- Volume 59 , Number 4
- Page: 558–68
Experience with WHO-recommended multidrug therapy (MDT) for multibacillary (MB) leprosy patients in the leprosy control program of the All Africa Leprosy and Rehabilitation Training Center in Ethiopia: appraisal of the recommended duration of MDT for MB patients
ABSTRACT
During 1981 a World Health Organization Study Group recommended that multibacillary (MB) leprosy patients should be given multidrug therapy (MDT) for at least 2 years and, wherever possible, until skin-smear negativity. This paper reports on the experience with MDT for MB patients under routine field conditions in the leprosy control program of the All Africa Leprosy and Rehabilitation Training Center (ALERT) in the Shoa Region of Ethiopia. The period of MDT to reach skin-smear negativity was evaluated for 348 new MB patients. Only 31.6% of these patients could be released after 26 four-weekly doses of MDT, and 19.8% needed over 5 years of MDT. The average period of MDT to reach skin-smear negativity was estimated at about 4 years. Of 3343 patients of cohorts which, almost exclusively, consisted of patients treated with dapsone before MDT, 72.8% were released after 26 four-weekly doses of MDT; whereas of 712 patients of cohorts which mainly included new patients, only 23.5% were released. It was estimated that if MDT would be stopped, regardless of skin-smear results, after 26 four-weekly doses of the drugs collected within a period of 3 years, about 80% of the patients would complete treatment. The operational problems with continuation of MDT until skin-smear negativity are discussed.Although as yet it has not been proven by study results that after 2 years of MDT the relapse rate will be low, the available knowledge indicates that this is likely to be the case. Based on a) probability, b) the finding that 2 years of MDT can be maintained in the majority of the patients, and c) the operational difficulties with the continuation of MDT until skin-smear negativity, it is recommended that MDT should be limited to 2 years. MDT of limited and fixed duration will facilitate the implementation and expansion of the treatment in parts of the world where most patients are not yet benefitting from this treatment.
RÉSUMÉ
Un groupe d'etude de l'Organisation Mondiale de la Santé recommanda en 1981 que les patients atteints de lèpre multibacillaire (MB) soient traités par polychimiothérapie (PCT) pour au moins 2 ans et, si possible, jusqu'à nègativation des frottis cutanés. Cet article rapporte l'expérience de PCT administrée aux patients MB dans les conditions habituelles de terrain à 1"'A11 Africa Leprosy and Rehabilitation Training Center" (ALERT) dans la Région de Shoa en Ethiopie. La durée de la PCT nécessaire pour obtenir la nègativation des frottis cutanés a été évaluée chez 348 nouveaux patients MB. Seulement 31, 6% de ces patients pouvaient arrêter leur traitement après 26 doses administrées à intervalle de quatre semaines, et 19, 8% avaient besoin de plus de 5 ans de PCT. La durée moyenne de PCT nécessaire pour obtenir la nègativation des frottis cutanés a été estimée à environ 4 ans. Parmi une cohorte de 3343 patients qui, pratiquement tous, avaient été traités à la dapsone avant de recevoir la PCT, 72, 8% ont été libérés après 26 doses de PCT administrées à quatre semaines d'intervalle, tandis que seulement, 23, 5% d'une cohorte de 712 patients comprenant principalement des nouveaux cas, ont pu stopper leur traitement. On a estimé que si la PCT était arrêtée indépendamment des frottis cutanés, après 26 doses mensuelles administrées en 3 ans, environ 80% des patients termineraient leur traitement. Les problèmes opérationnels de la continuation de la PCT jusqu'à la nègativation des frottis cutanés sont discutés.Bien qu'il n'ait pas encore été prouvé par les résultats de cette étude que le taux de rechute serait faible après 2 ans de PCT, les informations disponibles indiquent que ceci est vraisemblable. Sur la base a) de la probabilité, b) de l'observation qu'une PCT de 2 ans puet être administrée à la majorité des patients, et c) des problèmes opérationnels que pose la poursuite de la PCT jusqu'à la nègativation des frottis cutanés, il est recommandé que la PCT soit limitée à 2 ans. Une PCT de durée limitée et constante facilitera l'application et la dissémination du traitement dans les régions du monde où la majorité des patients n'en profitent pas encore.
