- Volume 59 , Number 4
- Page: 548–57
Intrabacterial sodium-to-potassium ratios and ATP contents of Mycobacterium leprae from ofloxacin-treated patients
ABSTRACT
In a clinical trial including 17 multibacillary leprosy patients the in vivo effectiveness of ofloxacin on Mycobacterium leprae was tested via mass spectrometric determination of intrabacterial ratios of the concentrations of the sodium and potassium ions of individual organisms and of the ATP content per 106 bacteria isolated from skin biopsies. After 3 months of treatment, the in vivo drug effect could be determined with at least one of the two methods in 14 cases. Both methods revealed that in two cases the bacteria definitely did not respond to a 3-month ofloxacin monotherapy (200 mg twice daily). In three further cases a nonresponse of the M. leprae organisms was suspected from the mass spectrometric measurements. In the responder cases, the M. leprae were severely impaired. From the intrabacterial cation ratios the percentage of viable organisms averaged over all untreated biopsies was determined to be 58% and the percentage-killing during the first 3 months of treatment was 72%.RÉSUMÉ
Au cours d'un essai clinique concernant 17 lépreux lépromateux, l'efficacité in vivo de l'ofloxacine vis-àvis de Mycobacterium leprae a été étudiée par détermination par spectrométrie de masse des ratios intrabactériens des ions sodium et potassium des bactéries, individuelles, et de la teneur en ATP par 106 bactéries isolées à partir de biopsies cutanées. Après 3 mois de traitement, l'effet du médicament in vivo pouvait être déterminé par au moins une des deux méthodes chez 14 patients. Les deux méthodes ont montré que chez deux patients les bactéries ne répondaient pas à trois mois de monothérapie à l'ofloxacine (200 mg deux fois par jour). Chez trois autres patients, on a suspecté une non-réponse de M. leprae à partir des mesures de spectrométrie de masse. Chez les patients où une réponse existait les M. leprae étaient sévèrement détériorés. Le pourcentage moyen des organismes viables obtenu à partir de toutes les biopsies en l'absence de traitement a été évalué à 58% à partir des ratios de cations intrabactériens, et le pourcentage de bactéries tuées durant les 3 premiers mois de traitement était de 72%.RESUMEN
Para analizar la efectividad in vivo de la ofloxacina sobre el Mycobacterium leprae en 17 pacientes con lepra multibacilar, se determinaron, por medio de la espectrometría de masas, las concentraciones intracclulares relativas de los iones sodio y potasio en organismos individuales y el contenido en ATP de 106 bacterias aisladas de biopsias de piel. Después de 3 meses de tratamiento, en 14 casos se pudo determinar el efecto in vivo de la droga por al menos uno de los dos métodos. Ambos métodos revelaron que en 2 casos la bacteria definitivamente no respondió al tratamiento de 3 meses con la ofloxacina (200 mg dos veces al día). En otros 3 casos, las mediciones hicieron sospechar la falta de respuesta del M. leprae. En los casos que sí respondieron, las bacterias estuvieron severamente afectadas. Las relaciones entre los cationes intrabacterianos en las muestras estudiadas indicaron que el porcentaje promedio de organismos vivos en biopsias de pacientes no tratados fue del 58% y que el porcentaje del efecto bactericida durante los primeros 3 meses de tratamiento con la droga fue del 72%.Since the early 1940s, monotherapy with dapsone has been the treatment of choice for multibacillary leprosy. However, the observation of increasing incidence of primary and secondary resistance to this drug (20) has urged many leprologists to search for new antileprosy drugs and drug combinations. To this end, Freeksen, et al. have, in a field trial in Malta (5), successfully demonstrated the therapeutic power of a multidrug therapy (MDT) containing isoniazid (INH), prothionamide (PTH), dapsone (DDS) and rifampin (RMP) known as Isoprodian®RMP. In 1981, the World Health Organization (WHO) recommended a multidrug regimen consisting of DDS, RMP, and clofazimine (CLF), according to a proposal by the WHO Study Group (29).
From the drugs DDS, RMP, INH, PTH, ETH, and CLF which were, or still are, used in multidrug antileprosy regimens, only RMP has a strong bactericidal activity (6). Furthermore, for all these drugs resistance-when applied as a monotherapy- and/or side effects are known (12). Therefore, new drugs have to be developed and tested under in vivo conditions (30).
