- Volume 59 , Number 1
- Page: 58–67
Proliferative response to seven affinity purified mycobacterial antigens in eight strains of inbred mice
ABSTRACT
This study compares the T-cell-stimulating ability of different mycobacterial antigens. The responses to crude culture filtrates and seven affinity-purified antigens were investigated in eight strains of inbred mice. Large differences in the stimulating abilities of the antigens were observed, and four antigens were found to give a powerful T-cell stimulation. Some antigens divided the strains into high and low responders, while a 17-kDa antigen was found to be exceedingly T-cell stimulatory in mice of all tested haplotypes. The responses of the eight strains were analyzed by comparing the response patterns of the strains. Using a statistical model based on antigen ranking, five strains were found to have a similar response pattern; three strains were found to differ. These results demonstrate the significance of the choice of mouse strain in studies of mycobacterial immunology and, furthermore, indicate that when research is conducted to develop new mycobacterial vaccines, it is important to include panels of antigens.RÉSUMÉ
Cette étude compare la capacité de différents antigènes mycobacterials de stimuler les cellules T. La réponse à des filtrats de culture non purifiés ont été étudiées chez, huit souches de souris d'élevage. De grand es différences ont été observées dans la capacité stimulante des antigènes, et quatre antigènes ont montré pouvoir provoquer une stimulation puissante des cellules T. Certains antigènes provoquaient une réponse forte ou faible selon les souches de souris, mais un antigène de 17-kDa se montra extrêmement stimulant pour les cellules T chez les souris de tous les haplotypes testés. Les réponses des huit souches ont été analysées en comparant les types de réponse des souches. En utilisant un modèle statistique basé sur le classement antigénique, cinq souches ont montré un type de réponse semblable; trois souches ont montré une réponse différente. Ces résultats démontrent l'importance du choix de la souche de souris dans les études d'immunologie des mycobactéries, et de plus, indiquent que quand une recherche est menée pour développer de nouveaux vaccins vis-à-vis des mycobactéries, il est important d'y inclure de nombreux antigènes.RESUMEN
En este estudióse compara la capacidad de diferentes antigenos micobacterianos para estimular a las células T. Se investigaron las respuestas a filtrados de cultivos micobacterianos crudos y a 7 antigenos purificados por afinidad en 8 cepas de ratones singénicos. Se encontraron grand es diferencias en la capacidad estimulante de los antígenos pero 4 de ellos resultaron fuertemente estimulantes. Algunos antígenos permitieron dividir a las cepas en altas y bajas respondedoras: un antígeno de 17 kDa resultó ser excesivamente estimulante para las células T de los ratones de todos los haplotipos probados. Las respuestas de las 8 cepas se analizaron comparand o sus patrones de respuesta. Usand o un modelo estadístico adecuado, se encontró que 5 cepas tuvieron patrones similares de respuesta; 3 cepas difirieron de las anteriores. Estos resultados demuestran la importancia de escoger las cepas de ratones en los estudios sobre la inmunología micobacteriana y, además, indican que cuand o se hace investigación conducente al desarrollo de nuevas vacunas micobacterianas, es importante incluir varios antigenos.T lymphocytes play a major role in the immunology of mycobacterial infections, and research is being conducted worldwide with the purpose of identifying mycobacterial antigens able to provide protection through stimulation of specific T lymphocytes.
Crude filtrate from Mycobacterium tuberculosis (3) or purified single antigens believed to be immunodominant provide the antigenic preparations which have preferentially been investigated (11,14,20). The differences in the experimental design chosen in many of these studies make comparison of the results obtained very difficult.
Most investigators use mice as experimental animals, and C57BL/6J is the mouse strain almost exclusively chosen to investigate T-cell responses (2,8,15). Strains of inbred mice vary in their susceptibility to mycobacterial infection (16,17) and can be divided into high and low responders to M. leprae (4). It might be expected that the T-cell-proliferative responses against individual mycobacterial antigens vary among strains.
