- Volume 59 , Number 1
- Page: 49–57
Major histocompatibility complex restriction of T-cell suppression of immune response to mycobacteria
ABSTRACT
In earlier work, intraperitoneal (i.p.) immunization with Mycobacterium vaccae was shown to generate a T-suppressor (Ts) response but intradermal (i.d.) immunization did not. We have now studied the major histocompatibility complex (MHC) restriction of this Ts response. The ability of C57BL/6 (H-2b), BALB/c (H-2d), and the (C57BL/6 x BALB/c) F1 mice to generate suppression after i.p. immunization with 10skilled M. vaccae was investigated. The BALB/c and the F1 mice generated suppression, but the C57BL/6 mice failed to do so. The suppression could be ascribed to Lyt-2 +, L3T4- antigen-specific T cells. The F1 suppressors generated after i.p. immunization could suppress the generation of T-cell responses to i.d. immunization with M. vaccae in the parental BALB/c but not in the C57BL/6 mice. Monoclonal anti-I-A antibody could suppress the antigen-induced proliferative response of mice primed i.d. with M. vaccae. In contrast, monoclonal anti-I-E antibody enhanced antigen-specific proliferation of spleen cells primed i.p. with M.vaccae. The suppressors generated by i.p. priming of mice with M. vaccae could also suppress the in vitro antigen-induced proliferative response of i.d.-primed spleen cells; the suppression could be blocked by anti-I-E antibody. Thus, the T-cell-mcdiated suppression in the above experimental model was I-E restricted. The inability of the C57BL/6 mice to generate suppression after i.p. immunization with M. vaccae was ascribed to the lack of I-E expression by mice of H-2b strain.RÉSUMÉ
Au cours de travaux antérieurs, on a montré que l'immunisation intrapéritonéalc par Mycobacterium vaccae engendrait une réponse des T suppresseurs (Ts), mais l'immunisation intradermique ne le faisait pas. Nous avons à présent étudié la restriction du complexe majeur d'histocompatibilité (CMH) à cette réponse Ts. La capacité des souris C57BL/6 (H-2b), BALB/c (H-2d), et (C57BL/6 x BALB/d) F1 de produire une suppression après immunisation intra-péritonéalc avec 10 M. vaccae tués a été examinée. Les souris BALB/c et F1 ont montré une suppression mais les souris C57BL/6 ne l'ont pas fait. La suppression pouvait être attribuée aux cellules T spécifiques pour les antigènes Lyt-2+ et L3T4-. Les suppresseurs F1 produits après immunisation intra-péritonéale pouvaient supprimer les réponses par les cellules T à l'immunisation intra-dermique avec M. vaccae chez la souche BALB/c mais pour les souris C57BL/6. Les anticorps monoclonaux anti-IA pouvaient supprimer la réponse proliferative induite par l'antigène des souris infectées en intra-dermique avec M. vaccae. Par contre, les anticorps monoclonaux anti-IE augmentaient la prolifération, spécifique pour l'antigène, des cellules spléniques après injection intra-péritonéale de M. vaccae. Les suppresseurs produits par l'injection intra-péritonéalc des M, vaccae à des souris pouvaient aussi supprimer in vitro la réponse proliferative de cellules spléniques à une injection intra-dermique de l'antigène; la suppression pouvait être bloquée par les anticorps anti-IE. Donc, la suppression due à la médiation des cellules T dans le modèle expérimental ci-dessus était restreinte par les I-E. L'incapacité des souris C57BL/6 de produire une suppression après immunisation intra-péritonéalc avec M. vaccae était attribuée au manque d'expression des I-E par les souris de la souche H-2b.RESUMEN
En un trabajo previo, se demostró que mientras que la inmunización intraperitoneal (i.p.) con Mycobacterium vaccae condujo a la generación de células T-supresoras (Ts), la inoculación intradérmica (i.d.) no lo hizo. Ahora hemos estudiado la restricción genética (complejo mayor de histocompatibilidad) de esta respuesta. Se investigó la capacidad de los ratones C5713L/6 (H-2b), BALB/c (H-2J) y de los F1 (C57BL/6 x BALB/ c), para generar supresión después de la inmunización M. vaccae muertos. Los ratones BALB/c y los F| generaron supresión pero los C57B1/6 no lo hicieron. La supresión pudo adscribirse a células T Lyt2 +, L3T4-, específicas para el antígeno. Las células supresoras