The new synthetic nalidixic acid derivative, ofloxacin, was shown to be active in vitro against both gram-negative and gram-positive bacteria in general (25) and against a broad spectrum of mycobacterial species (26). Several authors have proven a strong killing activity of ofloxacin against Mycobacteria leprae in mouse foot pad experiments (7, 19, 21). Its potency to combat mycobacterial infections in man has been shown in the case of tuberculosis (27), and very recently also for leprosy by Grosset, et al. (8). In the latter study, from mouse foot pad experiments with normal and nude mice, a reduction of viability of the M. leprae organisms by about 99.9% after 22 single doses of ofloxacin was observed in virtually all patients.
In this paper we present the results of a clinical trial with ofloxacin monotherapy on 17 multibacillary leprosy patients. The therapeutic effectiveness was monitored during the first 3 months of treatment by clinical controls [bacterial index (BI), morphological index (MI), and visual inspection], by the collection of skin biopsies, isolation of M. leprae from these, and determination of the degree of impairment by two independent methods: a) the mass spectrometric analysis of a limited number of individual bacterial organisms to determine the distribution of the intrabacterial sodium-to-potassium ratios (Na+,K+-ratios) and to register the fragment ion peaks originating from the organic cell matrix for mass fingerprint analysis, and b) the determination with a bioluminescence assay of the intrabacterial ATP content obtained as an average value from 106 organisms.
The measurement of these parameters is based on general metabolic processes. Living bacterial organisms actively maintain lower intracellular concentrations of Na+-ions and higher concentrations of K+-ions than are found in the extracellular fluid. Dead or impaired organisms are unable to maintain these concentration gradients. Therefore, the intrabacterial Na+,K+-ratio is lowest for unimpaired bacteria and increases with the degree of impairment (the upper limit of this ratio is given by the extrabacterial ratio). In previous studies (16, 23) we have shown that the Na+,K+-ratio is a sensitive indicator of the organisms' physiological state (viability), and that changes in this value correlate well with those observed with established microbiological techniques. The mass fingerprints have previously not only been proven to be species-specific 17) but also to reflect the degree of drug-induced impairment (9). The ATP content is highest in viable, unimpaired organisms and decreases with the degree of impairment (2). Both methods-the mass spectrometric analysis of individual cells and the ATP assay-have been successfully applied to monitor the in vivo efficacy of DDS in leprosy patients in an earlier study (24). In that trial also, a qualitative correlation between the results of these two parameters and those obtained from the mouse foot pad has been established. In two cases in which the alternative techniques gave evidence for DDS-resistance, this suspicion was confirmed later on by the mouse foot pad test.
MATERIALS AND METHODS
Patients and treatment. The study covered 17 multibacillary leprosy patients with a high bacterial index (BI > 3) who previously had not received any antileprosy treatment, including 10 females and 7 males aged between 14 and 75 (mean: 39) years. All of the patients were treated as inpatients with the supervised administration of 200 mg ofloxacin twice daily for 3 months, except patients nos. 2 and 13 who received only 200 mg once per day because of their age (14 years) and low body weight. Before treatment was started, patients had to undergo a general clinical check-up and baseline laboratory investigation (HCT, WBC, ESR, Diff. count, SGOT, SGPT, alkaline phosphatase, stool and urine examinations). During treatment the patients were seen by the same physician daily. Clinical photographs were taken and skin biopsies were collected in duplicate for each patient before and after 1 and 3 months of treatment, respectively. Parallel to biopsy sampling, skin smears from six sites per patient were taken to determine the bacterial and morphological index according to WHO guidelines (31).
For the patients' benefit and for ethical reasons treatment was continued after 3 months of ofloxacin by daily doses of 600 mg rifampin, 100 mg dapsone, and 100 mg clofazimine for 3 weeks. During this period, the patients were clinically controlled and then discharged to leprosy control clinics.
Biopsy sampling and shipment. Biopsies were obtained in duplicate from active lesions with a weight of typically 50-100 mg and immediately transferred to a freezer (-80ºC). Biopsies were then shipped by air on dry ice in styrofoam containers, arrived within 48 hr in frozen condition, and were stored at - 80ºC until preparation.