In this study, we compare the T-cell-stimulating capacity of seven affinity-purified mycobacterial antigens with molecular masses ranging from 17 kDa to 82 kDa. This screening of antigens for T-cell-stimulating capacity was done in eight strains of inbred mice to identify the possible strain differences in response to single antigens.
MATERIALS AND METHODS
Animals. Eight strains of inbred mice representing seven H-2q haplotypes were chosen: C57BL/6J (H-2b), DBA/1J (H-2q), BALB/cJ (H-2d), CBA/J (H-2k), A. CA/Sn (H-21), BALB. B10 (H-2b), C3H. OH (H-202), BlOA/SqSn (H-2a). With the exception of DBA/1J (obtained from Jackson Laboratories, Maine, U. S. A. ), all strains were bred at Statens Seruminstitut (SSI), Copenhagen, Denmark. Female mice, 8-12 weeks old, were used in all experiments.
Immunization. The mice received subcutaneous injections of modified Freund's complete adjuvant (mFCA) containing killed M. tuberculosis H37Rv mixed 1:2 with culture filtrate (CF) of the same strain. The subcutaneous injections were given in both sides at the base of the tail and in all four foot pads. The total quantity of mFCA/CF given to each mouse was 0. 2 ml containing 45 μg glutaraldehyde-killed H37Rv and 300 μg H37Rv CF protein.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and iminunoblotting. SDS-PAGE was carried out in a discontinous system using a 10%-20% acrylamide separation gel (10). The antigens were transferred onto nitrocellulose paper and incubated with polyclonal rabbit anti- M. tuberculosis immunoglobulin. The strips were screened for antibody binding by peroxidase-conjugated swine anti-rabbit immunoglobulin (P217; Dakopatts, Glostrup, Denmark) and subsequently stained with 5, 5', 3, 3' tetramethylbenzidine.
The rabbit anti-M. tuberculosis immunoglobulin was produced by immunization of rabbits 3-6 times with 1 mg M. tuberculosis culture filtrate in Freund's incomplete adjuvant. The immunoglobulins were purified from the serum by ammonium sulfate precipitation (pH 5. 0), followed by ion-exchange chromatography on a DEAE sephadex A50 column.
Bacterial antigens. CFs of M. tuberculosis H37Rv and M. bovis BCG grown in liquid Sauton medium were produced and concentrated by ammonium sulfate precipitation as described (1). Tuberculin PPD was obtained from the Tuberculin Department, SSI. Killed M. tuberculosis H37Rv used in mFCA were produced by incubating bacteria in 2% glutaraldehydc overnight.
Affinity-purified antigens were produced as described previously (17). Briefly, CF was passed through immunosorbent columns coupled with monoclonal antibodies. Eluates of bound material were dialyzed against phosphate buffered saline (PBS) and used as antigen in lymphocyte stimulation assays.
A large pool of each antigen preparation was aliquoted in small vials and kept frozen (-20ºC). Each vial contained the quantity of antigen to be used in one experiment. Control preparations for stimulation of lymphocytes were prepared by passing the eluting buffers through the columns which had not been loaded with CF prior to the elution. The eluting buffers were either 3 M KSCN (pH 7. 4) or 0. 1 M glycine hydrochloride (pH 2. 8). These mock eluates were dialyzed against PBS and processed like the antigen preparations.