RESUMEN
En 1981, un comité de la Organización Mundial de la Salud recomendó que los pacientes con lepra multibacilar (MB) deberían ser tratados con una terapia a base de múltiples drogas (TMD) cuando menos durante 2 años y, siempre que fuera posible, hasta la negatividad bacilar en los extendidos de linfa cutánea. Este trabajo se refiere a la experiencia, bajo condiciones rutinarias de campo, con la TMD administrada a pacientes MB dentro del programa de control contra la lepra del All Africa Leprosy and Rehabilitation Training Center (ALERT) en la región Shoa de Etiopía. Se evaluó el periodo de TMD requerido para alcanzar la negatividad bacilar en piel en 348 casos nuevos de lepra MB. Mientras que sólo el 31.6% de estos pacientes pudo ser liberado después de 26 dosis mensuales de TMD, el 19.8% de los mismos necesitó más de 5 años de tratamiento. Se calculó que el tiempo promedio de la TMD requerido para alcanzar la negatividad bacteriana en piel fue de aproximadamente 4 años. De 3343 pacientes tratados con dapsona antes de la TMD, el 72.8% fueron liberados después de 26 dosis mensuales de TMD, mientras que de 712 pacientes nuevos, sólo el 23.5% fueron liberados después de esc periodo de tratamiento. Se calculó que si la TMD se hubiera suspendido, independientemente del Índice bacteriano en piel, después de 26 dosis mensuales recogidas dentro de un periodo de 3 años, aproximadamente el 80% de los pacientes hubieran completado su tratamiento. Se discuten los problemas operacionales con la continuación de la TMD hasta alcanzar la negatividad bacteriana en piel.Aunque todavía no hay estudios que demuestren que después de 2 años de TMD la frecuencia de racaídas sería baja, la información que se tiene hasta el momento indica que éste es probablemente el caso. Con base en (a) la probabilidad, (b) el hallazgo de que la TMD durante 2 años puede ser mantenida en la mayoría de los pacientes, y (c) las dificultades operacionales para continuar la TMD hasta alcanzar la negatividad en piel, se recomienda que la TMD se limite a 2 años. La TMD de duración limitada y fija, facilitaría la implementación y la expansión del tratamiento en las regiones del mundo donde la mayoría de los pacientes todavía no se beneficia con este tratamiento.
During 1981 a World Health Organization (WHO) Study Group recommended that leprosy patients should be treated with multidrug therapy (MDT) (27). It was advised that multibacillary (MB) patients should be treated with MDT, including three drugs with a monthly supervised component, for at least 2 years and that the treatment should be continued, wherever possible, up to skin-smear negativity. The 2 years of MDT should be taken within a period of maximally 3 years. During 1988, it was recommended that at least 8 months of therapy have to be taken during each 12month period (30). Since the WHO Study Group made its recommendation there has been concern about the operational aspects of the implementation of MDT. Operational problems have been identified as one of the reasons for the slow pace in implementing MDT in several parts of the world (31-36).
A major operational problem of MDT for MB patients relates to the recommended duration of treatment (3). It is common experience that the decline in the bacterial index (BI) is not faster with MDT than with dapsone monotherapy, in the order of 0.5 to 1 point on the logarithmic scale per year(9, 12, 25, 32) Hence, continuation of MDT until skin-smear negativity implies that a proportion of the patients have to be treated for more than 2 years and part of them for more than 5 years.
This paper reports on the experience with MDT for MB patients in the leprosy control program of the All Africa Leprosy and Rehabilitation Training Center (ALERT) in the Shoa Region in Ethiopia. Data on the duration of MDT to reach skin-smear negativity and on the compliance of patients with clinic attendance are presented. Operational problems and implications of continuation of MDT until skin-smear negativity are discussed. Based on the observations in the ALERT leprosy control program, reports from other leprosy control programs, and theoretical and practical considerations of the effectiveness of MDT, an appraisal of the recommended duration of MDT for MB patients is made.
MATERIALS AND METHODS
The ALERT leprosy control program is responsible for leprosy control in the Shoa Region which is centrally located in Ethiopia. The region is divided into 11 urban districts and Addis Ababa. It covers about 85,000 sq. km., with an estimated (1989) population of 11 million. Since January 1983 MDT has been gradually introduced and by January 1988 MDT had been implemented in the whole control area. Prior to introduction of MDT the registered patients were clinically and bacteriologically examined. Patients classified as "B," according to the Madrid classification, were reclassified as either borderline tuberculoid (BT) or borderline lepromatous (BL) leprosy. In case of doubt, patients were considered BL. In two rural districts where MDT was introduced before a detailed manual on the implementation of MDT (') was written, all patients on dapsone were put on MDT. In the other districts, the release of patients on dapsone was carried out prior to implementation of MDT. MB patients who were clinically inactive, had negative skin smears, and had attended regularly for at least 10 years or for 15 years or more, regardless of the regularity of attendance, were released from dapsone monotherapy. Patients who did not fulfill the criteria for release from dapsone were given MDT.