Isolation of M. leprae and preparation for single cell mass analysis. The isolation procedures of M. leprae from tissue followed a modification of the protocol of Dhople and Storrs (4). Briefly, it includes the following steps: Removal of epidermis; homogenization in phosphate buffered saline (PBS) (0.05 M); removal of intact tissue via centrifugation (200 x g x 10 min, 4ºC); decontamination of M. leprae from remaining tissue by treatment with proteolytic enzymes (900 U collagenase, 225 U chymotrypsin, 83 U trypsin for 30 min at 20ºC in PBS); washing in PBS; decontamination from other bacterial genera with A'-acetylL-cysteine/NaOH-mixture (final NaOH concentration 1%, 20 min, 20ºC) according to Kubica, et al. (14); removal of extrabacterial sodium and potassium by two washing and centrifugation steps (3000 x g x 20 min, 4ºC) in distilled water; the preparation for mass spectrometry was done by transferring one drop of the concentrated bacterial suspension to a Formvar-coated copper mesh and draining off excess fluid with tissue paper to achieve a widespread distribution of bacteria, which allowed the evaporation of one single cell with each laser shot in the laser microprobc mass analyzer.
Isolation and preparation procedure for ATP assay. In principle, the same method as described above was used with the following modifications: Bacteria were decontaminated only with NaOH (final concentration 2%, 30 min, at 37ºC) followed by neutralization with 1 N HC1 before enzyme treatment. The bacteria were then exposed to Triton X-100 (final concentration 0.1%) for 10 min at room temperature. Nonbacterial ATP was removed by ATPasc digestion (final concentration 0.17%, containing 5 mM CaCl2) for 10 min at room temperature (4).
ATP assay. From the purified bacterial suspension in 50 mM Tris buffer, pH 7.7, ATP was extracted, by the method of Dhople and Hanks (3), using chloroform and heat. The final extract was suspended in Tris buffer solution, and 0.1 ml of the extract was injected into 0.1 ml of a luciferin-luciferase system (DuPont Company, Wilmington, Delaware, U.S.A.) supplemented with pure luciferin (Sigma Chemical Company, St. Louis, Missouri, U.S.A.). The peak height of the reaction was measured on a Chem-Glow Photometer (American Instrument Company, Silver Spring, Maryland, U.S.A.) and referred to the absolute number of M. leprae organisms determined by the pinhead method of Hanks, et al. (I0) to calculate the ATP content in pg/106 bacteria.
Single cell mass analysis. The mass spectrometric instrumentation, the laser micro-probe mass analyzer (LAMMA 500®; Ley-bold AG, Koln, Germany) has been described in detail elsewhere (' ')• Briefly, in the LAMMA instrument a high-energy, ultraviolet laser pulse is focused through the objective of a light microscope onto the sample to be analyzed and, in our experiment, evaporates one bacterial organism at one shot. The positive atomic and molecular (fragment) ions produced are registered by means of a time-of-flight mass spectrometer. The detection limits of the instrument for sodium and potassium are lower than 1018 g out of an analyzed volume of about 1 µm3 (= 10-12 g).
The intracellular Na+,K+-ratios were determined from the intensities of the 23 Na+ 39K+-ion peaks, respectively, from 200 single cell measurements in the average from each preparation. (The measured Na+,K+-ratios are only relative figures due to the different detection sensitivities for the two cations.) From the thus acquired 200 individual ratios, distributions within the populations were established. Due to the skewness of these distributions, instead of the average values the medians (50% values) were calculated.
Mass fingerprint analysis was used to classify, with so-called discriminant analysis, unknown samples as either unimpaired or impaired. The mathematical criterion according to which this classification can be achieved (discriminant function) is calculated from the two known classes "unimpaired" and "impaired" which arc made up by the untreated populations and those samples treated for 3 months and showing high drug response in both the cation ratio and the ATP content, respectively. Here, the calculation of the discriminant function was based on the analysis of 120 single cell spectra-registered in the mass range m/z 60 to 200 and normalized to total ion intensity-from each sample of the two classes (18).
RESULTS
The results for the influence of ofloxacin on the various measuring parameters (BI, MI, ATP content, and Na+,K+-ratios) are summarized in The Table and grouped according to the duration of treatment. A large number of biopsies from one shipment did not render évaluable samples for single cell mass analysis, due to insufficient activity of the enzyme mixture in the decontamination step by M. leprae from tissue. Therefore, these preparations had to be repeated and, consequently, no material was left for ATP determination. These cases are shown as ND (not determined). In those cases which are shown as NA (not analyzable), the number of well-separated M. leprae organisms on the Cu-meshes was not sufficient. Cases in which neither the ATP content nor the Na+,K+-ratio was evaluated are not included in The Table. The five cases marked with an asterisk did not show any response to a 3-month treatment with ofloxacin in either of the two parameters, ATP content or cation ratio, or in both, and are hereafter termed "suspected" and "definite nonresponder" cases, respectively.
