- Volume 59 , Number 1
- Page: 41–8
Effect of glucocorticoids and interferon-γ on the oxidative responses of monocytes f rom leprosy patients and normal donors
ABSTRACT
Leprosy patients suffering f rom erythema nodosum leprosum are frequently treated with glucocorticosteroids. The role glucocorticosteroids and interferon-gamma (IFN-γ) play in regulating the interaction of phagocytic cells with Mycobacterium leprae was examined. Monocytes f rom leprosy patients receiving prednisone therapy responded to lower concentrations of IFN-γ in vitro with enhanced superoxide anion release when challenged with M. leprae or M. bovis BCG than did monocytes f rom healthy subjects and other leprosy patients. Although the number of patients was small and the population heterogeneous, the data suggested that prednisone could alter IFN-γ efficacy and led to the examination of the effect of glucocorticosteroids on IFN-γ activation of monocytes. IFN-γ treatment following in vitro dexamcthasone pretreatment of monocytes f rom healthy subjects resulted in a greater enhancement of superoxide anion generation than that observed with IFN-γ treatment alone. These findings are important considerations in evaluating patient immune function because IFN-γ is being used in a number of clinical trials with leprosy patients.RÉSUMÉ
Les malades de la lèpre présentant un crythème noueux lépreux sont fréquemment traités avec des glycocorticostcroïdes. Le rôle que jouent les glycocorlicostéroïdes et l'interfcron-gamma (IFN-γ) dans la régulation de l'interaction des cellules phagocytaires avec Mycobacterium leprae a été examiné. Des monocytes de malades de la lèpre recevant un traitement á la prednisone répondaient in vitro à des concentrations plus faibles d'IFN-γ avec une augmentation de la libération d'anion superoxide en présence de M. leprae ou de BCG de M. bovis que des monocytes en provenance de sujets sains ou d'autres malades de la lèpre. Bien que le nombre de patients était limité et la population hétérogène, les données suggèrent que la prednisone pourrait modifier l'efficacité de l'IFN-γ et ont conduit á examiner l'effet des glycocorticostéroïdes sur ('activation des monocytes par l'IFN-γ. Le traitement par l'IFN-γ suivant un pré-traitement in vitro àla dexaméthasone des monocytes de sujets sains a résulté dans une augmentation de la production d'anion superoxide par rapport à celle observée suite à un traitement par l'IFN-γ seul. Ces observations sont importantes pour l'évaluation de la fonction immunitaire des malades parce que l'IFN-γ est utilisé dans un certain nombre d'essais cliniques avec des malades de la lèpre.RESUMEN
Los pacientes con eritema nudoso leproso frecuentemente son tratados con glucocorticocsteroides. Aquí se examina el papel que juegan los glucocorticocsteroides y el interferon gamma (IFN-γ) en la respuesta de las células fagocíticas al interaccionar con Mycobacterium leprae. Los monocitos de pacientes con lepra bajo tratamiento con prednisona, respondieron in vitro con la producción y liberación de anión superóxido (estimulada con M. leprae o con ,U. bovis. BCG) a concentraciones más bajas del IFN-γ que los monocitos de personas sanas o de otros pacientes con lepra. Aunque el número de pacientes fue pequeño y la población heterogénea, los datos sugirieron que la prednisona podría alterar la eficiencia del IFN-γ. Esto condujo al examen del efecto de los glucocorticocsteroides sobre la activación de los monocitos por el IFN-γ. El tratamiento con IFN-γ posterior al pretratamiento in vitro de los monocitos de individuos sanos con dexametasona, dio como resultado un mayor incremento en la generación de anión superóxido que el incremento observado cuand o el tratamiento fue con IFN-γ solo. Estos hallazgos pueden ser importantes porque el IFN-γ actualmente se está utilizand o en un buen número de ensayos clínicos con pacientes con lepra.A characteristic feature of leprosy is its spectrum of clinical symptoms correlating with the cellular immune responsiveness of the patient to Mycobacterium leprae. Tuberculoid leprosy patients, in contrast to lepromatous patients, exhibit normal cellmediated immune responses to M. leprae antigens. Mononuclear phagocytes in tuberculoid leprosy are able to ingest and destroy most microorganisms; however, in lepromatous leprosy, phagocytes fail to control the intracellular multiplication of M. leprae (9). Studies have shown that M. leprae are susceptible to oxygen radical killing (11). Macrophages from leprosy patients reportedly fail to present M. leprae antigens in an immunogenic form (6), are unable to provide accessory function for lymphocyte proliferation, and have reduced protein synthesis in the presence of M. leprae (26). It has been reported that T cells from lepromatous leprosy patients fail to produce interleukin-2 (IL-2) after exposure to M. leprae but respond by proliferation to M. leprae in the presence of exogenous IL-2 (4,5). Other studies point out that T cells fail to express IL-2 receptors upon challenge with M. leprae (15). More recently, it has been shown that T cells from lepromatous patients can respond to M. leprae if cultured in medium alone, which suggests that the failure of those T cells to respond to M. leprae in vivo may be due to the persistence of antigen (M). Interferon-gamma (IFN-γ ), an activator of human monocyte oxidative metabolism and antimicrobial activity (17,19), is produced in lower amounts by T lymphocytes from lepromatous patients than by normal T cells or T cells from tuberculoid patients (21). Patients with leprosy can spontaneously develop erythema nodosum leprosum (ENL) which is characterized by tender skin nodules, fever, malaise, nerve pain, bone pain, and pain in large joints (1,23). Current therapy for ENL includes the use of immunom odulators, such as thalidomide or prednisone.
