- Volume 59 , Number 1
- Page: 68–75
Rifampin in drug-incorporated diet: effect of duration and temperature of storage. Relevance to drug-susceptibility testing in mice inoculated with M. leprae
ABSTRACT
This paper is in two parts. Plasma concentrations of rifampin were assayed at 11 time points in 24 hr in mice fed one of three dosages of rifampin, either by gavage or by dietary incorporation. The drug-mixed diets had been stored for a maximum of 3 weeks at 4ºC or at room temperature (30ºC35ºC). The peak concentration of rifampin produced by gavage was approximately 1Vitimes higher than the maximum plasma concentration of the corresponding dosage in fresh diet. Plasma concentrations decreased with the increasing duration of storage of the drug-mixed diet, irrespective of whether the diet was stored at 4ºC or at room temperature. This decrease was less when the diet was stored at 4ºC than at room temperature. Drug levels were also assayed in another set of mice selected f rom ongoing drug-susceptibility experiments; these mice were fed a rifampin-incorporated diet stored at room temperature.The plasma concentrations in these mice, assayed at the time of foot pad harvest, were generally higher than in the 24-hr experiment. The harvest results f rom these mice were compared with the harvest results f rom a third set of mice, also f rom ongoing drug-susceptibility experiments, but fed a rifampin-mixed diet stored at 4ºC. Multiplicationof Mycobacterium leprae in mouse foot pads was prevented by rifampin mixed in the diet at a dosage of > 0. 003%, whether stored at room temperature or at 4ºC.
This study defines the criteria for rifampin resistance of M. leprae in the mouse foot pad by discussing methods of rifampin administration, the plasma concentration curves that result, and the effect of these on the multiplication of the organisms in the mouse foot pad.
RÉSUMÉ
Cet article comprend ducx parties. Les concentrations plasmatiques de rifampicine ont été dosées à 11 reprises au cours d'un intervalle de 24 h chez des souris auxquelles était administrée de la rifampicine (trois dosages différents ont été utilisés), soit par gavage, soit mélangée à la nourriture. Les aliments additionnés du médicament avaient été conservés pendant un maximum de 3 semaines à 4ºC, ou à température ambiante (30-35ºC). La concentration plasmatiquc maximale de rifampicine administrée par gavage était environ une fois et demi supérieure à celle obtenue avec un même dosage administré dans de la nourriture fraîche. Les concentrations plasmatiques diminuaient quand la durée de conservation des aliments additionnés du médicament augmentait, que la conservation ait été faite à 4ºC ou à température ambiante.Les concentrations du médicament ont également été dosées chez, un autre groupe de souris qui participaient à des essais sur la susceptibilité médicamenteuse; ces souris ont reçu un régime alimentaire additionné de rifampicine et conservé à température ambiante. Les concentrations plasmatiques observées chez ces souris, dosées au moment du prélèvement du coussinet plantaire, étaient généralement plus hautes que dans l'expérimentation de 24 h. Les coussinets plantaires de ces souris ont été comparés à ceux d'un troisième groupe de souris, participant également à des essais de susceptibilité médicamenteuse, mais dont la nourriture additionnée de rifampicine était conservée à 4ºC. La multiplication du Mycobacterium leprae était empêchée dans le coussinet plantaire des souris dont l'alimentation était additionnée de rifampicine à une concentration supérieure ou égale à 0. 003%, que celleci ait été conservée à température ambiante ou à 4ºC.
Ce travail définit les critères pour l'étude de la résistance à la rifampicine de M. leprae au niveau du coussinet plantaire de la souris, en discutant les modes d'administration de la rifampicine, leur influence sur les courbes de concentration plasmatiquc, ainsi que l'influence de la concentration plasmatiquc sur la multiplication des organismes dans le coussinet plantaire de la souris.
