the leprosy-specific, natural disaccharide-octyl-neoglycoprotein. Carbohydr. Res. 156(1986)39-56.
6. CHO, S .-N., FUJIWARA, T.,HUNTER, S. W., REA, T. H., GELBER, R.H. and BRENNAN, P.J. Use of an artificial antigen containing the 3, 6-di-Omethyl-β-D-glucopyranosyl epitope and use in serodiagnosis of leprosy.J. Infect. Dis. 150(1984)311-322.
7. CHO, S.-N., HUNTER, S.W., GELBER, R. H., REA, T.H. and BRENNAN, P.J. Quantitation of the phenolic glycolipid of Mycobacterium leprae and relevance to glycolipid antigencmia in leprosy.J. Infect. Dis.153(1986)560-569.
8. CHO, S .-N., SHIN, J. S.,CHOI, I.H. KIM, S. H., KIM, D. I . and KIM, J. D. Detection o f phenolic glycolipid I of Mycobacterium leprae and antibodies to the antigen in sera from leprosy patients and their contacts. Yonsei Med.J. 29(1988)219-224.
9. CHO, S .-N., YANAGIHARA, D . L.,HUNTER, S.W., GELBER, R.H. and BRENNAN, P.J. Serological specificity of phenolic glycolipid I from Mycobacterium leprae and use in serodiagnosis of leprosy. Infect. Immun. 41(1983)1077-1083.
10. DOUGLAS, J. T., HIRSCH, D. S.FAJARDO, T. T., CELLONA, R. V., ABALOS, R.M., DELA CRUZ, E. C, MADARANG, M. G., DE WIT, M. Y. L . and KLATSER, P. R. Evaluation of Mycobacteriumleprae antigens in the serological monitoring of a clofazimine-bascd chcmothcrapeutic study of dapsone resistant lepromatous leprosy patients in Cebu, Philippines. Lepr. Rev. 60(1988)8-19.
11. GRUGNI, A.,NADKARNI, N. J., KINI, M. S . and MEHTA, V. R. Relapses in paucibacillary leprosy after MDT-a clinical study. Int.J. Lepr. 58(1990)19-24.
12. HAWK.ES, R.,NIDAY, E. L. and GORDON, J. A dotimmunobinding assay for monoclonal and other antibodies. Anal. Biochem. 119(1982)142-147.
13. HUNTER, S. W. and BRENNAN, P.J. A novel phenolic glycolipid from Mycobacterium leprae possibly involved in immunogenicity and pathogenicity.J. Bacteriol. 147(1981)728-735.
14. HUNTER, S. W.,FUJIWARA, T. and BRENNAN, P. J. Structure and antigenicity of the major specific glycolipid antigen of Mycobacterium leprae. J. Biol. Chem. 257(1982)15072-15078.
15. KALDANY, R.-R.J. and NURLIGN, A. Development of a dot-ELISA for detection of leprosy antigenuria under field conditions. Lepr. Rev. 57Suppl.2(1986)95-100.
16. LEVIS, W. R.,MEEKER, H. C,SCHULLER-LEVIS, G., SERSEN, E. and SCHWERER, B. IgM and IgG antibodies to phenolic glycolipid I from Mycobacterium leprae in leprosy: insight into patient monitoring, erythema nodosum leprosum and bacillary persistence.J. Invest. Dermatol. 86(1986)529-534.
17. VOLLER, A.,BIDWELL, D. E. and BARTLETT, A. The enzyme-linked immunoassay (ELISA). Alexand ria, Virginia, U.S.A.: Dynatech Laboratories, Inc., 1979.
18. WHOEXPERT COMMITTEE ON LEPROSY. Sixth report. Geneva: World Health Organization, 1988. Tech. Rep. Ser. 768.
19. YOUNG, D. B. and BUCHANAN, T. M. A serological test for leprosy with a glycolipid specific for Mycobacterium leprae. Science 221(1983)1057-1059.
20. YOUNG, D. B.,DISSANAYAKE, S.,MILLER, R. A., KHANOLKAR, S. R. and BUCHANAN, T. M. Humans respond predominantly with IgM immunoglobulin to the species-specific glycolipid of Mycobacterium leprae. J. Infect. Dis. 149(1984)870-873.
21. YOUNG, D. B.,FOHN, M.J. and BUCHANAN, T. M. Use of polysulfone membrane support for immunochemical analysis of a glycolipid from Mycobacterium leprae. J. Immunol. Methods 79(1985)205-211.
22. YOUNG, D. B.,HARNISCH, J. P.,KNIGHT, J. and BUCHANAN, T. M. Detection of phenolic glycolipid I in sera from patients with lepromatous leprosy.J. Infect. Dis. 152(1985)1078-1081.
1. D.V.M., Ph.D., Assistant Professor; Department of Microbiology, Yonsei University, Seoul, Korea.
2. M.D., Ph.D., Professor, Department of Microbiology, Yonsei University, Seoul, Korea.
3. M.D., D.P.H. (Lond.), Chief, Epidemiology Branch; Leonard Wood Memorial, Cebu, The Philippines.
4. M.D., M.P.H., Chief, Clinical Research Branch; Leonard Wood Memorial, Cebu, The Philippines.
5. M.D., Pathologist; Leonard Wood Memorial, Cebu, The Philippines.
6. D.V.M., Chief, Vivarium; Leonard Wood Memorial, Cebu, The Philippines.
7. Ph.D., Director, Leonard Wood Memorial, Cebu, The Philippines.
8. Ph.D., Professor, Department of Microbiology, Colorado State University, Fort Collins, Colorado 80523, U.S.A.
Reprint request to Dr. S.-N. Cho, Department of Microbiology, Yonsei University College of Medicine, C.P.O. Box 8044, Seoul 120-752, Republic of Korea.
Received for publication on 22 June 1990.
Accepted for publication in revised form on 22 October 1990.