This study was undertaken to determine the effect of IFN-γ on superoxide anion (O ) production by monocytes from leprosy patients. In addition, in view of a recent report that corticosteroids can enhance the binding of IFN-γ to human monocytes in culture due to receptor biogenesis (30), and the use of corticosteroids in the treatment of ENL, we have examined the effects of both agents on monocyte O
) production by monocytes from leprosy patients. In addition, in view of a recent report that corticosteroids can enhance the binding of IFN-γ to human monocytes in culture due to receptor biogenesis (30), and the use of corticosteroids in the treatment of ENL, we have examined the effects of both agents on monocyte O release in response to various stimuli, including M. leprae.
release in response to various stimuli, including M. leprae.
MATERIALS AND METHODS
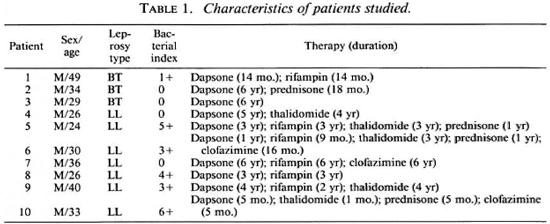
Subjects. Informed consent for a blood donation was given by normal adult laboratory personnel (control subjects) and by patients with a confirmed diagnosis of leprosy in the Hansen's Disease Clinic at the University of Illinois Medical Center. Disease classification was according to the criteria of Ridley and Jopling (24). Table 1 summarizes the characteristics of the patients used in this study. All were males with ages ranging from 24 to 49, and a median age of 33. Bacterial indices from biopsies performed 0-9 months prior to this study ranged from 0 to 6 +, with a median of 2 +. All the patients were on dapsone therapy, with some on multiple therapies as indicated in Table 1.
Cells. Peripheral blood was collected from donors as previously described (7) into 10 U/ml sterile heparin (ICN, Cleveland, Ohio, U.S.A.) and diluted with an equal volume of Hank's Balanced Salt Solution without phenol red, calcium or magnesium (HBSS; Gibco Laboratories, Grand Island, New York, U.S.A.) The blood was layered over Ficoll-Hypaque (Sigma Chemical Company, St. Louis, Missouri, U.S.A., and Winthrop-Breon Laboratories, New York, New York, U.S.A.), and centrifuged at 400 x g x 25 min. The mononuclear cell layer (MNC) was collected, and the cells washed with HBSS. The contaminating erythrocytes were hypotonically lysed. The percentage of monocytes in the MNC suspension was determined by counting the number of cells ingesting 0.8 μm latex particles (Difco Laboratories, Detroit, Michigan, U.S.A.). The monocytes were consistently > 98% viable, as determined by trypan blue dye exclusion. The cells were washed by centrifugation and resuspended at 2 x 106 monocytes/ml in medium which consisted of RPMI 1640 (Gibco) containing 10% heatinactivated fetal calf serum (Hyclone Laboratories, Logan, Utah, U.S.A.) and 50 μg/ml gentamicin (Sigma).
