- Volume 59 , Number 1
- Page: 32–40
On the value of sequential serology with a Mycobacterium leprae-specific antibody competition ELISA in monitoring leprosy chemotherapy
ABSTRACT
The Mycobacterium leprae-specific antibody assays-a serum antibody competition test (SACT-ELISA) for the epitope on the 35-kDa protein, and an enzyme immunoassay for the disaccharide epitope of phenolic glycolipid-I (PGDS-ELISA)-were evaluated as tools for the serological monitoring of chemotherapy in 20 lepromatous and 6 tuberculoid leprosy patients. In addition to estimates for M. leprae-speciñe antibodies, assessments of the bacterial index (BI) and clinical activity of the disease were also carried out prospectively in these patients on two to four occasions over a period of 19 months. In most cases, a decline in the BI, clinical scores, and antibody levels was observed during the course of treatment. The relative rate of decline was steepest and least variable with the SACT-ELISA, followed by the PGDS-ELISA and the BI. In some patients who showed a static or even an increased BI, despite marked clinical improvement, the antibody levels decreased. These data indicate that, unlike the BI, there is a greater dependence of specific antibody levels on the viability of M. leprae. This, combined with the fact that antibody titers would reflect the antigen load in the whole body, makes M. leprae-specific serology a promising tool for monitoring che motherapy in leprosy patients.RÉSUMÉ
Les tests pour les anticorps spécifiques vis-á-vis du Mycobacterium leprae- un test de competition pour les anticorps sériques (SACT-ELISA) vis-á-vis de l'épitope de la protéine de 35 kDa et un test immunoenzymatique pour l'épitope disaccharidique du glycolipidc phcnolique-I (PGDS-ELISA) -ontété evalúes en tant qu'instruments pour la surveillance sérologique de la chimiothérapie chez 20 malades lépromateux et 6 tuberculoïdes. En plus de la recherche des anticorps spécifiques vis-à-vis de M. leprae, des évaluations de l'indice bactérien (IB) et de l'activité clinique de la maladie ont été réalisées de manière prospective chez ces patients de deux à quatre reprises au cours d'une période de 19 mois. Dans la plupart des cas, une diminution de l'indice bactérien, des scores cliniques et des taux d'anticorps ont été observés au cours du traitement. Le taux relatif du déclin était le plus fort et le moins variable avec le SACT-ELISA, suivi par le PGDS-ELISA et l'indice bactérien. Chez certains patients qui ont montré un IB stable ou même en augmentation, malgré une amélioration clinique marquée, les taux d'anticorps ont diminué. Ces données indiquent que, au contraire de l'indice bactérien, il y a une plus grand e dépendance des taux d'anticorps spécifiques vis-à-vis de la viabilité de M. leprae. Ceci, combiné au fait que les titres d'anticorps refléteraient la charge antigénique dans l'ensemble de l'organisme, fait de la sérologie spécifique vis-à-vis de M. leprae un instrument prometteur pour la surveillance de la chimiothérapie chez les malades de la lèpre.RESUMEN
Se evaluaron, comparativamente, dos ensayos para determinar anticuerpos específicos contra Mycobacterium leprae: una prueba de competencia de anticueros por el epitope de la proteína de 35 kDa (SACT-ELISA), y un ensayo enzimático para el disacárido del glicolípido fenólico I (PGDS-ELISA). Los ensayos se usaron para visualizar el efecto de la quimioterapia en 20 pacientes lepromatosos y en 6 pacientes tuberculoides. Adicionalmentc se establecieron el indico bacteriano (IB) y la actividad clínica de la enfermedad de cada paciente en 2 a 4 ocasiones dentro de un periodo de 19 meses. Durate el curso del tratamiento, en la mayoría de los pacientes se observó una declinación en el IB, en los parámetros clínicos y en los niveles de anticuerpos. El grado relativo de declinación fue mas progresivo y menos variable cuand o se analizó por SACT-ELISA que por PGDS-ELISA o por el IB. En algunos pacientes que mostraron un IB estático o incluso incrementado, a pesar de una marcada mejoría clínica, los niveles de anticuerpos disminuyeron. Estos datos indican que los niveles de anticuerpos específicos dependen más de la viabilidad del M. leprae, que del IB. Esto, combinado con el hecho de que los títulos de anticuerpo reflejarían mejor la carga antigénica en todo el cuerpo, hacen a la scrología específica para M. leprae, una herramienta promisoria para visualizar la eficacia de la quimioterapia en los pacientes con lepra.The incidence of treatment failure in leprosy, due to reasons such as irregular treatment, drug resistance and persisters (22), has been alarmingly high in the era of dapsone monotherapy. Although this fear has been greatly reduced with the advent of multidrug therapy (MDT) for leprosy (9,21), careful monitoring of the patients is still necessary, not only for the detection of treatment failures at an early stage but also for further rationalization of treatment schedules so as to avoid longer-than-necessary treatment (10).