MB patients are patients who are clinically BL [including the few midborderline (BB)] or lepromatous (LL) leprosy patients who had a BI of 2 or more in at last one site. Since January 1990, the recommendation (29) of a WHO Expert Committee on Leprosy of 1988 was introduced to include the MB category, for the purpose of MDT, indeterminate, TT and BT patients showing skin-smear positivity. Some supervisors had already introduced this policy earlier.
The MDT regimen recommended by the WHO in 1981 is: one monthly supervised dose of 600 mg rifampin, one monthly supervised dose of 300 mg clofazimine, and daily 100 mg dapsone and 50 mg clofazimine self-administered (27). For patients younger than 15 years of age the dosages of the drugs are adapted according to age.
The minimal duration of treatment is 24 months. Because treatment is given at four weekly intervals, the minimal number of doses of MDT is 26. The 26 doses have to be taken within a period of 3 years. Clinical and bacteriological examination is done annually. MDT is stopped if the Bis of two consecutive sets of four skin smears, which should be taken within a period of 2-6 months, are negative. Prior to release from MDT, the supervisors examine the patients and record the findings on the Patient Record Card. Patients whose skin smears are positive after the 26 doses of MDT should continue the treatment until skin-smear negativity. Clinical and bacteriological examination is routinely done once per year until the patients can be released from the treatment.
Patients who fail to collect the 26 doses of MDT within 3 years, and who had negative skin smears, either prior to the start of MDT or during the annual review examination, have their treatment discontinued. These patients are recorded as "Treatment not Completed" (TnC).
Patients who fail to collect the 26 doses of MDT within 3 years, and whose last skin smears were positive should be traced and persuaded to attend regularly for their treatment. Those patients who continue to attend irregularly after the 3 years and for whom MDT cannot be terminated because of positive skin-smear results may, nevertheless, have their treatment stopped if they are either absent for 6 consecutive months or more or collected less than six doses of MDT during one calendar year.
Patients who leave the control area without a transfer letter or who refuse to continue antileprosy treatment are considered out of control (OC). In practice, patients who do not attend for treatment during 6 consecutive months and for whom tracing either cannot be done or has not been successful are considered OC.
At the time the skin smears are negative and, hence, MDT can be stopped, the decision as to whether or not the patient successfully completed MDT is based on the attendance of the patient during the whole treatment period. Patients who attend two thirds or more of the time they could have attended for treatment are recorded as "Release from treatment" (RFT). Those who attended less than two thirds of the time are recorded as TnC.
The monthly doses of rifampin and clofazimine are swallowed under strict supervision by the leprosy control field staff. Since 1987 blister packs of the drugs are, for a maximum period of 3 months, given to patients who are unable to attend a clinic, e.g., during the rainy season.
The period of treatment to reach skinsmear negativity was analyzed for new patients who started MDT in the two districts where MDT was introduced during 1983. All clinically and bacteriologically confirmed MB patients who had attended regularly, whose skin smears had been examined annually, and who by January 1990 either had completed MDT or had received at least 5 years of MDT are included in the analysis.
The results of completion of MDT are monitored for 6-month cohorts of patients. Because the 26 doses of the drugs have to be collected within a period of 3 years, the cohort reports are prepared 42 months after the start of a cohort. Findings at 3 years after the start of treatment are presented for all cohorts of patients who started treatment during the period January 1983 to December 1986. The results of cohorts which include almost exclusively patients who had been treated with dapsone before MDT, referred to as "first cohorts," are compared with the results of cohorts which consist mostly of new patients, referred to as "final cohorts." Estimates were made about the percentages of patients who would have completed MDT if this had been based on the collection of 26 four-weekly doses of the drugs within 3 years, regardless of the results of skin-smear examinations.
For patients who had to continue treatment after the initial 3 years, the results of completion of MDT should be reported 1 year later. Subsequently, reports have to be prepared each year for patients who-had to continue treatment. These reports have only been available since 1988, when the results of MDT of individual patients were computerized and the supervisors were given lists of patients for whom a follow-up report had to be prepared. For cohorts of patients who started MDT during the period January 1984 to July 1985 the results of completion of MDT at 3 years and at 4 years after the start of treatment are presented.