Culture conditions. The cells were plated in 96-well, flat-bottom, microtiter plates (Corning, Corning, New York, U.S.A.) at 2 x 105 monocytes/well, and allowed to adhere for 1 hr at 37ºC in 5% CO2 in air. The cells were washed with warm fresh medium to remove nonadherent cells, and then cultured overnight in medium alone or in medium containing 2 nM, 200 nM, or 20 μM dexamethasone (Rugby Laboratories, Rockville Centre, New York, U.S.A.). The cells were then washed and cultured in medium alone or in medium containing up to 500 U/ml IFN-γ (Sigma) overnight. The source of the IFN-γ was supernatants from A23187 and mizerein-induced human buffycoat cell preparations. In some experiments, the monocytes were cultured simultaneously with the dexamethasone and IFN-γ overnight in the concentrations mentioned above.
Superoxide anion assay. Superoxide dismutase-inhibitable production of O by monocytes was determined by a modification of the microassay technique of Pick and Mizel (22), measuring ferricytochrome c (Type III; Sigma) reduction as described (7). The absorbance was determined at 550 nm (EIA Reader, Burlington, Vermont, U.S.A.) immediately after the addition of reaction mixtures (0 time) and 150 min later. We chose to read the plates after 150 min instead of the conventional 90 min because we had previously shown (7) that M. leprae was a weak and slow stimulus of the metabolic burst of monocytes. The stimuli used in the assays included phorbol myristate acetate (PMA, 100 ng/ml; Sigma), M. leprae, and M. bovis BCG (50:1 bacteria-to-mono-cyte ratio). Following the assay, the wells were washed, and protein quantitation on the adherent cells was performed by the Lowry method (12) with bovine serum albumin used for the standard curve.
by monocytes was determined by a modification of the microassay technique of Pick and Mizel (22), measuring ferricytochrome c (Type III; Sigma) reduction as described (7). The absorbance was determined at 550 nm (EIA Reader, Burlington, Vermont, U.S.A.) immediately after the addition of reaction mixtures (0 time) and 150 min later. We chose to read the plates after 150 min instead of the conventional 90 min because we had previously shown (7) that M. leprae was a weak and slow stimulus of the metabolic burst of monocytes. The stimuli used in the assays included phorbol myristate acetate (PMA, 100 ng/ml; Sigma), M. leprae, and M. bovis BCG (50:1 bacteria-to-mono-cyte ratio). Following the assay, the wells were washed, and protein quantitation on the adherent cells was performed by the Lowry method (12) with bovine serum albumin used for the standard curve.
Bacteria. Armadillo-derived M. leprae were kindly provided by Dr. Patrick Brennan (Colorado State University, Fort Collins, Colorado, U.S.A.) through National Institutes of Health Contract No. AI-52582. The M. leprae were purified from irradiated (2.5 Mrad) armadillo spleens and livers and stored in phosphate buffered saline with 0.1 % Tween 80 (PBS-Tween) at - 70ºC. The M. leprae were washed by centrifugation before use in the O assay. M. bovis BCG were obtained from the University of Illinois at Chicago Institute for Tuberculosis Research as lyophilized vaccine, and was grown in Middlebrook broth with 10% OADC enrichment (oleic acid, albumin, dextrose, cat-alase) and 0.1% Tween 80 (Difco) at 37ºC for 4 weeks. The BCG were then heat killed by autoclaving at 121ºC for 15 min, and counted using fluorescence microscopy after staining with Auramine-O (Difco) as previously described (8). The BCG were stored in PBS-Tween at 4ºC, and washed by centrifugation prior to use in the O
assay. M. bovis BCG were obtained from the University of Illinois at Chicago Institute for Tuberculosis Research as lyophilized vaccine, and was grown in Middlebrook broth with 10% OADC enrichment (oleic acid, albumin, dextrose, cat-alase) and 0.1% Tween 80 (Difco) at 37ºC for 4 weeks. The BCG were then heat killed by autoclaving at 121ºC for 15 min, and counted using fluorescence microscopy after staining with Auramine-O (Difco) as previously described (8). The BCG were stored in PBS-Tween at 4ºC, and washed by centrifugation prior to use in the O assay.
assay.
Endotoxin contamination. A quantitative chromogenic limulus amebocyte lysate assay (Whittaker, Walkersville, Maryland, U.S.A.) with a sensitivity of 10 pg/ml was performed periodically on the reagents used in the preparation and culture of the monocytes. Less than 10 pg/ml of endotoxin was detected in the buffers, media, or reagents.
Statistical analysis. The differences between mean values were evaluated for statistical significance by Student's / test.