RESUMEN
Se administraron a ratones, por sonda gstrica o por incorporación de la droga en la dieta, 3 dosis de rifampina. Después de cada dosis se midieron las concentraciones plasmáticas de la droga en 11 ocasiones dentro de las 24 h siguientes a su administración. Las dietas adicionadas de la droga habían estado almacenadas por un máximo de 3 semanas a 4ºC o a temperatura ambiente (30-35ºC). El pico de máxima concentración de rifampina producido por sonda gástrica fue aproximadamente 1. 5 veces mayor que el obtenido por incorporación de la droga en la dieta. Las concentraciones en plasma disminuyeron cuand o se incrementó la duración del almacenamiento de la dicta con droga, independientemente de la temperatura de almacenamiento. La disminución fue menor cuand o el almacenamiento fue a 4ºC.También se examinaron los niveles de la droga en otra serie de ratones seleccionados de experimentos en desarrollo sobre susceptibilidad a las drogas; estos animales se alimentaron con una dicta adicionada de rafampinaque había sido almacenada a temperatura ambiente. Las concentraciones plasmáticas de rifampina en estos animales, medidas al momento de la remosión de sus almohadillas plantares, fueron generalmente mayores que en los experimentos de 24 h. Los hallazgos en las almohadillas plantares de estos animales se compararon con los de un tercer grupo de animales alimentados con una mezcla de rifampina y dicta almacenada a 4ºC. La multiplicación del Mycobacterium leprae en las almohadillas plantares del ratón fue evitada por la rifampina incorporada en la dieta a una concentración igual o menor al 0. 003%, independientemente de la temperatura de almacenamiento.
Este estudio define los criterios para resistencia del M. leprae a la rifampina en la almohadilla plantar del ratón, al discutir los métodos de administración de la droga, las curvas de concentración plasmática resultantes, y el efecto de esto sobre la multiplicación de los organismos en la almohadilla plantar del ratón.
Drug sensitivity or resistance of Mycobacterium leprae is routinely determined using the mouse foot pad technique. The minimum inhibitory concentration (MIC) and the minimum effective dosage (MED) of rifampin have not been as clearly established as for dapsone (9). A few studies have reported varied MICs and MEDs (6-8). Multiplication of M. leprae in mice treated once a week with 10 mg/kg of rifampin, by gavage, is an accepted criterion for determining rifampin resistance in the laboratory (5,11). However, definite criteria, when the drug is administered continuously in the diet, are lacking. This information would be useful, since dietary incorporation of drugs is a more convenient method of drug administration compared to the time- and labor-intensive gavage. Information on plasma concentrations in mice fed rifampin by various modes, and application of these results to actual bacillary counts at harvest of M. leprae-inoculaled mice from routine drug-susceptibility experiments, would help to define criteria since the potency of rifampin is stated to decrease when mixed in diet and stored (9).
MATERIALS AND METHODS
Ninety-nine rand omly bred CBA mice were divided into three groups of 33 mice each. Groups 1, 2 and 3 were fed 0. 01%, 0. 003% and 0. 001% of rifampin, respectively.
In addition, 792 CBA mice were divided into three equal groups (264 mice each) and fed a rifampin-incorporated diet. Each of the three groups received one concentration of the drug (0. 01%, 0. 003%, or 0. 001%). Each group was further divided into two subgroups of 132 mice each. One subgroup received a drug-incorporated diet stored at 30º-35ºC [room temperature (RT) feed] and the other received the same diet stored at 4ºC [cold feed (CF)]. Each subgroup was further divided into four smaller groups of 33 mice. Each group of 33 mice received feed stored for 1, 7, 14 or 21 days. Therefore, for any one concentration of rifampin mixed in the diet, eight groups of 33 mice received RT/CF feed stored for 1,7, 14, or 21 days. All mice were starved for 24 hr prior to feeding. Both feeding and bleeding were done on the same day, over a 24-hr period. Three mice per group of 33 were bled by the retroorbital route at 11 specific time-points over 24 hr (The Figure). Plasma was separated after centrifugation at 300 x g x 30 min, and stored at -20ºC.