F1 generadas después de la inmunización i.p., suprimieron las respuestas de las células T a la inmunización i.d. con M. vaccae en los ratones BALB/c pero no en los C57BL/6 paternos. El anticuerpo monoclonal anti-IA pudo suprimir la respuesta proliferativa de los esplenocitos de ratones preestimulados i.d. con M. vaccae. En contraste, el anticuerpo monoclonal anti-I-E aumentó la proliferación inducida por antígeno de las células de bazo estimuladas i.p. conM. vaccae. Las células supresoras generadas por la preinoculación i.p. de los ratones con M. vaccae, también suprimieron in vitro la respuesta proliferativa inducida por antígeno de las células de bazo generadas por inmunización i.d; la supresión pudo bloquearse por anticuerpo anti-I-E. Asi, la supresión mediada por células T en este modelo experimental fue restringida por I-E. La incapacidad de los ratones C57BL/6 para generar supresión después de la inmunización i.p. con M. vaccae, se adscribió a la falta de expresión de I-E por las células de los ratones de la cepa H-2bThe pathogenesis of T-cell nonresponsiveness in mycobacterial diseases such as leprosy is not clear. Two distinctly different mechanisms, namely, a genetic defect in antigen presentation (9) and selective generation of suppression as a function of the route of immunization (11), have been proposed to explain the nonresponder state. The latter investigators (11) employed the intraperitoneal (i.p.) route of immunization of mice to show that suppressors were generated. Employing the same animal model, we reported that the killed vaccines of rapidgrowing mycobacteria differed from those of slow growers in their immunogenicity to mice by the i. p. route, the former being nonimmunogenic (13). Failure of rapid growers to mount an immune response by the i. p. route could be due to two distinct mechanisms acting in isolation or combination, i. e., a) antigen presentation defect in the macrophage/antigen-presenting cell linked to major histocompatibility complex (MHC) class II or la antigens, and b) generation of suppressors.
In our earlier publications (12,14), we presented evidence in support of both mechanisms in the pathogenesis of nonresponsiveness of mice to the rapid-growing mycobacteria by the i. p. immunization route. Antigen presentation by the peritoneal macrophages was shown to be poor (12). Animals also generated Lyt-2+ T suppressors on i. p. immunization (14). In light of the poor antigen presentation by peritoneal macrophages, it was surprising that immunization with the same organisms by the same route also triggered a T-suppressor (Ts) response which itself has to depend upon efficient antigen presentation. The paradox can be resolved if one postulates the triggering requirements, such as MHC restriction and cofactors of T-cell stimulation, to be different for Ts and for T cells responsible for help (Th) and delayed-type hypersensitivity (DTH).
In this publication we present evidence to show that the Ts response of the i. p. immunized mice is 1-E restricted; whereas the Th response is I-A restricted. These data appear to support a role for both the socalled immune response (Ir) and immune suppressor (Is) genes in the pathogenesis of the nonresponder state, as has been proposed by Ottenhoff and de Vries 9).
MATERIALS AND METHODS
Organism. Mycobacterium vaccae was kindly supplied by Dr. J. L. Stanford of Middlesex Hospital, London.
Animals. Four-to-six-week-old, BALB/c (H-2d), C57BL/6 (H-2b), C3H (H-2k), and CBA (H-2k) mice, obtained from the National Institutes of Health, Bethesda, Maryland, U. S. A. and maintained at the Cancer Research Institute, Bombay, India, were used in the study. The (BALB/c x C57BL/ 6) F1 mice were bred in our animal house.
Preparation of sonic extract. A 1 -week old culture of rapid-growing M. vaccae was killed by 2. 4 Mrad gamma radiation in a 60Co source as described earlier (13). The killed cells were washed with phosphate buffered saline (PBS), disrupted in a sonicator (Branson Sonifier, B-30), and spun at 50,000 x g x 1 hr. The supernatant, i. e., the sonic extract, was employed as test antigen. The protein content of the test antigen was estimated as described earlier (13).