The data for the ATP contents and the Na+,K+-ratios in dependence on the duration of treatment are also presented graphically in Figure 1 which includes the values of the individual patients and the averages over all responder cases. The results show that the ATP content decreases and the values of the medians of the intrabacterial Na+,K+-ratios increases significantly statistically during the observation period of 3 months, indicating ofloxacin-induced impairment of the bacterial organisms. However, while the change in the ATP content is already significant after 1 month of treatment, the shift of the medians of the Na+,K+-ratios is not significant at that time.
Fig. 1. Scatter plot of the ATP content ( ) andmedians of the intrabacterial Na+,K+ ratios (
) andmedians of the intrabacterial Na+,K+ ratios (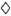 ) for M. leprae populations isolated from patients treated for 0,1, and 3 months, respectively, with 200 mg twice daily of ofloxacin. Each point represents one patient. Encircled values = nonresponder cases; mean values for each measuring parameter at each time for the responder cases are interconnected.
) for M. leprae populations isolated from patients treated for 0,1, and 3 months, respectively, with 200 mg twice daily of ofloxacin. Each point represents one patient. Encircled values = nonresponder cases; mean values for each measuring parameter at each time for the responder cases are interconnected.
In addition to the medians of the cation ratios, the single cell mass analysis renders information on the variation of the ratio (physiological state) within the bacterial populations. In Figure 2 the distribution of the Na+,K+-ratios within the various bacterial populations (untreated and treated for 1 or 3 months, respectively) averaged over all samples belonging to the same group (thus representing more than 1000 single organisms each) are plotted. Figure 2 shows, as was already obvious from Figure 1, that the medians of the distributions are shifted to higher values with increasing duration of treatment and that the distributions are broadened. This broadening already starts after 1 month of ofloxacin treatment. Clearly, the distribution obtained from M. leprae organisms isolated from the five nonresponder cases (according to the results from the cation ratios) after 3 months of treatment resembles that of the untreated patients with respect to its shape and position of the maximum.
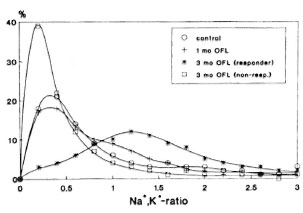
Fig. 2. Frequency distributions of the intrabacterial Na+,K+ ratios for all responder cases averaged over the respective periods of treatment (0, 1, and 3 months) and over the nonresponder cases after 3 months of treatment, respectively.
To further prove the nonresponsc of the bacteria isolated from these cases to the treatment, the influence of the drug on their organic matrix was also investigated in a two-group discriminant analysis of the fingerprint spectra (Fig. 3). For this, the fingerprint spectra from three untreated patients and from three responder cases (according to high intrabacterial Na+,K+-ratios and low ATP contents) after 3 months of treatment, respectively, were used to establish the discriminant function for the classification of the suspected nonresponder cases. Positive discriminant scores correspond to impaired samples and negative scores to unimpaired samples. The averaged fingerprint spectra obtained from the bacteria isolated from the five "definite" or "suspected" cases have exclusively negative discriminant scores and thus are classified as unimpaired. Furthermore, as can be taken from the values for the respective ATP contents and the Na+,K+-ratios included in Figure 3, the three suspected nonresponder cases showed a rather low response in the ATP values as compared to the responder cases.
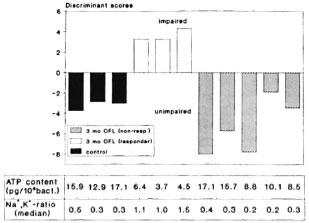
Fig. 3. Two-group discriminant analysis of the mass fingerprint spectra of M. leprae populations isolated from 3 responder cases after 0 and 3 months of treatment, respectively, and of 2 definite and 3 suspected nonresponder cases. Each bar represents the average over 120 single cell spectra. Below = corresponding values of the intrabacterial ATP contents and Na+,K+ ratios.