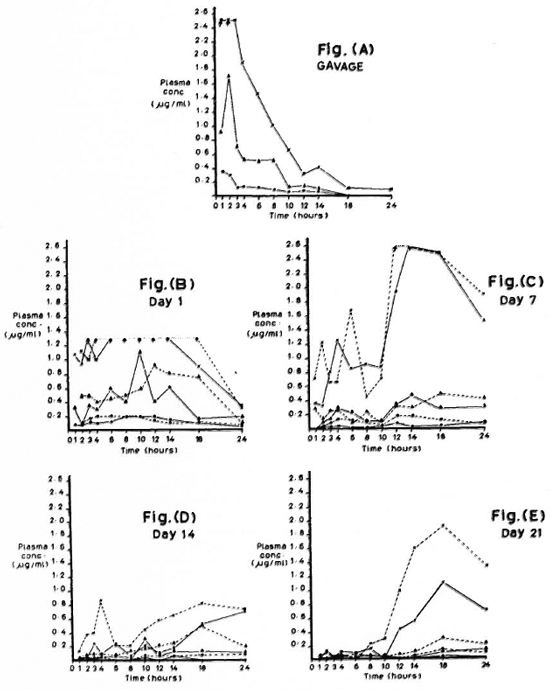
The figure. Plasma rifampin (RFP) concentrations over 24 hours in mice fed RFP by gavage and by dietary incorporation. Plasma concentration at each time-point is the mean of values from 3 mice; value per mouse is derived from mean diameter of quadruplicate zones of inhibition. (----) = diet stored at room temperature (RT); (- - -) = diet stored at 4ºC (CF); (x- - -x) = 0. 01% RFP; ( - - -
- - - ) = 0. 003% RFP; (
) = 0. 003% RFP; ( - - -
- - - ) = 0. 001% RFP; (↑. . . . . ↑) = above maximum stand ards used.
) = 0. 001% RFP; (↑. . . . . ↑) = above maximum stand ards used.
Plasma rifampin concentrations were determined by a microbiological assay (plate diffusion method3) using a test strain ofStaphylococcus aureus(NCTC 10702) sensitive only to rifampin. On receiving the test strain at our laboratory, the organisms were streaked on nutrient agar and grown for 24 hr at 37ºC. Two approximately similar-sized single colonies were then inoculated into 10 ml of nutrient broth and incubated for 18 hr at 37ºC. The broth culture was visually checked for turbidity, and 0. 3 ml aliquots of this culture were stored at - 70ºC. These aliquots were used in the assay to ensure uniformity, since the test samples were not measured simultaneously but in nine assays. A portion (0. 25 ml) of each aliquot was seeded into 350 ml of nutrient agar (8. 05 g of nutrient agar, Difco, Laboratories, Detroit, Michigan, U. S. A. ; 10. 5 ml of 1. 0 M KH2PO4 and 350 ml of triple distilled water adjusted to a pH of 6. 9 using 0. 4 N NaOH or 1 N HC1). The seeded agar was dispensed in 10 ml volumes into 90-mm diameter pe tri dishes. Four wells, each 3 mm in diameter, were cut in each petri dish and numbered serially.
Standards were prepared using rifampin from capsules (Rimactane®, 3-(4-methyl-lpiperazinyl-iminomethyl)-rifamycin SV; CIBA-GIEGY SA, Basel, Switzerland ). Sixteen mg of Rimactane® was dissolved in 0. 25 ml of 0. 1 N HC1 to which 1 ml of sterile triple-distilled water was added to make a concentration of 12,800 μg/ml. Serial 1 in 100 (128 μg/ml), 1 in 10 (12. 8 μg/ml), and 1 in 5 (2. 56 μg/ml) dilutions were made in distilled water for the first two dilutions and in pooled normal mouse plasma for the last dilution. Two-fold dilutions up to a concentration of 0. 02 μg/ml were then made using pooled mouse plasma. The concentrations of stand ards used in the assay ranged from 2. 56 μg/ml to 0. 02 μg/ml.
Pooled mouse plasma was used as a blank control. Stand ards, blank controls, and test samples were coded and loaded rand omly in quadruplicate. After incubation at 37ºC for 18 hr, zones of inhibition (inclusive of wells) were viewed through a WILD dissection microscope, at an objective magnification of 6 x and an ocular magnification of 8 x, and measured using a scale placed under the petri dish. Plasma concentrations of rifampin were calculated (with software developed for this purpose) using a formula derived from Dickinson, et al. (13):
concentration = of rifampin Regression coefficient for the stand ards
Drug levels were also assayed at harvest (using the same method as described above) in 125 CBA mice (27 controls and 98 test mice). These mice were from 17 ongoing drug-susceptibility experiments. The mice had been inoculated with M. leprae isolates obtained from skin biopsies of 17 leprosy patients with a BI > 2 +. All biopsies were processed and inoculated using Rees' technique (10). Of the 17 biopsies, M. leprae derived from 16 were wild (patient → mouse), while the M. leprae in one experiment had been passaged once (patient → mouse → mouse) prior to inoculation. All 17 isolates were tested against 0. 001%, 10 against 0. 003%, 11 against 0. 01%, and 14 against 0. 1% rifampin. The non-uniformity in testing was because mice from ongoing experiments were selected.