Reports on the regularity of attendance by MB patients were introduced in 1987. The regularity of clinic attendance is evaluated 24 months after the start of a 6-month cohort, which is 18 months after the intake of the last patient. A patient is considered a regular attender if he/she had collected at least two thirds of the doses of the drugs. The regularity of clinic attendance is presented for patients who started MDT during the period January 1985 to July 1986.
RESULTS
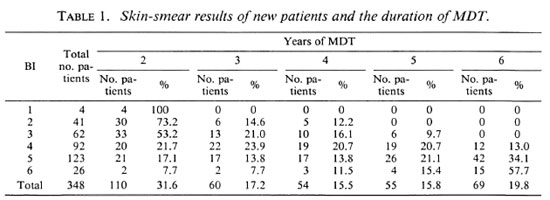
The duration of MDT for 348 new patients is presented in Table 1. The BI is the highest BI of the four sites from which skin smears are routinely examined; two earlobes and two lesions. One-hundred-seven patients (30.7%) had a BI of up to 3 and 241 patients (69.3%) had a BI of 4 or above. While for none of the patients with an initial BI of up to 3 did the period of MDT exceed 5 years, 13.0%, 34.1%, and 57.7% of the patients with Bis of 4, 5, and 6, respectively, still had positive skin smears after 5 years of MDT. By February 1990, when the analysis was made, only 6 of the 69 patients who had to continue MDT after 5 years had been treated long enough to reach skin-smear negativity. These six patients had become skin-smear negative after 6 years of MDT. The period of treatment for the remaining 63 patients was not yet known. If it is assumed that none of the patients will still be skin-smear positive after 10 years of MDT, and that after each additional year of MDT an equal proportion of the 69 patients, i.e., 20%, will have reached skin-smear negativity, the average treatment period of these 348 patients will be almost 4 years.
Of 627 clinically and bacteriologically confirmed MB patients who were diagnosed in the whole control area during the period July 1987 to January 1990, 174 patients (27.8%) had a BI of up to 3 and 453 (72.2%) a BI of 4 or above. This distribution of smear results does not differ significantly from that among the 348 patients (χ2 = 0.84, p = 0.4). Hence, comparable results with respect to the duration of MDT to reach skin-smear negativity can be expected in the other areas of the control program.
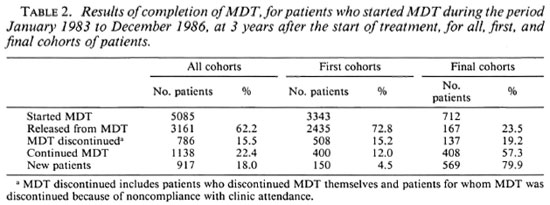
The results of completion of MDT 3 years after the start of the treatment for all, first, and final cohorts of patients are presented in Table 2. Of all cohorts, which include 18.0% new patients, 62.2% of the patients were released from MDT and 22.4% continued the treatment. Of the first cohorts, with only 4.5% new patients, 72.8% of the patients were released from MDT and 12.0% continued the treatment. This is quite different for the final cohorts which include 79.9% new patients. Of these cohorts only 23.5% of the patients were released from MDT and 57.3% of the patients continued the treatment.
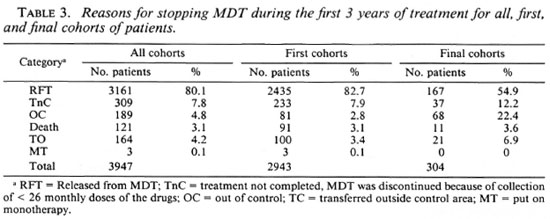
In Table 3 the reasons for stopping MDT during the first 3 years after the start of treatment are presented for the different cohorts of patients. While release from MDT is observed in 82.7% of the first cohorts, this was only the case in 54.9% of the patients of the final cohorts. This is a very significant difference (χ2 = 132, p < 0.001). Compared with the first cohorts, there are significantly more patients in the final cohorts who had their treatment stopped because of noncompliance with clinic attendance (χ2 = 5.99, p = 0.01), who were out of control (χ2 = 238, p < 0.001), and who were transferred to outside the control area (χ2 = 8.50, p = 0.004). This could mean that discontinuation of MDT is more of a problem among new patients than among those who have already been treated with dapsone before MDT. Although this possibility could not completely be ruled out, the drought and famine which had affected large parts of the area where about 90% of the patients of the final cohorts resided was identified as the most probable reason for this difference.