Lymphocyte proliferation assays. The popliteal, inguinal and axillary lymphnodes of mice were removed 8 days after immunization and single cell suspensions (LNL) prepared in RPMI 1640 supplemented with 5 x 10-5 M 2-mercaptoethanol, 100 IU penicillin/ml, 50 μg streptomycin/ml, nonessential amino acids, 1 mM glutamine and 0. 25% v/v fresh mouse serum. Cell cultures were established in 96-well, round-bottom, microtiter plates (Nunc, Denmark). Each well contained 2 x 105 cells in a volume of 200 μl. Based on dose-response studies (data not shown), the concentrations of antigens in the final cultures were chosen to be 20 μg/ml of the culture filtrates and PPD. The concentration in final cultures chosen for the affinity-purified antigen preparations were 2 μg/ml with the exception of the HYT27 antigen, which was used at 1 μg/ml. Concentrations which stimulated less than maximally were chosen to avoid highdose inhibition. Some cells were left unstimulated (controls) or were stimulated with mock eluates from the affinity columns. The mock eluates were used at a 10 x dilution, which approximately corresponds to the dilutions used for the antigens. Phytohemagglutinin (PHA; Wellcome) was used at a concentration of 10 μg/ml as a positive control for cell reactivity. All tests were carried out in triplicate. Cultures were incubated for 4 days at 37ºC, the last 22 hr in the presence of 1 μCi 3H-thymidine (TRA 120; Radiochemical Centre, Amersham, U. K. ) in an atmosphere of 5% CO2 in humidified air. The cells were harvested on fiber glass paper, and the incorporated radioactivity was measured in a liquid scintillation counter.
Statistical methods. Calculations were performed on the geometric means of the counts obtained from triplicate cultures. For each strain a two-way analysis of variance was carried out taking systematic differences between antigens and between experiments into account. The analyses show that a ratio between geometric means found for two strains for a given antigen should exceed 3. 16 to be significant at the 95% level.
For the four selected antigens the comparisons between the response patterns for the different strains were based on rankings. The ranks were obtained by comparing the counts per minute (cpm) values for four antigens in each experiment, assigning the value 4 to the antigen obtaining the highest cpm.
The reproducibility of the rankings was ascertained using Friedman's test for the rankings obtained for each strain. Mean ranks found for the different strains were compared as follows: denoting the mean ranks for the two strains r1. 1-41. 4 and r2. 1 -r2. 4 a squared distance, d2 (coefficient of dissimilarity) was calculated as:
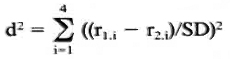
where SD equals the stand ard error of the rank difference. These d2 values approximately follow the chi-squared distribution and have three degrees of freedom. They are considered measures of the differences between response patterns found for each pair of strains.
RESULTS
Affinity-purified antigens. The purity of the antigens used in the proliferation assays was examined in SDS-PAGE followed by immunoblotting with a polyclonal rabbit antiserum against H37Rv CF. As seen in Figure 1, the affinity-purified antigens had these approximate molecular weights: HYT6 (17-19 kDa), HBT 11 (17 kDa), HBT2 (17 kDa), HBT4 (24 kDa), HYT27 (32-33 kDa), HBT 12 (38 kDa) and HBT1 (82 kDa). With the exception of HBT1, which contained a minor quantity of lower molecular weight material, the other antigens are seen to have a high degree of purity.
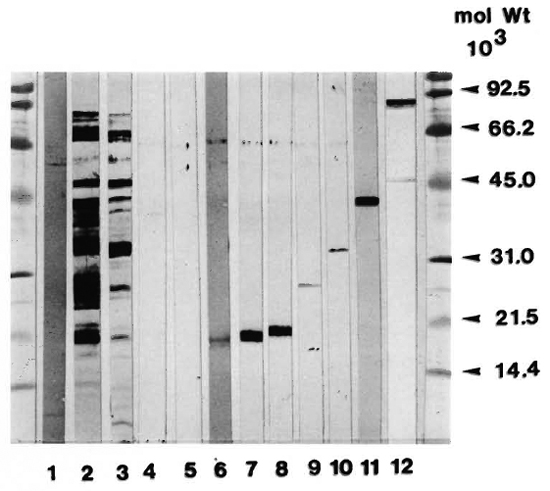
Fig. 1. Affinity-purified mycobacterial antigens were subjected to SDS-PAGE and electrotransferred onto nitrocellulose paper. After incubation with polyclonal rabbit anti- M. tuberculosis antibody, the reactivities were visualized by staining with a peroxidase-conjugated secondary antibody. Lanes: 1 = PPD; 2 = H37Rv CF; 3 = BCG CF; 4 = Gly. HCl (mock eluate); 5 = KSCN (mock eluate); 6 = HYT6 (17-19 kDa); 7 = HBT11 (17 kDa); 8 = HBT2 (17 kDa); 9 = HBT4 (24 kDa); 10 = HYT27 (32-33 kDa); 11 = HBT 12 (38 kDa); 12 = HBT1 (82 kDa).