The mice were fed rifampin-incorporated diet daily by the continuous technique (12) (dosages are in Table 3). The powder contained in rifampin capsules (same as used for stand ards, see above) was blended into commercial mice feed and stored at room temperature. Sufficient quantity of drug-incorporated feed was prepared to last for 3 weeks.
The criterion for selection of experiments for inclusion in the study was the detection of at least 5 x 105 acid-fast bacilli (AFB) per pooled paired foot pads in control mice. In each of the 17 experiments, the control and test mice were rand omly selected. The minimum number chosen was 1 control mouse and 1 test mouse from each available dietary dosage. The time of harvest of foot pads varied between 5 months and 12 months in the different experiments depending on when control mice showed 5 x 105 AFB/pooled, paired foot pads (see above). Harvests were routinely done at the end of the last feeding cycle, i. e., 24 hr after dispensing the drug-mixed feed. Harvest results were analyzed and correlated to plasma rifampin concentrations.
Harvest results were also analyzed in another set of 335 mice (157 controls and 178 test) which were fed rifampin-containing diets that were stored at 4ºC and renewed every 3 weeks. These mice were also from drugsusccptibility experiments, and the M. leprae isolates were from skin biopsies of 28 patients with a BI > 2 +. All isolates were wild.
RESULTS
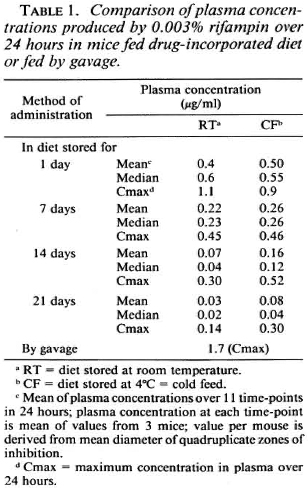
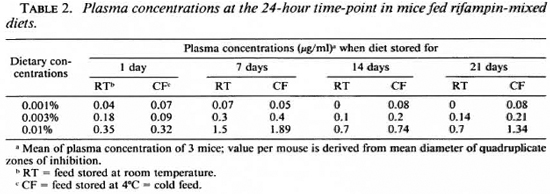
The Figure (A-E) shows plasma concentrations of rifampin at 11 time-points over 24 hr. Each point in The Figure (A to E) represents the mean of three mice, each assayed in quadruplicate. In A, tmax for 0. 003% (approximately 2 mg/kg body weight of mouse) and presumably for 0. 01% (approximately 6. 6 mg/kg of mouse) is 2 hr; whereas Grosset reported a tmax of 6 hr for a gavage dose of 10 mg/kg body weight of mouse (4). B to E show plasma concentrations in mice fed rifampin-mixed diets stored either at 4ºC or at room temperature for 1, 7, 14, and 21 days. Table 1 shows plasma rifampin concentrations for one of the dosages (0. 003%) with the different methods of rifampin administration.
The Figure (A to E) and Table 1 show that: a) the maximum concentration (cmax) produced by gavage is approximately 1½ times greater than the cmax achieved after 1 day ofstorage ofrifampin-mixed diet (Table 1); b) rifampin concentrations decrease over a 21-day period if mixed in diet and stored either at room temperature (RT) or at 4ºC; the decrease is greater with RT (The Fig. B to E; Table 1); c) over a 24-hr period there is no remarkable difference between the cmax obtained from CF and RT stored up to 7 days, after which CF values are higher than RT; at 21 days of storage, the CF values are slightly more than double that of RT (Table 1); d) 0. 001% consistently produces very low concentrations (The Fig. B to E).
Table 2 shows mean plasma concentrations at 24 hr in mice fed rifampin-mixed diets (same data as at 24-hr time-point in The Fig. B to E). This table provides reference values against which data from Table 3 may be interpreted.
Tables 3 and 4 display data derived from drug-susceptibility experiments in which rifampin incorporated in the diet was fed daily toM. /eprae-inoculated mice for a period ranging between 5 and 12 months. Table 3 shows plasma concentrations at harvest in mice fed rifampin-mixed diets stored at room temperature. These values, like those in Table 2, are also at the 24-hr time-point, since harvest and bleeding were performed 24 hr after the last dispensation of the drugincorporated diet. The values in Table 3 are mostly higher than those in Table 2.