A variety of laboratory methods have been developed for monitoring responses to antileprosy treatment, some of which are more time consuming, sophisticated, and .cumbersome than others. However, only bacterial index (BI) determination (15) and, to some extent, viability testing in foot pads of normal mice (17) have gained widespread acceptance. The BI determination technique has several inherent limitations, such as the lack of stand ardization, susceptibility to sampling and subjective errors, and the inability to distinguish between live and dead bacilli (26). The mouse foot pad technique, besides being expensive and time consuming, is not highly sensitive due to immune-mediated spontaneous killing of small numbers of viable bacilli present in a large pool of dead bacilli (5). A major limitation with most of the presently available monitoring methods is that they depend upon biopsies or samples from arbitrarily selected site(s) of the skin, which is neither uniformly infected (even in lepromatous leprosy patients) nor the only site of infection (25).
Assays for Mycobacterium leprae-specific antibodies (17) have been used for monitoring leprosy treatment on the premise that antibody titers would reflect the antigen load in the whole body. Nonetheless, most of the previous studies, including our own (18), have been cross-sectional in nature with wide individual variations in the BI, and antibody titers within the same class of patients have compromised the analysis and interpretation of the data. Longitudinal studies are likely to be more informative in determining the role of serology in this context, as evident from some recent reports based on extensive data (4,11). The majority of the published studies have dealt mainly with serology for M. leprae-specific antibodies directed against the disaccharide epitope of phenolic glycolipid-I (PGDS-ELISA) (1-4,11,12). We report here on the potentials of sequential serology with a monoclonal-antibody-based competition ELISA (19) for specific antibodies against the 35-kDa M. leprae antigen (8-20) in monitoring treatment. Serial determinations of PGDS-ELISA levels, the BI and clinical activity scores were also done simultaneously in leprosy patients subjected to this study.
MATERIALS AND METHODS
Study subjects. Twenty-six leprosy patients, 20 lepromatous (LL/BL) and 6 tuberculoid (TT/BT) according to Ridley-Jopling criteria (16), were included in the study. All patients were registered with the clinics of Central JALMA Institute for Leprosy (CJIL), Agra, India, and were put on multidrug therapy (MDT; dapsone, clofazimine and rifampin), with the exception of one patient. A total of 70 serum samples were collected prospectively from these patients at specified intervals over a period of 19 months. One LL patient who was examined and asked to collect medicine the next day turned up for treatment only after 9 months, and thus remained untreated over this period.
Sera from 82 nonleprosy subjects (healthy Indian = 32; healthy European = 30; pulmonary tuberculosis Indian = 20) served as controls for deciding the cut-off points when analyzing the antibody assay results (18). All sera were stored at - 20ºC until used.
Bacterial index (BI). Four slit-skin smears (from both earlobes and at least two active sites, generally on the arm and back) were used for serial determinations of the average BI in each patient, according to Ridley's logarithmic scale (15).
Clinical scoring. The clinical scoring and charting of the patients was done at specified intervals according to the method proposed by Ramanujam (13): the human body is divided into seven areas (head, trunk, buttocks, 2 upper and 2 lower extremities) and a clinical score (1 to 4) is given to each area, depending on the activity in skin lesions over it, and all scores are added up. For the present study, clinical improvement was arbitrarily graded as: mild improvement = 1+, a reduction in overall score by < 5 points; moderate improvement = 2+, a reduction of 5 to < 10 points; marked improvement = 3 +, a reduction of > 10 points; and complete regression = 4 +, zero score.