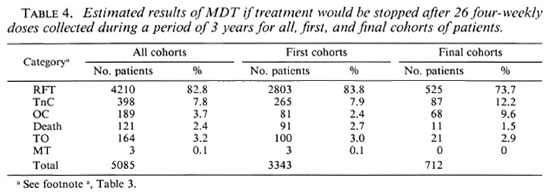
Estimates of the percentages of patients who would have completed treatment if this had been based on the collection of 26 doses of the drugs within 3 years are given in Table 4. These estimates are based on the assumption that the percentage of patients who failed to collect 26 doses of the drugs within 3 years is the same for patients whose MDT was discontinued after 3 years as for those who, because of positive skin smears, had to continue treatment. Of the 3947 patients of all cohorts, MDT was discontinued in 309 patients (7.8%) because they did not collect the 26 doses of MDT within 3'years (Table 3). In a sample of 224 patients who, because of positive skin smears, had to continue MDT after 3 years, it was observed that 16 patients (7.1%) had collected less than 26 doses of MDT within 3 years. The difference between these findings is not statistically significant (χ2 = 0.06, p = 0.8). Therefore, the above assumption appears justified. If MDT would have been stopped after 26 doses of the drugs collected within 3 years, 82.8% of all patients, 83.8% of the patients of the first cohorts, and 73.7% of the patients of the final cohorts would have completed treatment.
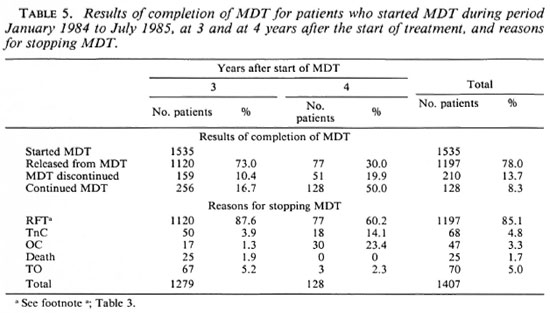
The results of completion of MDT for 1535 patients, at 3 and at 4 years after the start of the treatment, are given in Table 5. About 95% of these patients had already been treated with dapsone before MDT; 256 patients (16.7%) continued MDT after 3 years and 128 of these 256 patients (50%) or 8.3% of the initial 1535 patients continued the treatment after 4 years. In addition to the 1120 patients who were released from MDT during the first 3 years, 77 patients were released after 1 additional year of MDT, bringing the total number of released patients to 1197 or 78.0% of the 1535 patients. Table 5 also gives a subdivision of the reasons for discontinuing MDT during the first 3 years and during the fourth year after the start of treatment. During the first 3 years of MDT, release from treatment was observed in 87.6% of the patients. This was the case in only 60.2% of the patients during the fourth year of treatment. This is a very significant difference (χ2 = 66.7, p < 0.001). The percentage of patients who had their treatment discontinued because of noncompliance with clinic attendance (TnC) was significantly higher during the fourth year than during the first 3 years of MDT (χ2 = 23.9, p < 0.001). Because most of the patients recorded as TnC at the end of the fourth year were already irregular in their attendance during the first 3 years, but had to continue MDT because of positive skin smears, no definite conclusions from this finding should be drawn.
The percentage of patients who became out of control was very significantly higher during the fourth year than during the first 3 years after the start of MDT (χ2 = 169, p < 0.001). By early 1990, follow-up reports on the completion of MDT during the fifth and subsequent years after the start of MDT were not yet available. From treatment registers, it was observed that during the fifth and sixth year of MDT a substantial proportion of the patients became out of control. Of 64 patients who continued MDT after 4 years, 19 (29.7%) became out of control during the fifth year and of 38 patients who continued MDT after 5 years, 11 (31.6%) became out of control during the sixth year.
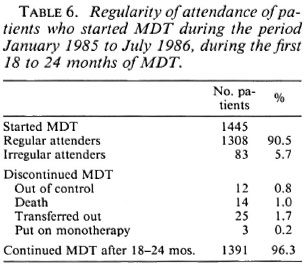
The regularity of attendance during the first 18 to 24 months of MDT is presented in Table 6; 90.5% of the patients had attended regularly, 5.7% attended irregularly, and 3.8% of the patients discontinued the treatment.