Immunization. A 1-week-old culture of M. vaccae was killed by irradiation, and the bacterial cells were harvested and washed in PBS (pH 7. 2). The washed bacterial cells were pelleted by centrifugation at 2200 X g x 1 hr. The supernatant was discarded, and the washed cell pellet was suspended in PBS to give 1% (v/v  109 bacilli/ml) suspension. The required number of bacilli were suspended in 0. 5 ml for i. p. immunization and 0. 05 ml for intradermal (i.d.) immunization on the back at two or three sites.
109 bacilli/ml) suspension. The required number of bacilli were suspended in 0. 5 ml for i. p. immunization and 0. 05 ml for intradermal (i.d.) immunization on the back at two or three sites.
DTH test. The delayed-typc hypersensitivity test (DTH) was done as described earlier (13). Sonicate containing 50 μg protein in 0. 03 ml volume was injected into the hind foot pad and measurements of foot pad thickness were taken before, 24 hr, and 48 hr after injection. The sonicate was also tested in nonimmune mice as controls. The results were expressed as corrected foot pad enlargement (CPE), which is the difference between the foot pad enlargements caused by the test sonicate in the immunized and control mice simultaneously tested. A statistical Student's t test was done on CPE values by comparing with zero.
Lymphoproliferation test. A lymphoproliferation test was carried out using stand ard microculture techniques (8). Briefly, each well of a microtitcr plate (Nunc, Denmark) received 2 x 105 primed spleen cells in 0. 2 ml culture medium (RPMI 1640) containing 2. 0 mM glutaminc, 10% fetal calf serum, 1% antibiotic antimycotic (all from GIBCO, Grand Island, New York, U. S. A. ), and 10 μg/ml of the M. vaccae sonicate. Cultures were pulsed 120 hr later with 0. 5 μCi of 3H-labeled thymidine for a period of 16 hr, harvested, and the incorporation of the label assayed and expressed as counts per minute (cpm). All tests were run in quadruplicate. When required, comparison of the cpm values was done by Student's t test.
Depletion of T cells, DTH effectors, and Ts. Depletion of T cells, DTH effectors, and Ts from the lymphoid cell suspensions was done by treatment with monoclonal anti-Thy-1. 2, anti-L3T4, and anti-Lyt-2. 2 antibodies and complement as described earlier (6). The monoclonal antibodies were kindly provided by Dr. C. S. Henney, Immunex Corporation, Carson City, Nevada, U. S. A.
Antibody to I-Abdq, Edk. Supernatants of the cultures of the rat IgG2b clone of the above anti-mouse anti-MHC class II specificity (2) were pooled. Antibody was precipitated with 33% (NH4), SO4, dissolved in PBS, and dialyzed extensively. The protein concentration was adjusted as desired by concentration or dilution.
RESULTS
DTH responses of C57BL/6, BALB/c, and F1 mice. The C57BL/6 (H-2b), the BALB/c (H-2d), and the (C57BL/6 x BALB/c) F1 mice were immunized with 108 killed M. vaccae by the i. p. and i. d. routes, and their DTH responses to the sonicate were tested 1 week later. The i. d. route of immunization generated a DTH response in all three strains. However, after i. p. immunization it was observed that only the C57BL/6 mice were successful in generating DTH responsiveness; the BALB/c and the F1 mice behaved as nonresponders (Table 1).

Immune suppression in C57BL/6, BALB/c, and F1 mice. In order to investigate if i. p. immunization of the above three strains of mice with rapid-growing mycobactcrium generates suppression, the following two protocols described in detail in our earlier publication (14) were employed.
The i. p. nonresponder BALB/c and the F1 mice were given 108 killed M. vaccae by the i. p. route as before. One week later they were given a second immunization of 108 M. vaccae by the i. d. route. Intraperitoneal immunization 1 week prior to i. d. immunization completely abrogated the ability of the two strains of mice to respond to i. d. immunization (Table 1).