RESULTS
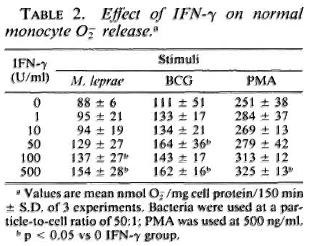
Response of monocytes to IFN-γ. To determine the optimal concentration of IFN-γ necessary to enhance the oxidative response of the monocytes, cells from control subjects were cultured with the indicated concentrations of IFN-γ for 24 hr. The enhancement of O release by IFN-y is shown in Table 2. IFN-γ at 500 U/ml significantly increased the monocyte O
release by IFN-y is shown in Table 2. IFN-γ at 500 U/ml significantly increased the monocyte O generation in response to all stimuli (PMA, BCG, and M. leprae) with 100 U/ml and 50 U/ml sufficient to augment O
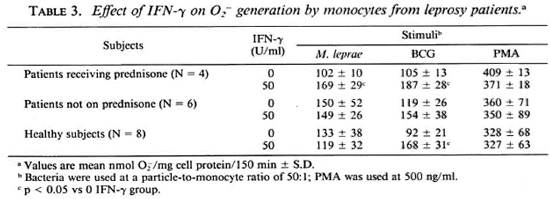
generation in response to all stimuli (PMA, BCG, and M. leprae) with 100 U/ml and 50 U/ml sufficient to augment O release in response to M. leprae and BCG, respectively. It was observed that when monocytes from leprosy patients were pretreated with 50 U/ml IFN-γ, those from patients on prednisone therapy responded with significantly enhanced O
release in response to M. leprae and BCG, respectively. It was observed that when monocytes from leprosy patients were pretreated with 50 U/ml IFN-γ, those from patients on prednisone therapy responded with significantly enhanced O release when challenged with M. leprae or with BCG (Table 3). Monocytes from patients not on prednisone responded as did the monocytes from healthy subjects, in that pretreatment with 50 U/ml IFN-γ did not enhance their O
release when challenged with M. leprae or with BCG (Table 3). Monocytes from patients not on prednisone responded as did the monocytes from healthy subjects, in that pretreatment with 50 U/ml IFN-γ did not enhance their O generation upon challenge with M. leprae.
generation upon challenge with M. leprae.
Effect of dexamethasone pretreatment on IFN-γ enhancement of monocyte release. Monocytes from healthy control subjects were cultured overnight in media containing 0, 2 nM, 200 nM, or 20 μM dexamethasone. They were then washed and cultured for an additional 24 hr in the presence or absence of 500 U/ml IFN-γ. The cells were then challenged with PMA or M. leprae. Monocytes pretreated with all concentrations of dexamethasone tested released less O in response to M. leprae than did untreated control monocytes (Fig. 1A). Cells pretreated with 200 nM or 2011Mdexamethasone but not with 2 nM dexamethasone showed a decrease in O
in response to M. leprae than did untreated control monocytes (Fig. 1A). Cells pretreated with 200 nM or 2011Mdexamethasone but not with 2 nM dexamethasone showed a decrease in O release when stimulated with PMA (Fig. 1B). IFN-γ enhanced the O
release when stimulated with PMA (Fig. 1B). IFN-γ enhanced the O generation of monocytes in response to M. leprae and to PMA whether or not the cells had been pretreated with dexamethasone (Fig. 1). IFN-γ restored O
generation of monocytes in response to M. leprae and to PMA whether or not the cells had been pretreated with dexamethasone (Fig. 1). IFN-γ restored O generation to normal levels. In addition, it appears that IFN-γ reversed the suppressive effects of dexamethasone.
generation to normal levels. In addition, it appears that IFN-γ reversed the suppressive effects of dexamethasone.
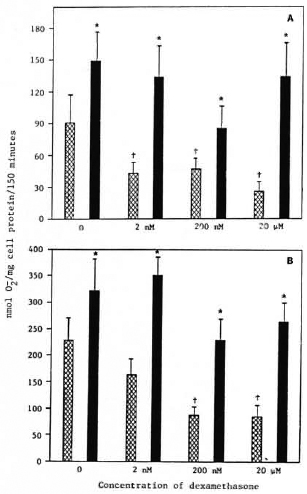
Fig. 1. Effect of dexamethasone treatment prior to interferon-γ treatment on monocyte O, release in response to M. leprae (A) or PMA (B). Cells were treated on the day of isolation with indicated concentrations of dexamethasone for 24 hr. Cells were then cultured in the presence  or absence
or absence  of IFN-γ (500 U/ml) for 24 hr. Results are means ± S.E. of 8 experiments, + = p < 0.05 when compared with untreated control monocytes; * = p < 0.05 when compared with no IFN-γ treatment.
of IFN-γ (500 U/ml) for 24 hr. Results are means ± S.E. of 8 experiments, + = p < 0.05 when compared with untreated control monocytes; * = p < 0.05 when compared with no IFN-γ treatment.