Serum antibody competition test (SACT). A SACT was performed according to the inhibition ELISA procedure reported by us 19), which is an adaptation of the RIA technique described earlier (20). Briefly, ELISA plates (Immunoplate I or II; Nunc, Denmark) were coated with soluble antigen (2.5 Mg/50 jd/well, at 4ºC, overnight) of M. leprae (of armadillo origin; a kind gift from IMMLEP provided by Dr. R. J. W. Rees). After removing the antigen, the plates were blocked (2 hr, ambient temperature) with 1% skimmed milk powder (Anikspray; Lipton India Ltd.) in Tris-buffered saline (0.01 M Tris, 0.15 M NaCl, pH 7.4) containing 0.05% Tween 20 (TBST). Antigen wells (in duplicate) were incubated (60 min, 37ºC) with serial tenfold dilutions (in 1% milk-TBST) of each scrum (25 μl/well). The incubation was continued for a further 120 min (37ºC) after the addition of a 1:1000 dilution (in 1% milk-TBST) of peroxidaseconjugated monoclonal antibody ML0 4 (25 μl/well). The plates were washed (with TBST) and color was developed with o-phenylenediamine (Sigma) substrate solution (50 μl/well; 20 min, 37ºC). The reaction was stopped by adding 2.5 N H2SO4 (50 μl/well). The optical densities (OD) were read at 492 nm using an ELISA reader (Titretek Multiskan Plus; Flow Laboratories).
Relative percent bindings were calculated (using 100% as the mean OD value for binding of P-ML04 alone to the antigen-coated wells), and plotted against the corresponding dilutions of a serum. The dilution which would cause 50% inhibition of P-ML04 binding is referred to as the ID50 titer of that serum. On the basis of the results obtained with nonleprosy controls (19 and this study), a serum with an ID50 titer of 10 (1:10) or more was regarded as SACT positive.
ELISA for anti-PGDS antibodies. The assay was performed according to the method of Cho, et al. (3) with minor modifications. Briefly, wells of an ELISA plate were coated with either ND-O-BSA (natural disaccharide-octyl-BSA conjugate; kindly provided by Dr. D. Chatterjee) synthetic antigen (1 ng carbohydrate/50 /xl/well, at 4ºC, overnight) or with a coating buffer. After blocking with 1% milk-TBST, a 1:300 dilution (in milk-TBST) of each serum was incubated (50 μl/well, 60 min, 37ºC) in four wells (a pair each of antigen coated and buffer coated). The plates were washed (with TBST), and incubated (50 μl/well, 60 min, 37ºC) with 1:2000 (diluted in milk-TBST) peroxidase-conjugated anti-human IgM (Dakopatts). The remaining steps (washing, color development, reading) were the same as described above for the SACT-ELISA. On the basis of the results obtained with nonleprosy controls (mean + 2 S.D.), a serum (at 1:300 dilution) was regarded as PGDS-ELISA positive if it showed an OD of > 0.20 (1 9 and this study).
RESULTS
Of the 20 lepromatous patients (Figs. 1 and 2) who had previously been untreated, 6 had received treatment for short periods ranging from 1-4 months (patients 1, 6, 7, 13, 15 and 16), and two others (patients 8 and 20) had been on treatment for unspecified periods. As mentioned earlier, one patient (no. 9) had remained untreated over a period of 9 months after inclusion in the study. All of the six tuberculoid leprosy patients (Fig. 3) had no previous treatment.