Lymphocyte proliferation assay. Figure 2 shows the lymphocyte proliferative responses to the antigen panel of seven individual immunized and two individual unimmunized mice of the BALB. B10 strain. As can be seen in the figure, the individual mice in general have similar response patterns but the levels of the responses vary somewhat. The unimmunized mice are seen to have a profile with a surprising similarity, but at much lower level. It appears from Figure 2 that HYT6 in particular has a powerful T-cell-stimulating capacity in nonimmunized mice as well as in immunized mice. This antigen either possesses epitopes crossreactive with antigens existing in the normal microbiological environment of the animals, or has mitogenic properties.
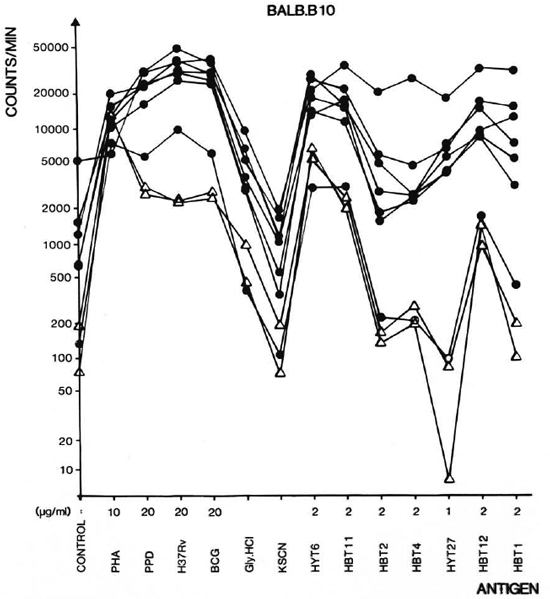
Fig. 2. Proliferative responses of single cell suspensions of lymph node lymphocytes (LNL) from 9 individual BALB. B10 mice to mycobacterial antigens. Mice were imunized with mFCA/H37Rv CF 8 days before isolation of LNL ( ). Two unimmunized animals served as negative controls (Δ). The culture filtrates are in the figure denoted H37Rv and BCG. The antigens were used in the concentrations indicated. The mock cluates Gly. HCl and KSCN from the affinity columns were diluted 10 times in the cultures.
). Two unimmunized animals served as negative controls (Δ). The culture filtrates are in the figure denoted H37Rv and BCG. The antigens were used in the concentrations indicated. The mock cluates Gly. HCl and KSCN from the affinity columns were diluted 10 times in the cultures.
Stimulating ability of the antigens. To compare the response patterns of eight strains after immunization, the geometrical mean of the cpm values was calculated for each antigen. The patterns obtained for each strain are shown in Figure 3. As seen in the figure, large differences in the T-cell-stimulating capacity of the antigens existed. were found to possess intermediate T-cell-H37Rv CF, BCG CF, and PPD, which constimulating abilities with responses between tain a large number of proteins and are used 2000 and 20,000 cpm. HBT2, HBT4, and in high concentrations, gave the highest reHYT27 gave no or very limited T-cell stimsponses. HBT1, HBT11, HBT12, and HYT6 ulation. The activities of these antigens were comparable to those of the mock eluates and therefore were not used for further calculation.