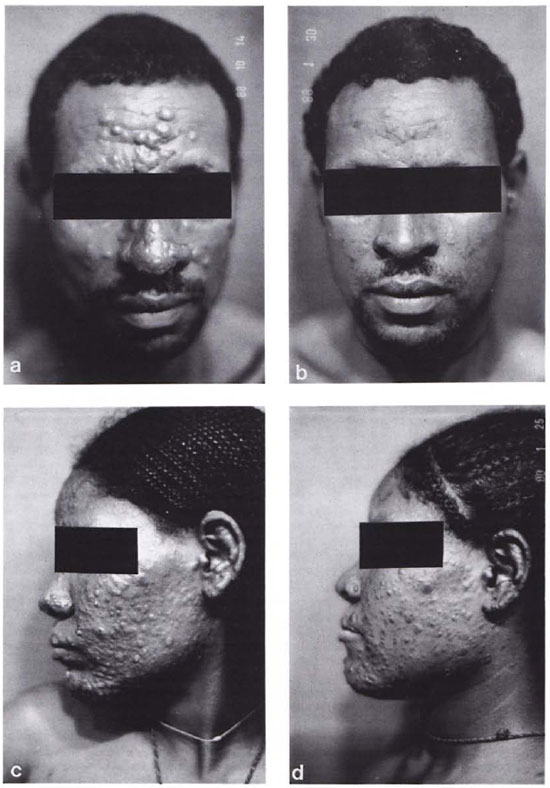
The clinical response to ofloxacin treatment is demonstrated by the photographs in Figure 4, showing in each case the patient before and after 3 months of treatment. Figure 4 a and b shows a clinically responding case for whom the measuring parameters showed significant changes. Figure 4 c and d is a clinically nonresponding case for whom no changes in the ATP and cation values could be observed. It must be pointed out, however, that not in all cases was such a clear-cut correlation between the clinical response and the measuring parameters possible.
Fig. 4. Photographs of a responder case and a nonresponder case before (a and c) and after (b and d) 3 months of ofloxacin treatment. respectively.
A particular problem to be solved in connection with the application of the cation measurements to therapy control is the determination of the percentage of viable organisms in a human skin biopsy. This value cannot directly be derived from the measurements described above, because they allow only statements on changes in the physiological state relative to the untreated control. The quantification of the percentage of viable organisms requires the determination of the intrabacterial Na+,K+-ratio up to which M. leprae are viable (limiting value). Briefly, we approached this problem in the following way: We correlated, for the same experiment, the relative cumulative distributions of the Na+,K+-ratios with the percentage of viable organisms determined as colony forming units (cfu) per total cell number in 49 independent experiments with various cultivable bacterial species and genera treated with differently acting drugs, resulting in a limiting value of 0.43 ± 0.03 (mean ± SEM). Under the assumption that this limiting value is valid also for M. leprae, the percentage of viable organisms in treated and untreated biopsies can be determined. For this, the relative cumulative distributions of the Na+K+-ratio were evaluated for the responder cases at 0, 1, and 3 months of treatment, respectively (Fig. 5). For the limiting value of 0.43, the following percentages of viable organisms were obtained: 58% for the untreated biopsies, 48% after 1 month and 16% after 3 months of treatment, respectively. From these figures it follows that 17% of the organisms viable at time 0 were killed during the first month of treatment and 72% during a 3-month period of treatment, respectively.
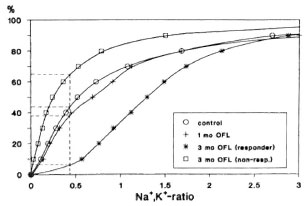
Fig. 5. Relative cumulative distributions of the intrabacterial Na+,K+ ratios for all responder cases averaged over the respective periods of treatment (0, 1, and 3 months) and over the nonresponder cases after 3 months of treatment, respectively. Vertical dashed line = upper limit of the Na+,K+ ratio up to which the organisms arc viable (0.43); horizontal dashed lines = readings of the respective percentages of viable organisms.
DISCUSSION
In this study we have applied the mass spectrometric measurement of the intrabacterial Na+,K+-ratios and of the mass fingerprint spectra of limited numbers of individual M. leprae organisms and a bioluminescence assay for determining the average ATP content from at least 106 bacteria to monitor the in vivo antilepfosy effectiveness of ofloxacin. Our study included 17 multibacillary leprosy patients who were treated for 3 months with 200 mg ofloxacin twice daily. The bacterial samples isolated from two patients did not show changes in the mass spectrometrically determined intrabacterial cation ratios or in the ATP content and, therefore, were classified as nonresponder cases (The Table, Fig. 1). In three additional cases the isolated M. leprae organisms did not show a drug-induced increase of the Na+,K+-ratios but did show a decrease of intrabacterial ATP which was, however, less pronounced than in typical responder cases (Fig. 3). Therefore, these cases were suspected to be nonresponders.