In order to investigate the characteristics of cells responsible for suppression in BALB/c and F1 mice, the mice were immunized by the i. p. route as before. One week later their spleen cells were harvested and 4. 0 x 107 cells were transferred to syngeneic recipients. The latter were then challenged by i. d. immunization. Mice receiving i. p. -primed spleen cells failed to mount an immune response to the i. d. immunization with M. vaccae, indicating generation of suppressor cells by i. p. priming. The ability of i. p. -primed spleen cells to adoptively transfer suppression could be completely abrogated by removal of cells having Thy-1 and Lyt-2 markers by anti-Thy-1. 2 and anti-Lyt-2. 2 antibody and complement, indicating that the suppressors were in fact Ts cells. In Table 2, the data on F1 mice are presented. The BALB/c mice also showedsimilar behavior (data not shown here; re-ported earlier14).
The experimental protocol of prior i. p. immunization suppressing the subsequenti. d. immunization could not be employedfor testing the ability of C57BL/6 mice togenerate suppression because they were re-sponders to M. vaccae by the i. p. route. Hence, a different protocol (described earlier 14) was employed. C57BL/6 mice wereimmunized i. p. with 108 Al. vaccae. One week later their spleen cells were harvested, and the DTH effectors were eliminated withanti-L3T4 antiserum and complement treatment. Spleen cells (4 x 107) depleted of cells with the L3T4 marker were transferred to syngeneic recipients which were then immunized i. d. with 108 M. vaccae, and their DTH responses were tested 1 weeklater. The DTH response of the recipients was of the same order as that of the control mice simultaneously immunized by the i. d. route and tested; no suppression was observed (Table 3).
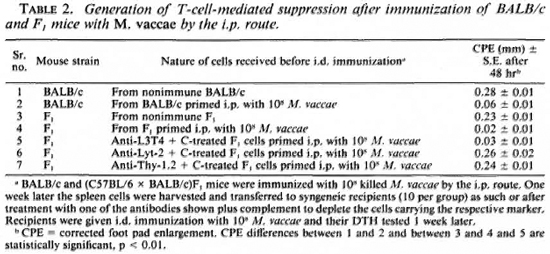
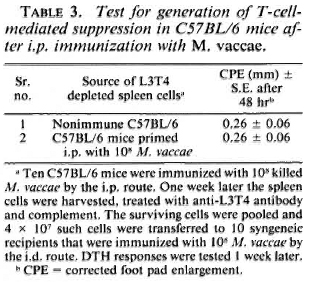
From these experimental data, it could be concluded that i. p. immunization with M. vaccae generated T-cell-mediated suppression in BALB/c and F1 mice but not in C57BL/6 mice.
MHC restriction of suppression. The F1 mice were immunized i. p. with 10s M. vaccae. One week later their spleen cells'were harvested and transferred to syngeneic F1, parental BALB/c mice and the C57BL/6 mice (4 x 107 cells/mouse). The recipients were then immunized i. d. with 108 M. vaccae, and their DTH responses were tested 1 week later. The C57BL/6 mice showed a DTH response comparable to that of the control mice simultaneously immunized. In contrast, the BALB/c and the F1 mice failed to generate a DT H response (Table 4).
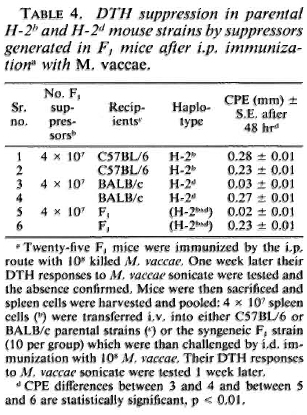
The MHC restriction of suppression by the F1 cells was also investigated in an in vitro lymphoproliferative response. C57BL/6 and BALB/c mice were immunized i. d., and the F1 mice were immunized i. p. with killed 108 M. vaccae: 2 x 105 i. d. primed parental spleen cells were cocultured with 5 x 104 splenic-T cells of i. p. primed F1 mice, and a 6-day lymphoproliferative assay against M. vaccae sonicate was performed. The i. p. -primed F1 splenic-T cells could suppress the proliferative response of the BALB/c (50% reduction in cpm); whereas the response of the C57BL/6 mice was unaffected (Fig. 1).