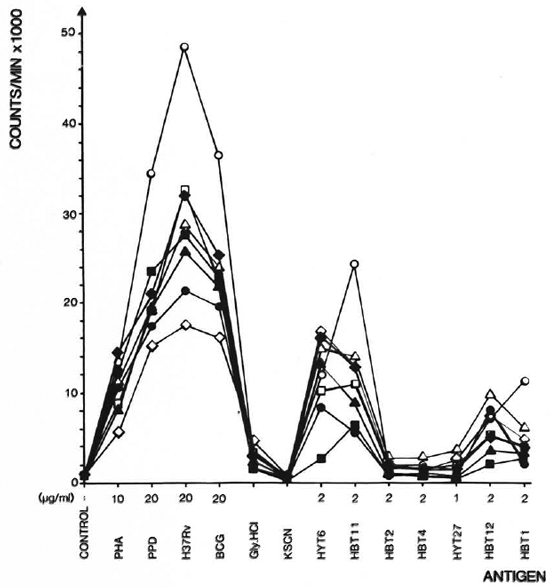
Fig. 3. Proliferative responses of 8 strains of mice to mycobacterial antigens. Responses arc geometrical means of 7-9 experiments. The culture filtrates are in the figure denoted H37Rv and BCG. The antigens were used in the concentrations indicated. The mock eluatcs Gly. HCl and KSCN from the affinity columns were diluted 10 times in the cultures.  = A. CA/Sn;
= A. CA/Sn;  = B. 10A/SqSn;
= B. 10A/SqSn;  = CBA/J;
= CBA/J;  = BALB/cj;
= BALB/cj;  = DBA/1J; Δ= BALB. B10;
= DBA/1J; Δ= BALB. B10;  = C3H. OH;
= C3H. OH;  = C57B1/6J.
= C57B1/6J.
Response patterns of eight inbred strains of mice. Having identified four antigens with stimulatory properties (HBT1, HBT11, HBT12, HYT6), we compared the responses of the eight strains to these antigens. This analysis was divided in two parts: a) the possible existence of high and low responders to the different antigens, and b) a comparison of the response patterns of the strains. Results of the latter analysis are independent of differences in response levels. As seen in Figure 3, most of the strains responded very similarly, but the levels of responses to HBT1 and HYT6 differed for some of the strains. The ratios between the geometric means for two strains were found to be significantly different from the geometric means of the remaining strains. Thus, the DBA/1J is identified as a high responder to HBT1, while CBA/J is a low responder to HYT6.
The analyses of the response patterns were done using a statistical model based on antigen ranking as described in Statistical Methods. Mean ranks obtained on the basis of data from 7-9 experiments are shown in Figure 4. The ranks of strains were com pared pairwise in all possible combinations, and the differences between the strains were expressed as coefficient of dissimilarity (Fig. 5). Values of 7. 8 and above indicate significantly dissimilar response patterns at the 95% level. The strains sharing values within the framed area were found to have similar response patterns, while DBA/1J, C57BL/ 6J and A. CA/Sn were found to differ from this pattern.
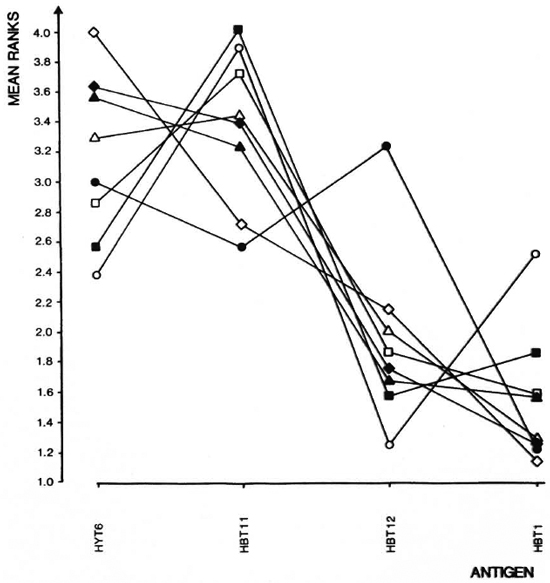
Fig. 4. Ranking of responses to indicated antigens as described in Statistical Methods were done on data from experiments summarized in Fig. 3. Mean ranks for each strain and each antigen are shown.  = A. CA/ Sn;
= A. CA/ Sn;  = B. 10A/SqSn;
= B. 10A/SqSn;  = CBA/J;
= CBA/J;  = BALB/cJ;
= BALB/cJ;  = DBA/1J; Δ = BALB. B10;
= DBA/1J; Δ = BALB. B10;  = C3H. OH;
= C3H. OH;  = C57BL/6J.