The evaluation of the mass fingerprint spectra of these three suspected nonresponder cases in a two-group discriminant analysis classified them-as it classified the two definite nonresponder cases-as unimpaired (Fig. 3). The clinical photographs give further evidence supporting this classification. Thus, the skin lesions of a patient (Fig. 4a) clearly defined as a responder case resolved nearly completely (Fig. 4b); whereas in the case of a suspected nonresponse (Fig. 4c), the fading was rather marginal (Fig. 4d). It should be considered, however, that the fading of skin lesions is only vague evidence for drug effectiveness, particularly within an observation period of only 3 months, and that not in all cases, including the responders, was such an apparent correlation possible.
Also, the bacterial index (BI) and the morphological index (MI) provide no further helpful information. As expected, the BI remained, on the average, nearly unchanged during the observation period. The MI already had declined drastically during the first month of treatment. However, Mis of 0 were also determined before the beginning of treatment and for the nonresponder cases after 3 months of treatment (The Table). This is not surprising considering published data(1, 13, 22 ) stating that non-solid organisms are also viable. Very recently, we provided proof for this by a direct correlation between the morphology of individual organisms and their physiological state as deduced from their intrabacterial Na+,K+ratios (data not shown).
At present we have no conclusive explanation for the discrepancy in the number of nonresponder cases as obtained from the cation measurements and the ATP assay, respectively. For this, it would have been favorable to keep the three suspected nonresponder cases under ofloxacin monotherapy for a longer time. However, for ethical reasons after 3 months treatment was continued with MDT for all patients.
In the responder cases the ofloxacin treatment led to a significant impairment of the M. leprae organisms in 12 of these patients as was deduced from the decrease of the intrabacterial ATP content and the increase of the Na+,K+-ratio and depended on the duration of treatment (Figs. 1 and 2).
An estimation of the percentage of viable M. leprae organisms in a skin biopsy on the basis of a limiting value of the intrabacterial cation ratio up to which the organisms are viable (able to multiply)-determined for cultivable mycobacteria-resulted in a mean value for untreated M. leprae of 58%. For the responder cases this value was reduced after 3 months of treatment to 16%, corresponding to a killing rate of 72%. The respective data after 1 month of treatment are less favorable (48% viable, 17% killing).
The percentage of viable bacteria in untreated biopsies determined from the Na+,K+-ratios is by at least 1 order of magnitude higher than the values published for the mouse foot pad test (6, 28). Thus, for example, Grosset, et al. (8) state a mean value of less than 1% in their ofloxacin study at day 0 of treatment. If only 1% (3 bacteria viable out of 300) were viable at the beginning of treatment, the considerable changes in the distributions of the intrabacterial Na+,K+-ratio obtained from the analysis of only 300 organisms would not be understandable. A possible explanation for the low value deduced from the mouse foot pad test could be adaptation problems of the M. leprae organisms when transferred to the foot pads, leading to an underestimation of the percentage of viable organisms. The obvious discrepancy between the two methods will be further investigated in collaboration with Dr. Louis Levy (Jerusalem, Israel) by comparing the most probable number of variables derived from mouse foot pad tests with the cation ratios obtained from the same bacterial populations.
A similar explanation as above for the discrepancy in the percentage of viable organisms may also hold for that in the killing rates: whereas Grosset, et al. found a reduction in the percentage of viable organisms during the first 28 days of ofloxacin treatment of 99.9%, we determined a killing rate of only 17% within the same period. Even after 3 months of treatment only 72% of the bacteria viable at day 0 were killed. For us, the lower killing rate seems rather reasonable considering the low metabolic activity and the long generation time of M. leprae during exponential growth in the mouse (15). In particular, for a drug acting as an inhibitor of DNA replication, like ofloxacin, this characteristic precludes a fast kinetic rate of impairment.
The mode of action of ofloxacin can also explain the occurrence of nonresponder cases, assuming that in these cases the bacterial organisms do not proliferate during drug exposure. Also, Tsukamura and coworkers have, in their clinical trial with tuberculosis patients, stated the occurrence of a significant number of resistant cases (26) which would be included in our study under the term "nonresponder." The occurrence of a secondary resistance in a significant number of patients after only 3 months of treatment seems rather unlikely.