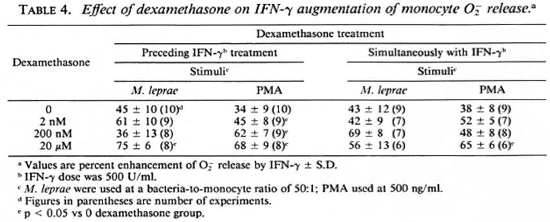
Degree of metabolic burst enhancement by IFN-γ. The percent enhancement of monocyte O release by IFN-γ shown in Table 4 was calculated from data obtained when the monocytes were simultaneously treated with dexamethasone and IFN-γ, and is from the data in Figure 1. Synergism is suggested by the data when dexamethasone treatment preceded IFN-γ treatment. When PMA was the stimulus, all concentrations of dexamethasone appeared to enhance the augmentation of monocyte O release. When M. leprae was used as a stimulus for monocyte O
release by IFN-γ shown in Table 4 was calculated from data obtained when the monocytes were simultaneously treated with dexamethasone and IFN-γ, and is from the data in Figure 1. Synergism is suggested by the data when dexamethasone treatment preceded IFN-γ treatment. When PMA was the stimulus, all concentrations of dexamethasone appeared to enhance the augmentation of monocyte O release. When M. leprae was used as a stimulus for monocyte O release, 20 μM dexamethasone increased the enhancement by IFN-γ. Under culture conditions where dexamethasone and IFN-γ treatment was simultaneous, only the highest concentration of dexamethasone enhanced the IFN-γ activation of monocytes in response to PMA (Table 4).
release, 20 μM dexamethasone increased the enhancement by IFN-γ. Under culture conditions where dexamethasone and IFN-γ treatment was simultaneous, only the highest concentration of dexamethasone enhanced the IFN-γ activation of monocytes in response to PMA (Table 4).
DISCUSSION
Lymphokines are known to enhance, and hydrocortisone to inhibit, the ability of monocytes to secrete reactive oxygen intermediates (18). IFN-γ is the lymphokinc responsible for the activation of macrophages that enhances oxidative killing of intracellular parasites (17,19,20,25). Lymphocytes from patients with the lepromatous form of Hansen's disease are unable to produce normal amounts of IFN-γ upon challenge with M. leprae (21). Kaplan, et al. (10) have reported that monocyte-derived macrophages from 10 to 14 lepromatous patients studied responded to recombinant IFN-γ with enhanced ability to generate H2O2. More recently, it has been shown that macrophages from leprosy patients have an enhanced ability to kill M. leprae in the presence of IFN-γ (2) or delipidified cell components of M. leprae (13). Our study shows that monocytes of patients receiving prednisone therapy at the time peripheral blood was drawn responded to lower concentrations of IFN-γ with an enhanced O production when stimulated with M. leprae or BCG, than did monocytes from patients not on prednisone therapy or monocytes from healthy subjects. Even though the number of patients in the study was small and the patient population was heterogeneous, the data suggested a prednisone effect and led us to examine the effect of dexamethasone on IFN-γ activation of normal monocytes in vitro.
production when stimulated with M. leprae or BCG, than did monocytes from patients not on prednisone therapy or monocytes from healthy subjects. Even though the number of patients in the study was small and the patient population was heterogeneous, the data suggested a prednisone effect and led us to examine the effect of dexamethasone on IFN-γ activation of normal monocytes in vitro.