= C57BL/6J.
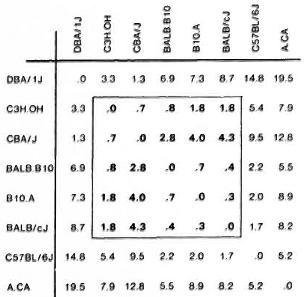
Fig. 5. Dissimilarities between response patterns for eight strains of mice. Strains are compared pairwisc in all combinations. Based on the mean ranks the difference between two strains is calculated and expressed as a coefficient of dissimilarity. Values of 7. 8 and above indicate significantly dissimilar response patterns at the 95% level. Strains sharing values within the framed area were found to have similar response patterns.
DISCUSSION
Current studies of purified mycobacterial antigens are predominately thorough examinations of single antigens at a time and, particularly, the 65-kDa antigen has received a lot of interest (8,13,14). The reason for choosing these particular antigens is not always obvious.
Apart from recent studies from our laboratory (5,18) no previous study, to our knowledge, describes the screening of a larger panel of potential T-cell-stimulating antigens. In our view, a comparison between antigens' T-cell immunogenicity seems important before single antigens arc chosen for detailed analyses.
In this study we have compared seven affinity-purified antigens and some heterogenous antigen preparations in a lymphocyte proliferation assay. It was found that two antigens in the 17-19 kDa region (HYT6, HBT11) possess exceedingly powerful T-cell-stimulating ability. The 17-kDa antigen HBT11 has recently been produced in our laboratory (19) and has, to our knowledge, not been investigated by others. Interestingly, this antigen was found to be a superior T-cell antigen in mice of all haplotypes.
Two other antigens of approximate molecular masses 38 kDa and 81 kDa stimulated more moderately (HBT12, HBT1); while antigens of the molecular mass 19 kDa, 24 kDAand 32 kDa (HBT4, HBT2, HYT27) were shown to have none or very limited T-cell immunogenic activity in the mouse. Large differences in the stimulatory properties of HBT11 and HBT2 were seen for all strains (Fig. 3). The corresponding monoclonal antibodies were previously found to identify the same molecule (19). However, the position of the two antigens in SDS-PAGE is not exactly the same (Fig. 1). The results might indicate that the two molecules share a potent B-cell epitope but that differences exist in the rest of the molecules, which determine the differences in the stimulating capacity of their T-cell epitopes.
To select potentially interesting antigens solely on the basis of their ability to stimulate lymphocytes isolated from animals immunized in a particular way is obviously debatable. Animals infected with live mycobacteria might give a different result. The choice of antigen should be based on investigation of other parameters as well, e. g., the amount of intcrferon-gamma produced. Such supplementary parameters are the subject of current studies in this laboratory.
The influence of several genetic factors for responses against mycobacteria in mice have been described, among which the bcg gene and major histocompatibility complex (MHC) seem to be of major importance (6,16). The effect of different H-2 haplotypes on the immune response to mycobacteria has been investigated by several different approaches. Using congenic strains of mice, Ivanyi, et al. reported control by H-2 genes of the antibody response to mycobacterial epitopes (7). Ljungqvist, et al. have shown major differences in the repertoire of antibody responses among strains (12). By investigating the polyclonal antibody response to mycobacterial antigens, it could be shown that antibody specificities present in large quantities in one strain were undetectable in other strains. The authors suggest differences in H-2 haplotypes as a possible explanation to these qualitative differences (12).