Table 3 also shows harvest results along with plasma concentrations. Of all dosages, 0. 001% alone permitted multiplication of a few isolates; the ranges of plasma concentrations were similar in mice in which M. leprae multiplied and in those in which it did not (data not shown). Nine out of the 17 isolates in Table 3 were tested against all four dosages (0. 001%, 0. 003%, 0. 01%, and 0. 1%) simultaneously; 8 were susceptible to all four dosages, while 1 multiplied when tested against 0. 001%. None of the isolates tested against 0. 003% (10/10), 0. 01% (11/ 11), or 0. 1% (14/14) multiplied. Fourteen of 17 isolates tested against 0. 001 % also did not multiply, while the remaining 3 multiplied; 1 of these 3 did not multiply when simultaneously tested against 0. 003%. The other two were not tested against 0. 003%. All three were subcultured into mice fed 0. 001% rifampin-containing diets, but showed no multiplication at the end of 12 months, suggesting that the number of viable AFI3 in the inocula were too few to multiply.
Table 4 shows harvest results in mice fed different dosages of rifampin-mixed diets stored at 4ºC. Only two dosages, 0. 003% and 0. 01% were used, and none of the 28 isolates tested showed multiplication.
DISCUSSION
The potency of rifampin when incorporated in the diet is expected to decrease over time (9). Our study shows that plasma concentrations in mice fed rifampin-mixed diets stored up to 3 weeks decreased with the increasing duration of storage. This decrease is greater with diet stored at room temperature than at 4ºC. However, the study also shows that multiplication of M. leprae is consistently prevented by rifampin mixed in the diet, whether stored at room temperature or at 4ºC at concentrations > 0. 003%.
Therefore, the therapeutic end-point, lack of multiplication of M. leprae in mouse foot pads, is achieved in spite of: a) the decreasing plasma concentrations which, in a routine drug-susceptibility experiment, would occur cyclically every 21 days (since feed is renewed every 3 weeks), and b) the much lower peak plasma concentrations produced by dietary incorporation as compared to gavage.
Since biologic assays, in this instance the plate diffusion assay, measure the activity of unbound drug (10), all of the values may be assumed with reasonable certainty to be the amount of drug actually available for exerting bactericidal activity. Even though bioavailability of rifampin is reduced by concomitant administration of food (2), an accumulation of rifampin is to be expected, since drug administered by a continuous technique would result in an exponential accumulation up to a plateau (1). It is possible that the continuous method of rifampin administration (sec Materials and Methods), which provided rifampin for the entire duration of the drug-susceptibility experiment, adequately compensates for the periodic decreases and fluctuations in the amount of the drug. This would explain the generally higher values seen in Table 3 compared to those shown in The Figure (B to E) and in Tables 1 and 2. Evidence that accumulation occurs in the mouse when fed by gavagc over days is present (4). This is attributed to the incomplete elimination of the drug within 24 hours (4). Our results from dietary incorporation (The Fig. B to E) also show that plasma concentrations do not reach zero at the end of 24 hours. Gros set suggests that the incomplete elimination of the drug within 24 hours is due to metabolism and excretion being more limited in mouse than in man (4).
Another finding is that 0. 001% rifampin in diet produces very low plasma concentrations (The Fig. B to E; Tables 2 and 3). This explains why 0. 001% is unable to consistently prevent multiplication of M. leprae isolates.
It is clear that the dietary dosage of0. 003% prevented multiplication of all 38 wild isolates of M. leprae (10 in Table 3 and 28 in Table 4), irrespective of whether the diet was stored at room temperature or at 4ºC. An earlier publication refers to 0. 003% in diet (plasma concentration, 0. 3ng/m\)as the minimal effective dosage of rifampin (l3). However, that publication did not address the potential problem of decreased potency of rifampin on storage or the possible effect this decrease could have on the multiplication of M. leprae. This study describes the effects of duration and temperature of storage of rifampin-mixed diets on the multiplication of M. leprae in the mouse foot pad, thus describing the laboratory conditions under which a wild isolate of M. leprae may be defined as rifampin resistant.
The results of drug-susceptibility testing of M. leprae isolates are used in the management of leprosy patients and in leprosy control activities. The act of defining rifampin resistance in the mouse foot pad system, therefore, provides a tool with which control programs using multidrug therapy (which includes rifampin) may be assessed. This is important in the current situation where M. leprae is still nonculti vable in vitro and where more and more patients are being covered by the multidrug therapy program.