Monocytes pretreated with dexamethasone only released less O than did control cells. IFN-γ enhanced the Oj generation of monocytes whether or not the cells had been pretreated with dexamethasone. In fact, synergism is suggested by these results since when dexamethasone treatment preceded IFN-γ treatment, the IFN-γ enhancement of O
than did control cells. IFN-γ enhanced the Oj generation of monocytes whether or not the cells had been pretreated with dexamethasone. In fact, synergism is suggested by these results since when dexamethasone treatment preceded IFN-γ treatment, the IFN-γ enhancement of O release was further augmented. When PMA was the stimulus, all concentrations of dexamethasone appeared to enhance the IFN-γ augmentation of monocyte O^ release. When M. leprae was used as the challenge stimulus, the highest concentration of dexamethasone exerted its potentiating effect.
release was further augmented. When PMA was the stimulus, all concentrations of dexamethasone appeared to enhance the IFN-γ augmentation of monocyte O^ release. When M. leprae was used as the challenge stimulus, the highest concentration of dexamethasone exerted its potentiating effect.
A dexamethasone-induced increase in receptors for IFN-γ on monocytes has been reported (30). We found that IFN-γ augmentation of monocyte O release was greater in dexamethasone-pretreated cells than in untreated cells. This would be consistent with an increase in IFN-γ receptors on the monocytes. It appears that pretreatment of monocytes with dexamethasone was more effective in increasing the augmentation of O
release was greater in dexamethasone-pretreated cells than in untreated cells. This would be consistent with an increase in IFN-γ receptors on the monocytes. It appears that pretreatment of monocytes with dexamethasone was more effective in increasing the augmentation of O by IFN-γ than simultaneous treatment with both agents. This is in agreement with observations that 24 hours or longer was needed for dexamethasone to exert its effects (27). It has been shown that the IFN-γ enhancement of the macrophage respiratory burst could not be prevented by dexamethasone (16), even at the concentrations of dexamethasone used that suppressed the antimicrobial activity of human monocytes (27). Others (31) have reported that IFN-γ can block the O
by IFN-γ than simultaneous treatment with both agents. This is in agreement with observations that 24 hours or longer was needed for dexamethasone to exert its effects (27). It has been shown that the IFN-γ enhancement of the macrophage respiratory burst could not be prevented by dexamethasone (16), even at the concentrations of dexamethasone used that suppressed the antimicrobial activity of human monocytes (27). Others (31) have reported that IFN-γ can block the O inhibitory activity of various glucocorticoids. Moreover, dexamethasone has been shown to augment IFN-γ effects on human monocyte effector function as measured by antibody-dependent cellular cytotoxicity (29) and to enhance IFN-γ augmentation of monocyte IgG Fc receptor expression (3-32). Although it was recently reported that dexamethasone did not suppress H2O2 release by monocyte-de-rived macrophages (28), our studies, using 24- to 48-hour monocyte cultures, show that dexamethasone can suppress O
inhibitory activity of various glucocorticoids. Moreover, dexamethasone has been shown to augment IFN-γ effects on human monocyte effector function as measured by antibody-dependent cellular cytotoxicity (29) and to enhance IFN-γ augmentation of monocyte IgG Fc receptor expression (3-32). Although it was recently reported that dexamethasone did not suppress H2O2 release by monocyte-de-rived macrophages (28), our studies, using 24- to 48-hour monocyte cultures, show that dexamethasone can suppress O release as measured by cytochrome c reduction in a sensitive microassay.
release as measured by cytochrome c reduction in a sensitive microassay.
IFN-y and dexamethasone are both important immunomodulators. In our studies, IFN-y reversed the immunosuppressive effect of dexamethasone on monocytes. In fact, our findings suggest that enhanced effects of IFN-y may perhaps be achieved by glucocorticoid pretreatment.
Acknowledgment. This work was supported by the University of Illinois Earl M. Bane Trust Fund, the Veterans Administration Merit Review Grant MRSI034, and National Institutes of Health Grant AI-21354.
We express our appreciation to Sophie Worobec, M. D., and to the personnel of the Hansen's Disease Clinic, University of Illinois, for referral of patients. We thank Patrick Brennan, Ph.D., Colorado State University, for M. leprae; Vijai Moses, University of Illinois, for statistical analysis; and Bca Wozniak for secretarial assistance.
REFERENCES
1. BLOOM, B. R. and MEHRA, V. Immunological unresponsiveness in leprosy. Immunol. Rev. 80( 1984)5-28.
2. Desai, S. D., Birdi, T.J. and Antia, N. H. Correlation between macrophage activation and bactericidal function and Mycobacterium leprae antigen presentation in macrophages of leprosy patients and normal individuals. Infect. Immun. 57(1989)1311-1317.
3. Girard, M. T., Hjaltadottir, S., Fejes-Toth, A. N. and Guyre, P.M. Glucocorticoids enhance the γ-interferon augmentation of human monocyte immunoglobulin G Fc receptor expression.J. Immunol. 138(1987)3235-3241.