Further clinical trials seem to be necessary with ofloxacin to assess the optimal daily dose, the duration of treatment, and the combined action and possible side effects in a MDT regimen. Also, the influence of ofloxacin treatment on the occurrence of type 1 and type 2 reactions has to be studied.
Acknowledgment. We thank Mrs. G. Nargorny for her skillful technical assistance. We gratefully acknowledge the financial support by the German Leprosy Relief Association (GLRA) and the Federal Minister of Research and Technology (grant No. 03 8667/4). Hoechst AG (Frankfurt/Main, Germany) supplied us with the drugs; Armauer Hansen Research Institute (AHRI), Addis Ababa, Ethopia, assisted with supply of dry' ice and facilities for deep freezing. We thank the ALERT/AHRI Research Committee for allowing us to do this research.
REFERENCES
1. DESIKAN, K. V. Correlation of morphology with viability of Mycobacterium leprae. Int. J. Lepr. 48(1976)391-397.
2. DHOPLE, A. M. Adenosine triphosphate content of Mycobacterium leprae from leprosy patients. Int. J. Lepr. 52(1984)183-188.
3. DHOPLE, A. M. and HANKS, J. H. Quantitative extraction of adenosine triphosphate from cultivable and host-grown microbes: calculations of adenosine triphosphate pools. Appl. Microbiol. 26(1973)399-403.
4. DHOPLE, A. M. and STORRS, E. E. Adenosinetriphosphate content of M. leprae: effect of purifi cation procedures. Int. J. Lepr. 50(1982)83-89.
5. FREERKSEN, E. and ROSENFELD, M. Leprosy eradication project of Malta. Chemotherapy 23(1977)356-386.
6. GROSSET, J. H. Recent developments in the field of multidrug therapy and future research in chemotherapy of leprosy. Lepr. Rev. 57(1986)223-234.
7. GROSSET, J. H., GUELPA-LAURAS, C.-C, PERANI, E. G. and BEOLETTO, C. Activity of ofloxacin against Mycobacterium leprae in the mouse. Int. J. Lepr. 56(1988)259-264.
8. GROSSET, J. H., JI, B., GUELPA-LAURAS, C.-C, PERANI, G. and N'DELI, C. N. Clinical trial of pefloxacin and ofloxacin in the treatment of lepromatous leprosy. Int. J. Lepr. 58(1990)259-264.
9. HAAS, M., LINDNER, B., DIETZ, M., TEBEBE, Y. B. and SEYDEL, U . Determination of in vivo and in vitro drug effects on mycobacteria from the mass spectrometric analysis of single organisms. Int. J. Lepr. 59(1991)262-270.
10. HANKS, J. H., CHATTERJEE, B. R. and LECHAT, M. F. A guide to the counting of mycobacteria in clinical and experimental materials. Int. J. Lepr. 32(1964)156-167.
11. HEINEN, H. J., HlLLENKAMP, F., KAUFMANN, R., SCHRODER, W . and WECHSUNG, R. LAMMA: a new laser microprobe mass analyzer for biomedicine and material analysis. In: Recent Developments in Mass Spectrometry in Biochemistry and Medicine, Vol. 6. Frigerio, A. and McCamish, M., eds. Amsterdam: Elsevier Science Publishers, 1980, pp. 435-459.
12. JI, B. Drug resistance in leprosy-a review. Lepr.Rev. 56(1985)265-278.
13. KATOCH, V. M., KATOCH, K., RAMU, G., SHARMA, V. D., DATTA, A. K. and SHIVANNAVAR, C. T. In vitro methods for determination of viability of mycobacteria: comparison of ATP content, morphological index and FDA-EB fluorescent staining in Mycobacterium leprae. Lepr. Rev. 59(1988)137-143.
14. KUBICA, G. P., DYE, W. E., COHN, M. L. and MIDDLEBROOK, G. Sputum digestion and decontamination with N-acetyl-L-cysteine-sodium hydroxide for culture of mycobacteria. Am. Rev. Respir. Dis. 87 (1963) 775-779.
15. LEVY, L. Studies of the mouse footpad technique for cultivation of Mycobacterium leprae. 3. Doubling time during logarithmic multiplication. Lepr. Rev. 47(1976)103-106.