On basis of the T-cell-prolifcrativc response to M. leprae, congenic strains of mice could be divided into high and low responders. Genes located in the D region of the H-2 complex were shown to be responsible for this effect (4). In the present study, some of the eight strains were characterized as either high or low respondcrs to some of the antigens, but none of the antigens had selective-strain, T-cell-stimulating capacity. This is suggested to be due to the large size of the antigens, each probably containing several epitopes. The qualitative differences in antibody responses observed by Ljungqvist, using the same strains of mice could be due to the fact that only some of the epitopes initiating T-cell proliferation arc T-hclper-cell epitopes (9).
The response patterns of the eight strains in the present paper were analyzed using the ranking of the four stimulating antigens. This comparison of the immunogenicity of the antigens in different strains is independent of the response level and, using this approach, five strains were found to share a common response pattern while three strains differed. A. CA/Sn is the strain which differed most markedly from the rest, reacting significantly differently from 5 out of 7 strains (Fig. 5).
A question of major importance in vaccine development is the genetically determined differences in the protective efficacy of chosen antigens. The existence of high and low responders to single antigens and marked differences in response patterns suggests that the composition of potential, new, mycobacterial vaccines will have to be based on several antigens. An antigen such as the 17-kDa antigen HBT11, found to stimulate all tested haplotypes, could perhaps be suggested as a possible component of this mixture.
Acknowledgments. We wish to thank Annette Hansen for excellent technical assistance. Bodil Hansen and Marianne S. Schroder are also thanked for typing the manuscript, and Susan Wedel for doing the art work. This study was supported by grants from the World Health Organization Programme for Vaccine Development. Peter Andersen is the rcccipicnt of a postgraduate scholarship from the Royal Veterinary and Agricultural University, Copenhagen.
REFERENCES
1. ANDERSEN, A. B. ,YUAN, Z. L., HASLOV, K., VERG-MANN, B. and BENNEDSEN, J. Interspecies rcactivy of five monoclonal antibodies to Mycobacterium tuberculosis as examined by immunoblotting and enzyme-linked immunosorbent assay. J. Clin. Microbiol. 23(1986)446-451.
2. BOOM, W. H. ,HUSSON, R. N. ,YOUNG, R. A. ,DAVID, J. R. and PIESSENS, W. F. In vivo and in vitro characterization of murine T-cell clones reactive to Mycobacterium tuberculosis. Infect. Immun. 55(1987)2223-2229.
3. COLLINS, F. M. ,LAMB, J. R. and YOUNG, D. B. Biological activity of protein antigens isolated from Mycobacterium tuberculosis culture filtrate. Infect. Immun. 56(1988)1260-1266.
4. DOUGLAS-JONES, A. G. and WATSON, J. D. Immunity to leprosy. II. Genetic control of murine T-cell proliferative responses to Mycobacterium leprae. J. Immunol. 135(1985)2824-2828.
5. HASLØV, K., ANDERSEN, Â. B. LJUNGQVIST, L. and WEIS BENTZON, M. Comparison of the immunological activity of five defined antigens from Mycobacterium tuberculosis in seven inbred guinea pig strains. The 38 kDa antigen is immunodominant. Scand . J. Immunol. 1990 (in press).
6. IVANYI, J. Pathogenic and protective interactions in mycobacterial infections. Clin. Immunol. Allergy 6(1986)127-157.
7. IVANYI, J. and SHARP, K. Control by H-2 genes of murine antibody responses to protein antigens of Mycobacterium tuberculosis. Immunology 59(1986)329-332.
8. KAUFMANN, S. H. E. ,VATH, U. ,THOLE, J. E. R, VAN EMBDEN, J. D. A. and EMMRICH, F. Enumeration of T cells reactive with Mycobacterium tuberculosis organisms and specific for the recombinant mycobacterial 64-kDa protein. Eur. J. Immunol. 17(1987)351-357.
9. KRZYCH, U. ,FOWLER, A. V., MILLER, A. and SER-CARZ, E. E. Repertoires of T cells directed against a large protein antigen, bcta-galactosidase. I. Helper cells have a more restricted specificity repertoire than proliferative cells. J. Immunol. 128(1982)1529-1534.