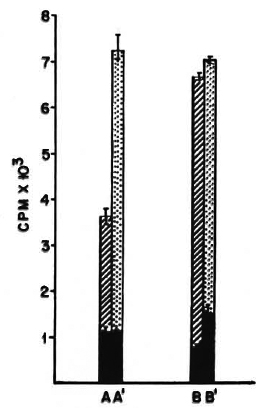
Fig. 1. In vitro suppression of parental lympho-proliferative response by suppressors generated in F1 mice by i. p. immunization with M. vaccae . BALB/c and C57BL/6 mice were immunized i. d. and(C57BL/6 x BALB/c) F1 mice were immunized i. p. with 108 killed cells of M. vaccae: 2 x 105 spleen cellsof i. d. -primed parental strains were co-cultured with either 5 x 104 splenic-T cells of i. p. -primed F1 miceor 5 x 104 splenic-T cells from nonimmune mice, anda standard 6-day LTT performed with 10 μg/ml M. vaccae sonicate.  = A and B show proliferative re-sponses of i. d. -primed spleen cells from BALB/c or C57BL/6 mice, respectively, in the presence of 5 x 104 splenic-T cells from i. p. -primed F1 mice;
= A and B show proliferative re-sponses of i. d. -primed spleen cells from BALB/c or C57BL/6 mice, respectively, in the presence of 5 x 104 splenic-T cells from i. p. -primed F1 mice;  = A' and B' show proliferative responses of i. d. -primed spleencells from BALB/c or C57BL/6 mice, respectively, inthe presence of 5 x 104 splenic-T cells from nonprimedF1 mice;
= A' and B' show proliferative responses of i. d. -primed spleencells from BALB/c or C57BL/6 mice, respectively, inthe presence of 5 x 104 splenic-T cells from nonprimedF1 mice;  = proliferation in the absence of antigen. Difference between A and A' is statistically significant, p < 0. 01.
= proliferation in the absence of antigen. Difference between A and A' is statistically significant, p < 0. 01.
From these data it could be concluded that the suppressor-T cells of the F1 mice could induce suppression in the H-2d but not in the H-2b strain. Since the latter do not express I-E coded MHC class II antigens, the above data support a conclusion that the suppression was I-E restricted.
Effect of anti-I-Abdq, Edk antibody. The BALB/c (H-2d) mice were immunized i. d. and the C3H and CBA (H-2d) mice were immunized i. p. with 108 killed M. vaccae. The DTH responses of the C3H and CBA mice to M. vaccae were very similar to those of the BALB/c mice, i. e., the organism was immunogenic by the i. d. route and nonimmunogenic by the i. p. route. Further i. p. immunization generated T-cell-mediated suppression (data not shown). One week after immunization, the spleen cells were harvested and a lymphoproliferative assay against M. vaccae sonicate was performed in the presence or absence of monoclonal antibody of the specificity anti-I-Abdq, Edk at a final concentration of 100 μg/ml. Control cultures received normal mouse globulin at the same concentration. The antibody depressed the proliferative responseof i. d. -primed spleen cells of BALB/c mice (40% reduction in cpm); whereas it enhanced the response of i. p. -primed spleencells of the C3H and CBA mice (2. 5- to 3-fold increase in cpm; Fig. 2).
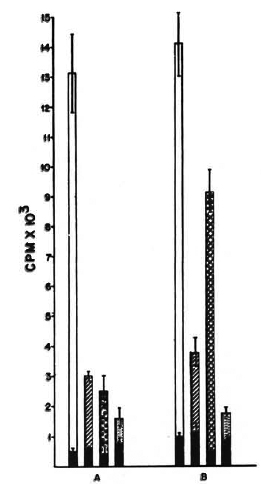
Fig. 2. Differential effect of anti-la (IAbdq, IEdk) antibody on in vitro proliferation of i. p. and i. d. M. vaccae-primed spleen cells of H-2d and H-2k haplotypes. Proliferation of 2 x 105 spleen cells of BALB/c (A) and C3H (B) mice primed i. p. or i. d. with M. vaccae, in the presence or absence of anti-la (IAbdq, IEdk) anti-body.  = Proliferation in absence
= Proliferation in absence  of antigen;
of antigen;  =proliferation of i. d. -primed spleen cells with antigen,without antibody;
=proliferation of i. d. -primed spleen cells with antigen,without antibody;  = proliferation of i. p. -primedspleen cells with antigen, without antibody;
= proliferation of i. p. -primedspleen cells with antigen, without antibody;  = proliferation of i. d. -primed spleen cells with antigen andanti-la antibody;
= proliferation of i. d. -primed spleen cells with antigen andanti-la antibody;  = nonprimed spleen cells and antigen. Differences in proliferative responses of i. p. - and i. d. -primed spleen cells (
= nonprimed spleen cells and antigen. Differences in proliferative responses of i. p. - and i. d. -primed spleen cells ( and
and  ), of i. d. -primed spleen cells of BALB/c with andwithout antila antibody (
), of i. d. -primed spleen cells of BALB/c with andwithout antila antibody ( and
and  ), and of i. p. -primedspleen cells of C3H mice with and without anti-la an-tibody (
), and of i. p. -primedspleen cells of C3H mice with and without anti-la an-tibody ( and
and  ) are statistically significant, p < 0. 01.