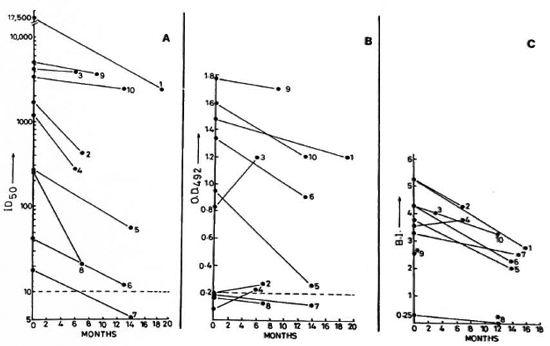
Fig. 1. Sequential value for: A SACT-ELISA (ID50); B PGDS-ELISA (OD492); C bacterial index in leprosy patients following treatment. Figure shows data on lepromatous leprosy patients who were investigated on two occasions over a maximum period of 19 months. (---) in A and B = cut-off values for corresponding antibody assays; *8 = timings of BI and serology determinations arc not identical; *9 = only initial BI is known.
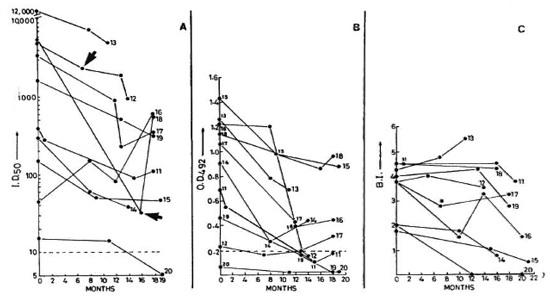
Fig. 2. Sequential values for SACT-ELISA, PGDS-ELISA, and BI in lepromatous patients investigated on three to four occasions;  = points of time at which patients 12 and 18 were experiencing ENL (see Fig. 1 legend for details).
= points of time at which patients 12 and 18 were experiencing ENL (see Fig. 1 legend for details).
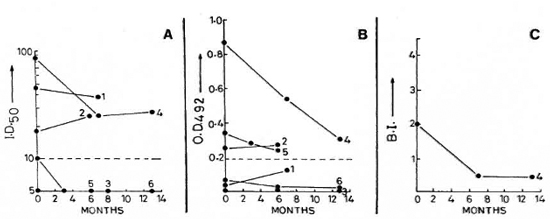
Fig. 3. Sequential value for SACT-ELISA, PGDS-ELISA, and BI in tuberculoid leprosy patients investigated on two to three occasions (see Fig. 1 legend for details).
An appreciable clinical recovery in response to treatment was generally accompanied by a net reduction in the BI and in the levels of specific antibodies in a majority of the patients. The decline in the levels of specific antibodies was continuous in some of the cases, while it was interrupted in others. This was also true for the BI.
Serological assays. Initially, the SACT was positive in all of the 20 lepromatous and in 4 of the 6 tuberculoid patients; whereas the PGDS-ELISA was positive in only 15 lepromatous and 3 tuberculoid cases (Figs. 1-3). Nonetheless, an overall positive correlation (r = 0.52, p < 0.01) was noted between the corresponding values of the SACT-ELISA and the PGDS-ELISA.
Lepromatous patients. The trends in the BI and the serology results among the lepromatous leprosy patients are shown in Table 1. The patients had consistently high initial BI values (3.78 ± 0.97), but there were great individual variations in the rate of its decline in response to treatment (0.73 ± 0.81 BI units per year). These variations were equally prominent even if the decline was expressed in relative terms, i.e., percent of corresponding initial BI (21.73 ± 22.38). In contrast to the BI, the initial values for specific antibodies measured either by the SACT-ELISA or by the PGDS-ELISA showed wide variations (3829.23 ± 5319.64 and 0.97 ± 0.46, respectively), and so did the corresponding absolute values for their decline per year (2283.92 ± 3380.42 and 0.33 ± 0.17, respectively). However, expression in relative terms revealed a striking consistency in the rates of decline, especially by the SACT-ELISA (60.98 ± 16.10% per year).