4. Haregewoin, A., Godal, T.,Mustafa, A. S., Belehu A. and Yemaneberhan, T. T-cell conditioned media reverse T-cell unresponsiveness in lepromatous leprosy. Nature 303(1983)342-344.
5. Haregewoin, A., Mustafa, A. S., Helle, I.,Waters, M. F. R.,Leiker, D. L. and Godal, T. Reversal by intcrlcukin-2 of the T cell unresponsiveness of lepromatous leprosy to Mycobacterium leprae. Immunol. Rev. 80(1984)77-86.
6. Hirschberg, H. The role of macrophages in the lymphoprolifcrative response to Mycobacterium leprae in vitro. Clin. Exp. Immunol.34(1978)46-51.
7. Holzer, T.J., Kizlaitis, L.,Vachula, M., Weaver, C.W. and Andersen, B. R. Human phagocytic cell responses to Mycobacterium leprae and Mycobacterium bovis BCG: an in vitro comparison of the leprosy vaccine components.J. Immunol. 141(1988)1701-1708.
8. Holzer, T.J., Nelson, K. E.,Crispen, R. G. and Andersen, B. R. Mycobacterium leprae fails to stimulate phagocytic cell superoxide anion generation. Infect. Immun. 51(1988)514-520.
9. Kaplan, G. and Cohn, Z. Cellular immunity in lepromatous and tuberculoid leprosy. Immunol. Lett.11(1985)205-209.
10. Kaplan, G.,Nathan, C. F.,Gandhi, R.,Horwitz, M. A., Levis, W. R. and Cohn, Z. A. Effect of recombinant interferon-gamma on hydrogen peroxide-releasing capacity of monocyte-derived macrophages from patients with lepromatous leprosy.J. Immunol. 137(1986)983-987.
11. Klebanoff, S. J. and Shepard, C. C. Toxic effect of the peroxidase-hydrogen pcroxide-halide antimicrobial system on Mycobacterium leprae. Infect. Immun. 44(1984)534-536.
12. Lowry, O. H.,Rosenbrough, N.J., Farr, A. L. and Rand all, R.J. Protein measurement with the Folin-phenol reagent.J. Biol. Chem.193(1951)265-275.
13. Marolia, J., Robinson, P. and Mahadevan, P. R.A complex component modulating immunedeficient cells in leprosy patients leading to loss of viability of Mycobacterium leprae-a possible vaccine. Clin. Exp. Immunol. 79(1990)7-14.
14. Mohagheghpour, N.,Gelber, R. H. and Engelman, E. G. T cell defect in lepromatous leprosy is reversible in vitro in the absence of exogenous growth factors.J. Immunol. 138(1987)570-574.
15. Mohagheghpour, N., Gelber, R. H.,Larrick, J. W., Sasaki, D. T.,Brennan, P. J. and Engelman, E. G. Defective cell-mediated immunity in leprosy: failure of T cells from lepromatous leprosy patients to respond to Mycobacterium leprae is associated with defective expression of intcrlcukin 2 receptors and is not reconstituted by interleukin 2.J. Immunol.135(1985)1443-1449.
16. Mokoena, T. and Gordon, S. Human macrophage activation. Modulation of mannosyl, fucosyl receptor activity in vitro by lymphokincs, gamma and gamma-intcrfcrons, and dexamcthasone.J. Clin. Invest.75(1985)624-631.
17. Murray, H.W., Rubin, B. Y. and Rothermal, C. D. Killing of intracellular Leishmania donovani by lymphokine-stimulated human mononuclear phagocytes. Evidence that intcrferon-7 is the activating lymphokine.J. Clin. Invest. 72(1983)1506-1510.
18. Nakagawara, A., de Santis, N. M., Nogueira, N. and Nathan C. F. Lymphokincs enhance the capacity of human monocytes to secrete reactive oxygen intermediates.J. Clin. Invest. 70(1982)1042-1048.
19. Nathan, C. F.,Murray, H.W., Wiebe, M. E. and Rubin, B. Y. Identification of interferon-7 as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity.J. Exp. Med.158(1983)670-689.
20. Nathan, C. F.,Prendergast, T.J., Weibe, M. E.,Stanley, E. R.,Platzer, E.,Remold, H. G., Welte, K.,Rubin, B. Y. and Murray, H.W. Activation of human macrophages. Comparison of other cytokines with intcrferon-7.J. Exp. Med. 160(1984)600-605.