) are statistically significant, p < 0. 01.
This antibody is reactive with both I-A and I-E glycoproteins of BALB/c (H-2d); whereas in C3H and CBA (H-2k), it is reactive with only I-E (2). Hence, it could be concluded that the antibody suppressed the response of i. d. -primed spleen cells if the antibody recognized the I-A specificities, and enhanced the proliferative response if it recognized the I-E specificities.
Blockade of in vitro T-cell-mediated suppression by anti-I-Abdq, Edk antibody. CBA and C3H mice were immunized i. p. and i. d. with 108 M. vaccae: 2 x 105 i. d. -primed spleen cells were cocultured with 5 x 104 i. p. -primed syngeneic splenic-T cells withor without anti-I-Abdq, Edk antibody at a final concentration of 100 μg/ml. The controlcultures received normal mouse immuno-globulin at the same concentration. A stan-dard 6-day lymphoproliferative test was performed against M. vaccae sonicate. The proliferative response of i. d. -primed cells was suppressed by splenic-T cells of i. p. -primed mice (60% reduction in cpm). When the above antibody was added to the culture, suppression was significantly blocked (Fig. 3).

Fig. 3. Blockade of Ts-induced in vitro suppression of lymphoproliferative response by anti-I-E antibody. C3H mice were primed i. d. and i. p. with M. vaccae.  = Proliferation of 2 x 105 i. d. -primed spleen cells inpresence of 5 x 104 syngeneic normal spleen cells andantigen;
= Proliferation of 2 x 105 i. d. -primed spleen cells inpresence of 5 x 104 syngeneic normal spleen cells andantigen;  = proliferation of 2 x 105 i. d. -primed spleencells in the presence of 5 x 104 syngeneic i. p. -primed spleen cells and antigen;
= proliferation of 2 x 105 i. d. -primed spleencells in the presence of 5 x 104 syngeneic i. p. -primed spleen cells and antigen;  = proliferation of 2 x 105 i. d. -primed spleen cells in the presence of 5 x 104 syngeneic i. p. -primed spleen cells, antigen, and anti-la anti serum;
= proliferation of 2 x 105 i. d. -primed spleen cells in the presence of 5 x 104 syngeneic i. p. -primed spleen cells, antigen, and anti-la anti serum;  = proliferation of different cells with out antigen. Differences between A and B and between Band C are statistically significant, p < 0. 01.
= proliferation of different cells with out antigen. Differences between A and B and between Band C are statistically significant, p < 0. 01.
DISCUSSION
In our previous publication (13) it was reported that the killed vaccines of rapidgrowing mycobacteria differed from those of slow growers in their immunogenicity to mice by the i. p. route, the rapid growers being nonimmunogenic. Two main factors which influence immunogenicity, namely, antigen presentation and generation of T-cell-mediated suppression, were investigated (12,14).
So far as antigen presentation is concerned, the peritoneal macrophages behaved discriminatingly for slow- and rapid-growing mycobacteria. Whereas the slow-grower antigens were presented efficiently, rapid-grower antigens were not (12). In contrast, both groups of mycobacteria were observed to generate T-cell-mediated suppression (14). The resultant outcome of these two factors affected i. p. immunogenicity of the two groups of mycobacteria differently, the slow growers being immunogenic and the rapid growers, nonimmunogenic (13).