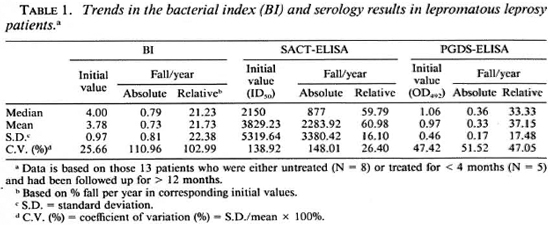
The per annum fall in the BI and serology values in eight patients who showed markedto-complete clinical recovery over the study period are compiled in Table 2. The rate of relative decline in the serological value of specific antibodies in these patients (Table 1) were also noticeably steeper and more consistent (54.15 ± 15.13 for the SACT-ELISA and 38.58 ± 17.17 for the PGDS-ELISA) than that in the BI (22.63 ± 26.31).
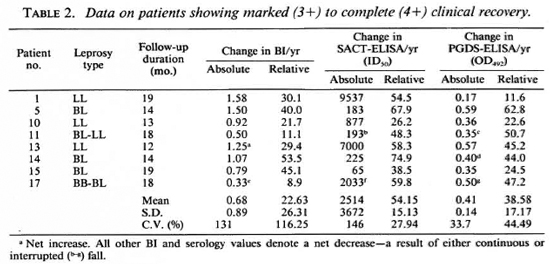
Table 3 shows the data on three patients who had varying degrees of clinical improvement along with a marked reduction in antibody titers (especially by the SACT-ELISA), but no bacteriological improvement. In fact, in one of them (no. 13) an increase (29.4%) in the BI value was recorded despite clinical improvement.
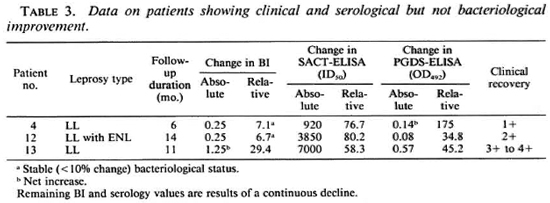
Two patients did not show any change in clinical activity even after 18 months of treatment, although they were not being considered refractory to therapy as yet (Table 4). One of them showed a net 1233% increase in the SACT-ELISA titers, while his PGDS-ELISA and the BI values declined. The other patient (no. 18) showed a sharp rise in the SACT-ELISA titers (1775%) over the level 3 months previously, the point at which he was experiencing an episode of erythema nodosum leprosum (ENL) (Fig. 2). However, this patient was also a case of irregular treatment.
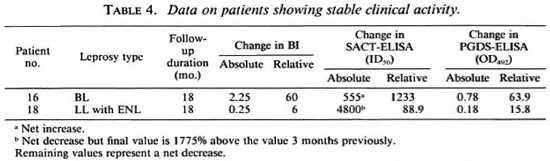
Patient no. 9 also showed a reduction in antibody levels, particularly with the SACT-ELISA (28% relative decline), despite remaining untreated over a period of 9 months between the initial and second examination.
At the end of the study period, 15 lepromatous patients still had a BI of > 2 (Figs. 1 and 2). Two patients (nos. 7 and 20) had become negative by both antibody assays, although one of them was still positive with a BI of 2.5. Four other patients finally showed negativity for the PGDS-ELISA but not for the SACT-ELISA.
Tuberculoid patients. Of the six tuberculoid leprosy patients, only one was positive for both the BI and antibodies (patient no. 4, Fig. 3). In this group, the initial antibody titers in the four patients who were positive for the SACT-ELISA (three of whom were also positive for the PGDS-ELISA) were significantly lower than those of the lepromatous group. A gradual fall in titers was noted in the antibody-positive tuberculoid patients, along with a progressive decline in disease activity.