10. LAEMMLI, U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 277(1970)680-685.
11. LAMB, J. R. ,REES, A. D. M. ,BAL, V. IKEDA, H., WILKINSON, D. ,DE VRIES, R. R. P. and ROTHBARD, J. B. Prediction and identification of an HLADR-restricted T-cell determinant in the 19-kDa protein of Mycobacterium tuberculosis. Eur. J. Immunol. 18(1988)973-976.
12. LJUNGQVIST, L. ,WORSAAE, A. and HERON, I. Antibody responses against Mycobacterium tuberculosis in 11 strains of inbred mice: novel monoclonal antibody specificities generated by fusions, using spleens from BALB. B10 and CBA/J mice. Infect. Immun. 56(1988)1994-1998.
13. OFTUNG, F., MUSTAFA, A. S., SHINNICK, T. M., HOUGHTEN, R. A. ,KVALHEIM, G. ,DEGRE, M., LUNDIN, K. E. A. and GODAL, T. Epitopes of the Mycobacterium tuberculosis 65 kilodalton protein antigen as recognized by human T-cells. J. Immunol. 141 (1988) 2749-2754.
14. OTTENHOFF, T. H. M. ,KALE AB, B., VAN EMBDEN, J. D. A. ,THOLE, J. E. R. and KIESSLING, R. The recombinant 65 kD heat shock protein of Mycobacterium bovis bacillus Calmette-Guerin/ M. tuberculosis is a target molecule for CD4+ cytotoxic T lymphocytes that lysc human monocytes. J. Exp. Med. 168(1988)1947-1952.
15. PEDRAZZINI, T. ,HUG, K. and LOUIS, J. A. Importance of L3T4+ and Lyt-2+ cells in the immunologic control of infection with Mycobacterium bovis strain bacillus Calmette-Guerin in mice; assessment by elimination of T-cell subsets in vivo. J. Immunol. 139(1987)2032-2037.
16. SKAMENE, E. Genetic control of resistance to mycobacterial infection. Curr. Top. Microbiol. Immunol. 124(1986)49-66.
17. STIFFEL, C, IBANEZ, O. M., RIBEIRO, O. G. ,DECREUSEFOND, C, SIQUEIRA, M., MOUTON, D. and Biozzi, G. Genetic regulation of the specific and non-specific component of immunity. Immunol. Lett. 16(1987)205-217.
18. WORSAAE, A., LJUNGQVIST, L., HASLOV, K., HERON, I. and BENNEDSEN, J. Allergenic and blastogcnic reactivity of three antigens from Mycobacterium tuberculosis in sensitized guinea pigs. Infect. Immun. 55(1987)2922-2927.
19. WORSAAE, A., LJUNGQVIST, L. and HERON, I. Monoclonal antibodies produced in BALB. B10 mice define new antigenic determinants in culture filtrate preparations of Mycobacterium tuberculosis. J. Clin. Microbiol. 26(1988)2608-2614.
20. YOUNG, D., KENT, L., REES, A., LAMB, J. and IVANYI, J. Immunological activity of a 38-kilo dalton protein purified from Mycobacterium tuberculosis. Infect. Immun. 54(1986)177-183.
1. D. V. M. ; Biostatistical Department, Statens Seruminstitute, DK-2300 Copenhagen S, Denmark.
2. M. Sc, Biostatistical Department, Statens Seruminstitute, DK-2300 Copenhagen S, Denmark.
3. M. D., D. Sci., Head, Vaccine Department; Biostatistical Department, Statens Seruminstitute, DK-2300 Copenhagen S, Denmark.
4. Pharmacist, Mycobacteria Department; Biostatistical Department, Statens Seruminstitute, DK-2300 Copenhagen S, Denmark.
5. Statistician, Biostatistical Department, Statens Seruminstitute, DK-2300 Copenhagen S, Denmark.
Reprint requests to Dr. Andersen.
Received for publication on 18 June 1990.
Accepted for publication on 14 August 1990.