16. LINDNER, B. and SEYDEL, U . Mass spectrometric analysis of drug-induced changes in Na+ and K+ contents of single bacterial cells. J. Gen. Microbiol. 129(1983)51-55.
17. LINDNER, B. and SEYDEL, U . Results on taxonomy and physiological state of bacteria derived from laser-induced single cell mass analysis. J. Phys. Colloq. (France) 45C-21984)565-568.
18. LINDNER, B. and SEYDEL, U . Pattern recognition as a complementary tool for the evaluation of complex LAMMA data. Microbeam Analysis 1989.
19. PATTYN, S. R. Activity of ofloxacin and Pefloxacin against Mycobacterium leprae in mice. (Letter) Antimicrob. Agents Chemother. 31(1987)671-673.
20. PEARSON, J. M. H. The problem of dapsone-resistant leprosy. Int. J. Lepr. 49(1981)417-420.
21. SAITO, H., TOMIOKA, H. and NAGASHIMA, K. In vitro and in vivo activities of ofloxacin against Mycobacterium leprae infection induced in mice. Int. J. Lepr. 54(1986)560-562.
22. SATHISH, M., PRASAD, H. K., MITTAL, A. and NATH, I. Lack of correlation between morphological index and viability as assessed by the uptake of3H-thymidine by macrophage resident M. leprae. Lepr. India 54(1982)420-427.
23. SEYDEL, U . and LINDNER, B. Single bacterial cell mass analysis: a rapid test method in leprosy therapy control. Lepr. Rev. 57(1986)163-170.
24. SEYDEL, U., LINDNER, B. and DHOPLE, A. M. Results from cation and mass fingerprint analysis of single cells and from ATP measurements of M. leprae for drug sensitivity testing: a comparison. Int. J. Lepr. 53(1985)365-372.
25. SMITH, J. T. Awakening the slumbering potential of 4-quinolone antibacterials. Pharmaceut. J. 233(1984)299-305.
26. TSUKAMURA, M. In vitro antimycobacterial activity of a new antibacterial sustance DL-8280-differentiation between some species of mycobacteria and related organisms by the DL-8280 susceptibility test. Microbiol. Immunol. 27(1983)1129-1132.
27. TSUKAMURA, M., NAKAMURA, E., YOSHII, S. and AMANO, H. Therapeutic effect of a new antibacterial substance ofloxacin (DL8280) on pulmonary tuberculosis. Am. Rev. Respir. Dis. 131(1985)352-356.
28. WELCH, T. M., GELBER, R. H., MURRAY, L. P., NG, H., O'NEILL, S. M. and LEVY, L. Viability of Mycobacterium leprae after multiplication in mice. Infect. Immun. 30(1980)325-328.
29. WORLD HEALTH ORGANIZATION. Chemotherapy of leprosy for control programmes. Geneva: World Health Organization, 1982. Tech. Rep. Ser. 675.
30. WORLD HEALTH ORGANIZATION. Report of the fourth meeting of the Ad Hoc Drug Development Subgroup of the Scientific Working Group on the Chemotherapy of Leprosy, Geneva, 10-11 October 1983. Geneva: World Health Organization, 1983. TDR/THELEP/Drug. Dev./83.3.
31. WORLD HEALTH ORGANIZATION. A Guide to Leprosy Control. 2nd ed. Geneva: World Health Organization, 1988.
1. M.D., Ph.D., All-Africa Leprosy & Rehabilitation Training Center (ALERT), P.O. Box 165, Addis Ababa, Ethiopia.
2. M.D., D.D.V., Ph.D., All-Africa Leprosy & Rehabilitation Training Center (ALERT), P.O. Box 165, Addis Ababa, Ethiopia.
3. Dipl.Biol., Professor, Div. of Biophysics, Forschungsinstitut Borstel, D-2061 Borstel, Germany.
4. Ph.D., Professor, Div. of Biophysics, Forschungsinstitut Borstel, D-2061 Borstel, Germany.
5. Ph.D., Professor, Div. of Biophysics, Forschungsinstitut Borstel, D-2061 Borstel, Germany.
6. Ph.D., Professor, Department of Biological Sciences, Florida Institute of Technology, Melbourne, Florida 32901-6988, U.S.A.
Reprint requests to Professor U. Seydel.
Received for publication on 15 March 1991
Accepted for publication in revised form on 16 July 1991.