DISCUSSION
It has been repeatedly recorded that rifampin, the crucial component of1V1DT, kills M. leprae with exceptional speed (> 99% kill within a few days); whereas the BI shows a slow and steady decline of about 0.6 to 1 log per year (14-26). This is thought to be due to the persistence of dead bacilli in the skin for long periods awaiting clearance by normal body mechanisms (10). The rate of this clearance may vary from patient to patient, as indicated by the data presented in this study. Against this backdrop, the recommended (26) continuation of MDT till attainment of bacterial negativity could lead to unnecessary treatment for long durations (even up to a few years) in some of the patients (10). Serology forM./<?prae-specific antibodies, especially when performed serially, has shown the potential to provide a speedy and reliable method for monitoring the load of active infection in the body (1,4,11,12)
In this study, the expression in relative (to the corresponding initial values) rather than absolute terms revealed a greater consistency in the rates of decline of antibody levels following MDT . Similar observations were made in a recent report based on PG-ELISA (11). The reason for this could be the fact that the fall in titers was rapid in some of the patients who had initially high antibody levels and slow in some others who had low initial levels. The rate of relative decline was higher and less variable for the SACT-ELISA compared to the PGDS-ELISA. The rate of fall in the BI was, however, lowest and inconsistent. This observation, combined with the known efficacy of MDT, suggests that serology could provide sensitive pointers to progression under treatment. It also implies that the pool of corresponding M. /cprae-specific antigens (PGL-I and 35-kDa protein) in the host is largely dependent on the quantum of live bacilli. A sharp decline in a circulating pool of PGL-I (in the absence of any appreciable change in the BI) is known to occur within a few months ofinitiation of MDT (2). However, the fall in the levels of anti-PGL-I antibodies has been reported to be slower, a maximum decline being in the first year under therapy (4). PGL-I persisting in the skin (23) has been held responsible for the maintenance of low antibody titers over a considerable period of time. It remains to be seen whether the pool of 35-kDa antigen is also generated and maintained in an identical manner, although its protcinaccous nature and rapid decline in corresponding antibodies (SACT-ELISA levels) following MDT would suggest a faster clearance of this antigen than PGL-I.
The value of clinical activity scores (13) in monitoring response to treatment is apparent from studies in which a concurrent and steep progressive fall in the morphological index (24), viability of M. leprae as assessed by the mouse foot pad technique (18), and clinical scores were noted (in the absence of any appreciable change in the 131 values) within a few weeks of beginning rifampin therapy in lepromatous leprosy patients (6,7,14). However, the assessment of clinical activity by itself cannot serve as a tool for monitoring treatment, since the method is even less stand ardized, needs greater expertise, and can be more subjective than BI determinations. The present study indicates that M. leprae-specific serology, as an adjunct to periodical clinical assessment, may impart a degree of objectivity and reliability to the latter. The patients showing a variable degree of clinical recovery also showed a steep and consistent rate of relative decline in specific antibody titers, particularly by the SACT-ELISA.
In two patients who did not show a perceptible change in their clinical scores over the studied duration of treatment, the SACT-ELISA titers increased sharply. Although it is tempting to take it as yet another indication for a role of serology in detecting unresponsiveness to treatment (4) there are certain constraints. Firstly, this group is very small. Secondly, one of the patients was undergoing an episode of ENL which is thought to cause fluctuations in antibody levels. However, such fluctuations, if any (11), are not likely to be as great as seen in this case. In fact, another ENL patient in our study who was clinically responding to treatment did not show such results (Fig. 2, Table 3).
The fact that, despite a steep decline, many patients still had high antibody levels at the end of the study period needs to be considered specifically. It has been documented that patients continue to manifest clinicobacteriological improvement even after a fixed duration (coinciding with maximum follow-up duration in this study) of MDT 10). Quite likely, a longer follow-up of our patients would have seen further reduction in their antibody levels up to negativity or near-negativity. Whether such progressively declining trends leading to substantially lower antibody titers at the end of a treatment schedule are indicative of success of a treatment or not, can be ascertained by similar studies on an extended scale.
The SACT-ELISA has shown greater sensitivity compared to the PGDS-ELISA in the present and previous (19) studies. It also showed the highest and least variable rate of relative decline in patients showing a marked clinical improvement following treatment. Nonetheless, these observations may partly be attributed to the differences in commonly practiced methodology of the two assays. The SACT involves titration of antibody in the scrum which may reflect antibody concentrations in a better way than the PGDS-ELISA which is performed at a fixed dilution in which the relationship between concentration and OD is unlikely to be linear in the higher range.