This prompted us to investigate further the relative importance of antigen presentation vis-a-vis suppression in affecting the outcome of immunization in the experimental model. Since presentation of rapidgrower antigen by the peritoneal macrophages was poor (12), it was surprising that the i. p. immunization with them generated T-suppression (14), because T-suppression is also dependent upon antigen presentation and MHC restriction. Hence, the MHC restriction requirement of T suppression was investigated.
Unlike the BALB/c and several other inbred mice strains and the outbred Swiss white mice, C57BL/6 mice could be immunized to rapid growers by the i. p. route. The efficiency of presentation of M. vaccae sonicate by the peritoneal cells of the C57BL/6 mice was not significantly different from that of the BALB/c mice (data not shown). Hence, it was logical to conclude that the difference in behavior of the C57BL/6 and the BALB/c mice to i. p. immunization with a rapid-growing mycobacteria such as M. vaccae was not due to a difference in the efficiency of antigen presentation by the peritoneal cells of the two respective mouse strains. The difference was apparently due to nongeneration of T-cellmediatcd suppression by the C57BL/6 mice. Further, based on the following evidence, it could be concluded that the T suppression generated by i. p. immunization was I-E restricted; whereas the T-helper response was I-A restricted.
a) The C57BL/6 mice which failed to generate suppression are I-E deficient (7). b) The (C57BL/6 x BALB/c) F1 mice behaved like BALB/c, i. e., they generated T suppression, c) The F1 T suppressors could suppress the immune response in parental BALB/c (H-2d) but not in the C57BL/6 (H-2b) mice. Since the F1 mice are expected to express the I-E of the BALB/c (H-2d) haplotype only, it could be concluded that the F1 suppressors were restricted by the I-E of H-2d specificity, d) The anti-I-A antibody could block the lymphoproliferative response of i. d. primed spleen cells; whereas the anti-I-E antibody enhanced the proliferative response of the i. p. -primed spleen cells, e) The suppression of in vitro proliferative responses of i. d. -primed spleen cells by the i. p. -primed suppressors could be blocked by the anti-I-E antibody. Similar observations regarding I-E restriction of T-suppression have been reported for other antigens, such as LDH-β (1), and parasitic antigens of Trichinella spiralis and Nippostrongylus dubius (16).
These data support a major role for antigen presentation and MHC restriction in the immunogenicity of mycobacteria. Antigen presentation for both Th/DTH and Ts responses seems to play a role in the outcome of immunization. Since in this model two responses are regulated by different MHC class II locus glycoproteins, the outcome of immunization by a given route would be dependent upon their interactions. When the Th presentation was efficient, as with slow growers (12), despite suppression, i. p. immunization succeeded in generating DTH responsiveness (14). On the other hand, when the Th pesentation was poor, as with rapid growers (12), the outcome of immunization depended upon suppression. When suppression was absent, as with C57BL/6 mice (due to lack of I-E expression), i. p. immunization with M. vaccae generated DTH responsiveness, and when suppression was present, as with BALB/c mice, the i. p. immunization failed.
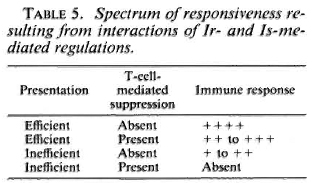
Several studies linking HLA and nonresponder states in leprosy have also suggested two distinctly different pathogenic mechanisms. In family and population studies (4,5,9,10,15) a significant deficit in HLA-class D phenotype, such as DR-3, was observed among BL-LL leprosy patients. In contrast, both family and population studies have shown preferential inheritance of DQwl by BL-LL cases (3,9). The pathology in the former situation can be visualized to be due to an antigen-presentation defect. In the latter situation, the most likely pathology would be selective generation of T-cell-mediated suppression induced by the particular HLA-class II glycoproteins. Thus, the data seem to suggest a role for both the socalled immune response (Ir) and immune suppressor (Is) genes. The spectrum of responsiveness seen in leprosy may also reflect the interactions of Ir and Is gene-mediated regulation as shown in Table 5.
Acknowledgments. The authors gratefully acknowledge the financial assistance to this study from the Department of Science and Technology, Government of India. The authors are also grateful to Dr. C. S. Henney of Immuncx Corporation, U. S. A., for the gift of monoclonal antibodies against T subsets.