It is suggested that M. leprae isolates which multiply in the foot pads of mice fed rifampin incorporated in diet, stored at either 4ºC or room temperature (30ºC-35ºC), by the continuous technique at a dosage of 0. 003% may be defined rifampin resistant.
Acknowledgments. This work was supported by the Karigiri Research Fund. The authors acknowledge the following: Mrs. S. Arumugam and Miss D. Chitra for the mouse foot pad work; Miss V. Shyla for secretarial assistance; Mrs. S. Devadoss for word processing and Mr. S. Rces for modifying the software used for calculating plasma rifampin concentrations; the Tuberculosis Research Centre, Madras, India, for the supply of the Staphylococcus aureus strain, for the original software used for calculating plasma rifampin concentrations, and for permitting one of us to learn the plate diffusion assay from Dr. P. Gurumurthy, Senior Research Officer (Biochemistry); Prof. J. H. Grosset, Laboratoire Central de Bactériologie-Virologie, Institut Pasteur, Paris, for helpful discussions.
M. leprae isolates were from biopsies received from the Branch of Epidemiology and Leprosy Control, and the Branch of Medicine, Schieffelin Leprosy Research & Training Centre, Karigiri, India.
REFERENCES
1. BOCHNER, F. ,CARRUTHERS, G., KAMPMANN, J. and STEINER, J., cds. Handbook of Clinical Pharmacology. Boston: Little, Brown and Co., 1978, p. 68.
2. BOCHNER, F. ,CARRUTHERS, G., KAMPMANN, J. and STEINER, J., eds. Handbook of Clinical Pharmacology. Boston: Little, Brown and Co., 1978, p. 272.
3. DICKINSON, J. M. ,ABER, V. R., ALLEN, B. W., ELLARD, G. A. and MITCHISON, D. A. Assay of rifampicin in scrum. J. Clin. Pathol. 27(1974)457-462.
4. GROSSET, J. H. Pharmacokinetics in drug screening. Int. J. Lepr. 55(Suppl. (1987)S852-S856.
5. GUELPA-LAURAS, C. -C,GROSSET, J. -H., CONSTANT-DESPORTES, M. and BRUCKER, G. Nine cases of rifampin-resistant leprosy. (Letter) Int. J. Lepr. 52(1984)101-102.
6. HILSON, G. R. F. ,BANNERJEE, D. K. and HOLMES, I. B. The activity of various antitubcrculous drugs in suppressing experimental Mycobacterium leprae infection in mice. Int. J. Lepr. 39(1971)349-353.
7. HOLMES, I. B. Minimum inhibitory and bactericidal dosages of rifampin against Mycobacterium leprae in the mouse foot pad: relationship to scrum rifampin concentrations. Int. J. Lepr. 42(1974)289-296.
8. HOLMES, I. B. and HILSON, G. R. F. The effect of rifampicin and dapsone on experimental Mycobacterium leprae infections: minimum inhibitory concentrations and bactericidal action. J. Med. Microbiol. 5(1972)251-261.
9. Ji, B. Drug susceptibility testing of Mycobacterium leprae. Int. J. Lepr. 55 Suppl. (1987)S830-S836.
10. ROWLAND, M. Drug administration and regimens. In: Clinical Pharmacology; Basic Principles in Therapeutics. 2nd ed. Melmon,K. L. and Morrelli, H. F., eds. New York: Macmillan Publishing Co., Inc. . 1978, p. 39.
11. WHO SCIENTIFIC WORKING GROUP ON THE CHEMOTHERAPY OF LEPROSY. Fifth report. Geneva: World Health Organization, 1986, p. 4. TDR/ THELEP-SWG (5)/86. 3.
12. WORLD HEALTH ORGANIZATION. Laboratory Techniques for Leprosy. Geneva: World Health Organization, 1987, pp. 65-78.
13. WORLD HEALTH ORGANIZATION. Laboratory Techniques for Leprosy. Geneva: World Health Organization, 1987, p. 79.
1. M. B. B. S., Ph. D., Research Fellow; Schieffelin Leprosy Research & Training Centre, Karigiri 632 106, India.
2. M. B. B. S., M. D., Research Pathologist; Schieffelin Leprosy Research & Training Centre, Karigiri 632 106, India.
3. M. B. B. S., M. D., Ph. D., Head, Branch of Laboratories, Schieffelin Leprosy Research & Training Centre, Karigiri 632 106, India.
Received for publication on 19 January 1989.
Accepted for publication in revised form on 23 August 1990.