In conclusion, since there are considerable individual variations in the severity of infection and response to treatment even within a class of leprosy patients (more so, when classified simply as multibacillary or paucibacillary), M. leprae-specific serology with the SACT-ELISA may serve as a helpful adjunct to routine clinico-bacteriological methods for deciding treatment schedules for a given case. It is likely to be particularly useful when marked clinical regression is not accompanied by a similar improvement in the BI. In post-treatment surveillance, serology may be helpful in detecting reactivation of residual or hidden foci of infection. However, serology may not be particularly helpful or necessary in the management of tuberculoid (or paucibacillary) leprosy patients.
Acknowledgments. We are grateful to Dr. R. J. W. Rees, National Institute for Medical Research, London, for the supply of M. leprae antigen from the WHO-IMMLEP Bank; to Dr. J. Ivanyi, MRC Tuberculosis and Related Infections Unit, London, for the supply of monoclonal antibodies against M. leprae; and to Dr. Delphi Chattcrjce, Colorado State University, Fort Collins, Colorado, U.S.A., for providing ND-O-BSA. Skillful technical assistance was provided by Mr. H. O. Agarwal and secretarial help was rendered by Mr. A. K. Chopra. We arc also grateful to Dr. H. Srinivasan for helpful suggestions during the preparation of the manuscript. In addition, certain materials used in this study were generous gifts of LEPRA, U.K.
REFERENCES
1. BACH, M.-A., WALLACH, D., FLAGEUL, B., HOFFENBACH, A. and COTTENOT, F. Antibodies to phenolic glycolipid-I and to whole M. leprae in leprosy patients: evolution during therapy. Int. J. Lepr. 54(1986)256-267.
2. CHANTEAU, S., CARTEL, J.-L., CELERIER, R. P., DESFORGES, S. and Roux, J. PGL-I antigen and antibody detection in leprosy patients: evolution under chemotherapy. Int. J. Lepr. 57(1989)735-743.
3. CHO, S.-N., FUJIWARA, T., HUNTER, S. W., REA, T. H., GELBER, R. H. and BRENNAN, P. J. Use of an artificial antigen containing the 3,6-di-O-methyl-β-D-glucopyranosyl epitope for the seriodiagnosis of leprosy. J. Infect. Dis. 150(1984)311-322.
4. GELBER, R. H., FUTÍAN, L., CHO, S.-N., BYRD, S„ RAJAGOPALAN, K. and BRENNAN, P. J. Serum antibodies to defined carbohydrate antigens during the course of treated leprosy. Int. J. Lepr. 57(1989)744-751.
5. GELBER, R. H., H UMPHRES, R. C. and FIELDSTEEL, A. H. Superiority of the nconatally thymcctomi/.ed rat (NTLR) to monitor a clinical trial in lepromatous leprosy of the two regimens of rifampin and dapsone. Int. J. Lepr. 54(1986)273-283.
6. GELBER, R. H., WATERS, M. F. R., PEARSON, J. M. H., REES, R. J. W. and MCDOUGALL, A. C. Dapsone alone compared with dapsone plus rifampicin in short-term therapy of lepromatous leprosy. Lepr. Rev. 48(1977)223-229.
7. GIRDHAR, B. K., RAMU, G., S REEVATSA and DE-SIKAN, K. V. Introductory rifampin therapy in lepromatous leprosy; a six month follow-up study. Lepr. India 50(1978)363-370.
8. IVANYI, J., MORRIS, J. A. and KEEN, M. Studies with monoclonal antibodies to mycobacteria. In: Monoclonal A ntibodies Against Bacteria. Macario, A. J. L. and Macario, E. C, eds. New York: Academic Press, 1985, pp. 59-90.
9. Ji, B. Drug resistance in leprosy-a review. Lepr. Rev. 56(1985)265-278.
10. JOPLING, W. H. A report on two follow-up investigations of the Malta project: 1983 and 1986. Lepr. Rev. 57Suppl.3(1986)47-52.
11. KLATSER, P. R.,DE WIT, M. Y. L., F AJARDO, T. T., C ELLONA, R. V., A BALOS, R. M.,DELA CRUZ, E. C, M ADARANG, M. G., HIRSCH, D. S. and DOUGLAS, J. T. Evaluation of M.leprae antigen in the monitoring of a dapsone based chemotherapy of previously untreated lepromatous patients in Cebu, Philippines. Lepr. Rev. 60(1989)178-186.