REFERENCES
1. BAXEVANIS, C. N., ISHII, N., NAGY, Z. A. and KLEIN, J. Role of Ek molecule in the generation of suppressor T cells in response to LDHβ. Scand. J. Immun. 16(1982)25-31.
2. BHATTACHARYA, A. ,DORF, M. E. and SPRINGER, T. A. A shared alloantigcnic determinant on la antigens encoded by I-A and I-E subregions: evidence for I region gene duplication. J. Immunol. 127(1981)2488-2495.
3. DE VRIES, R. R. P., SERJEANTSON, S. W. and LARISSE, Z. Leprosy. In: Histocompatibility Testing, 1984. Albert, E. D., Baur,M. P. and MAYR, W. R. ,EDS. HEIDELBERG: SPRINGER VERLAG, 1984, PP. 362-367.
4. DE VRIES, R. R. P., VAN EDEN, W. and OTTENHOFF, T. H. M. HLA Class II immune response genes and products in leprosy. Prog. Allergy 36(1985)95-113.
5. DE VRIES, R. R. P. ,VAN EDEN, W. and VAN ROOD, J. J. HLA linked control of course of M. leprae infections. Lepr. Rev. 52Suppl. (1981)109-119.
6. GEORGE, A. ,RATH, S. and KAMAT, R. S. Differential regulation of effector responses of cell mediated immunity in experimental salmonellosis. Clin. Exp. Immun. 63(1986)327-333.
7. MURPHY, D. B., JONES, P. P. ,LOKEN, M. R. and MCDEVITT, H. O. Intraction between I region loci influences the expression of a cell surface la antigen. Proc. Natl. Acad. Sci. U. S. A. 77(1980)5404-5408.
8. OPPENHEIM, J. J. and SCHECHTER, B. Lymphocyte transformation. In: Manual of Clinical Immunology. Rose, N. R. and FRIEDMAN, FL,EDS. WASHINGTON, D. C. : AMERICAN SOCIETY FOR MICROBIOLOGY, 1976, p. 81.
9. OTTENHOFF, T. H. M. and DE VRIES, R. R. P. HLA class II immune response and suppression genes in leprosy. Int. J. Lepr. 55(1987)521-534.
10. SERJEANTSON, S. W. HLA and susceptibility to leprosy. Immun. Rev. 70(1983)24-27.
11. SHEPARD, C. C, WALKER, L. L., VAN LANDINGHAM, R. M. and YE, S. Z. Sensitization or tolerance to Mycobacterium leprae antigen by route of injection. Infect. Immun. 38(1982)673-680.
12. SHROFF, K. E., SAINIS, K. B., SENGUPTA, S. R. and KAMAT, R. S. Variation in immunogenccity of mycobacteria: role of antigen presenting cells. Int. J. Lepr. 58(1990)58-64.
13. SHROFF K. E., SENGUPTA, S. R. and KAMAT, R. S. Route related variation in immunogenccity of mycobacteria. Int. J. Lepr. 58(1990)44-49.
14. SHROFF K. E., SENGUPTA, S. R. and KAMAT, R. S. Pathogenesis of route related variation in T-suppressor response on immunization with mycobacteria. Int. J. Lepr. 58(1990)50-57.
15. VAN EDEN, W. and DE VRIES, R. R. P. HLA and leprosy: a re-evaluation. Lepr. Rev. 55(1984)89-104.
16. WASSOM, D. L., KRCO, C. J. and DAVID, C. S. I -E expression and susceptibility to parasite infection. Immunol. Today 8(1987)39-43.
1. Ph. D., Research Fellow; Department of Immunology, Haffkinc Institute, Parel, Bombay 400012, India.
2. M. D., Director; Department of Immunology, Haffkinc Institute, Parel, Bombay 400012, India.
3. M. D., Assistant Director and Head, Department of Immunology, Haffkinc Institute, Parel, Bombay 400012, India.
4. Ph. D., Scientific Officer (SOSS), Molecular Biology and Agriculture Division, Biology Group, Bhabha Atomic Research Center, Trombay, Bombay, India.
Reprint request to Dr. Kamat.
Received for publication on 11 May 1990.
Accepted for publication in revised form on 15 November 1990.