21. Nogueira, N., Kaplan, G.,Levy, E.,Sarno, E. N., Kushner, P., Granelli-Piperno, A.,Vieira, L.,ColomerGould, V., Levis, W., Steinman, R.,Yip, Y. K. and Cohn, Z. A. Defective 7-interferon production in leprosy. Reversal with antigen and interleukin 2.J. Exp. Med.158(1983)2165-2170.
22. Pick, E. and Mizel, D. Rapid microassays for the measurement of superoxide and hydrogen peroxide production by macrophages in culture using an automatic enzyme immunoassay reader.J. Immunol. Meth.46(1981)211-226.
23. Rea, T. H. and Levan, N. E. Erythema nodosum leprosum in a general hospital. Arch. Dermatol. 111(1975)1575-1577.
24. Ridley, D.S. and Jopling, W. H. Classification of leprosy according to immunity; a five-group system. Int.J. Lepr.34(1966)255-273.
25. Rothermal, C. D.,Rubin, B. Y. and Murray, H.W. Gamma-interferon is the factor in lymphokine that activates human macrophages to inhibit intracellular Chlamydia psittaci replication. J. Immunol.131(1983)2542-2544.
26. Sálgame, P. R.,Birdi, T.J., Mahadevan, P. R. and Antia, N. H. Role of macrophages in defective cell mediated immunity in lepromatous leprosy. I. Factor(s) from macrophages affecting protein synthesis and lymphocyte transformation. Int. J. Lepr. 48(1980)172-176.
27. SCHAFFNER, A. Therapeutic concentrations of glucocorticoids suppress the antimicrobial activity of human macrophages without impairing their responsiveness to gamma-interfcron. J. Clin. Invest. 76(1985)1755-1764.
28. SHAFFNER, A. and RELLSTAB, P. γ-Interferon restores listericidal activity and concurrently enhances release of reactive oxygen metabolites in dexamethasone-treated human monocytes. J. Clin. Invest. 82(1988))913-919.
29. SHEN, L., GUYRE, P. M., BALL, E. D. and FANGER, M. W. Glucocorticoid enhances gamma interferon effects on human monocyte antigen expression and ADCC. Clin. Exp. Immunol. 65 (1986) 387-395.
30. STRICKLAND, R. W., WAHL, L. M. and FINBLOOM, D. S. Corticosteroids enhance the binding of recombinant interferon-7 to cultured human monocytes. J. Immunol. 137(1986)1577-1580.
31. SZEFLER, S. J., NORTON, C. E., BALL, B., GROSS, J. M., AIDA, Y. and PABST, M. J. IFN-γ and LPS overcome glucocorticoid inhibition of priming for superoxide release in human monocytes. Evidence that secretion of IL-1 and tumor necrosis factor-7 is not essential for monocyte priming. J. Immunol. 142(1989)3985-3992.
32. WARREN, M. K. and VOGEL, S. N. Opposing effects of glucocorticoids on interferon-γ-induced murine macrophage Fc receptor and la antigen expression. J. Immunol. 134(1985)2462-2469.
1. Ph.D., Departments of Medicine and Microbiology/Immunology, University of Illinois at Chicago, Chicago, Illinois.
2. Ph.D., Department of Microbiology/Immunology, University of Illinois at Chicago and West Side Veterans Administration Medical Center, Chicago, Illinois.
3. M.D., Department of Medicine, University of Illinois at Chicago, Chicago, Illinois.
4. M.D., Departments of Medicine and Microbiology/Immunology, University of Illinois at Chicago, and West Side Veterans Administration Medical Center, Chicago, Illinois, U.S.A.
Reprint requests to: Dr. Burton Andersen, Chief, Section of Infectious Diseases, M/P 151, West Side VA Medical Center, 820 South Damen Avenue, Chicago, Illinois 60612, U.S.A.
Present address for Dr. Vachula: Baxter Healthcare, WG2-2S, Route 120 and Wilson Road, Round Lake, Illinois 60073, U.S.A.
Present address for Dr. Holzer: Abbott Laboratories, 1400 Sheridan Road, North Chicago, Illinois 60064, U.S.A.
Present address for Dr. Nelson: The Johns Hopkins University, 615 N. Wolfe Street, Baltimore, Maryland 21205, U.S.A.
Received for publication on 26 March 1990.
Accepted for publication in revised form on 15 November 1990.