12. MILLER, R. A., G ORDER, D. and HARNISCH, J. P. Antibodies to phenolic glycolipid-I during longterm therapy: serial measurements in individual patients. Int. J. Lepr. 55(1987)633-636.
13. RAMANUJAM, K. Discussion on criteria for the assessment of drug activity. Lepr. Rev. 46Suppl.(1975)223-224.
14. REES, R. J. W., PEARSON, J. M. H. and WATERS, M. F. R. Experimental and clinical studies on rifampicin in treatment of leprosy. Br. Med. J. 1(1975)89-92.
15. RIDLEY, D. S. Bacterial indices. In: Leprosyin Theory and Practice. Cochrane, R. G. and Davey, T. E., eds. Baltimore: Williams and Wilkins, 1964, pp. 620-622.
16. RIDLEY, D. S. and J OPLING, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1986)255-273.
17. SEROLOGICAL TESTS FOR LEPROSY. (Editorial) Lancet 1(1986)533-535.
18. SHEPARD, C. C. A kinetic method for the study of activity of drugs against Mycobacterium leprae in mice. Int. J. Lepr. 35(1967)429-435.
19. SINHA, S., MCENTEGART, A., GIRDHAR, B. K., BHATIA, A. S. and S ENGUPTA, U. Appraisal of two Mycobacterium leprae-specific serological assays for monitoring chemotherapy in lepromatous (LL/ BL) leprosy patients. Int. J. Lepr. 57(1989)24-32.
20. SINHA, S., S ENGUPTA, U., RAMU, G. and IVANYI, J. A serological test for leprosy based on competitive inhibition of monoclonal antibody binding to the MY2a determinant of M. leprae. Trans. R. Soc. Trop. Med. Hyg. 77(1983)869-871.
21. SUBCOMMITTEE ON CLINICAL TRIALS OF THE CHEMOTHERAPY O FLEPROSY (THELEP) S CIENTIFIC WORKING G ROUP OF THE UNDP/WORLD BANK/ WHO SPECIAL PROGRAMME FOR RESEARCH AN D T RAINING IN TROPICAL DISEASES. Persisting M. leprae among THELEP trial patients in Bamako and Chingleput. Lepr. Rev. 58(1987)325-337.
22. TOMAN, K. Bacterial persistancc in leprosy. Int. J. Lepr. 49(1981)205-217.
23. VENKATESAN, K., SINGH, H., BHARADWAJ, V. P. and RAMU, G. Isolation, purification and quantification of phenolic glycolipid I from human leprosy skin tissues. Trans. R. Soc. Trop. Med. Hyg. 82(1988)321-323.
24. WATERS, M. F. R. and REES, R. J. W. Changes in the morphology of Mycobacterium leprae in patients under treatment. Int. J. Lepr. 30(1962)266-277.
25. WATERS, M. F. R., RIDLEY, D. S. and RIDLEY, M. J. Clinical problems in initiation and assessment of multidrug therapy. Lepr. Rev. 57Suppl. 3(1986)92-100.
26. WHO E XPERT C OMMITTEE O N LEPROSY. Sixth report. Geneva: World Health Organization, 1968. Tech. Rep. Ser. 768.
1. Ph.D.; Central JALMA Institute for Leprosy (ICMR), P.O. Box 31, Taj Ganj, Agra 282001, India.
2. Ph.D.; Central JALMA Institute for Leprosy (ICMR), P.O. Box 31, Taj Ganj, Agra 282001, India.
3. M.D.; Central JALMA Institute for Leprosy (ICMR), P.O. Box 31, Taj Ganj, Agra 282001, India.
4. Ph.D., Central JALMA Institute for Leprosy (ICMR), P.O. Box 31, Taj Ganj, Agra 282001, India.
Reprint requests to Dr. Sinha.
Received for publication on 9 May 1990.
Accepted for publication in revised form on 15 November 1990.