DISCUSSION
A major advantage of MDT over dapsone monotherapy is the limited duration of the treatment. However, if skin-smear negativity is chosen as the end point of treatment, many patients have to continue MDT after completion of 26 four-weekly doses of the drugs. In particular, a substantial proportion of patients who were not treated with dapsone before MDT need more than 26 doses of MDT. These are especially those with a BI of 4 or above.
Observations on the duration of MDT similar to those presented in this paper have been reported by others. In Karigiri, India, it was observed that only 38.4% of smear-positive patients had become negative at the end of the second year of the treatment (32). Chattopadhyay, et al. reported that 25.8% of the patients had become skin-smear negative after 24 months of MDT (4). Ganapati, et al. found that 27.7% of their patients with an average BI of 4 and above at the start of MDT were still skin-smear positive after 60 monthly doses of MDT (5). In the material presented here, 28.6% of the patients with a BI of 4 or above still had positive skin smears after 5 years of MDT (Table 1).
The estimated completion of MDT by 82.8% of all patients and 83.8% of first-cohort patients, if the treatment would be stopped after collection of 26 four-weekly doses of the drugs within 3 years (Table 4), regardless of skin-smear results, would be satisfactory results for a field program. The probability that these results can be obtained is supported by the high percentage of patients (90.5%) who attended regularly during the first 18 to 24 months (Table 6). Also, considering the very difficult circumstances of the patients, the 73.7% completion of 26 doses of MDT within 3 years for the final-cohort patients is a satisfactory result. These data show that in the majority of patients regular treatment can be maintained for 2-3 years even under adverse conditions. Others also reported a high percentage of regularity of attendance, 90% or more, during the first years of the treatment (24,32).
Continuation of MDT until skin-smear negativity presents, however, several operational problems:
1. With the extension of the treatment period the compliance of the patients with clinic attendance decreases. This was illustrated by the significantly higher percentage of patients becoming out of control during the fourth year after the start of MDT as compared with the first 3 years (Table 5). Although follow-up reports on completion of MDT during the fifth and subsequent years after the start of treatment were not yet available, observations from treatment registers indicate that also during the fifth and sixth year after the start of MDT a substantial proportion of the patients become out of control. The effect of this on cohorts which mainly include patients treated with dapsone before MDT will be very limited, because 72.8% of the patients of the first cohorts could already be released from treatment after 26 doses of MDT (Table 2). If only 50% of the patients who had to continue the treatment after 26 doses would comply with clinic attendance until skin-smear negativity, almost 80% of the patients (72.8% + ½ x 12.0%) will complete the treatment. However, a similar result among cohorts of new patients will substantially decrease the percentage of patients who will complete the course of MDT. With 23.5% of patients of the final cohorts who were released after 26 doses of MDT and 57.3% of the patients who had to continue the treatment (Table 2), this will result in just over 50% of the patients (23.5% + ½ x 57.3%) completing the prescribed course of MDT. Because patients who fail to attend for their treatment have to be traced, this puts a burden on the services.
2. Monitoring the completion of MDT will be very difficult. The proportion of MB patients on regular MDT during a year is recommended as an operational indicator for monitoring case management(28, 30). However, in the control of infectious diseases it is important to monitor what proportion of the patients who started treatment completed it. This is usually done through an analysis of completion of treatment in cohorts of patients. Because cohorts of patients have to be followed for several years, the open-ended treatment period makes monitoring of the proportion of the patients who completed MDT out of those who started the treatment very difficult. It was the experience in the ALERT leprosy control program that monitoring of MDT completion at 36 months after the start of treatment can, after some training, be done reliably by the leprosy control field staff. Follow-up reports were usually incomplete and could only be prepared after the staff were given lists of patients for whom a report was required.
3. Rather complicated instructions for the requirements of attendance by patients have to be defined and applied. The decision of whether or not a patient has successfully completed MDT should be based not only on the finding of negative skin smears, but also on the attendance for treatment. The requirement for attendance could either be the collection of the first 24 months of MDT within 3 years, regardless of the attendance thereafter, or at last two thirds attendance during the whole treatment period, or at least two thirds attendance during each year of the treatment period. How to register and report patients who did not fulfill the requirement of attendance should be specified. In order to prevent patients remaining on irregular treatment for several years, instruction for stopping the treatment should be defined.
4. A network of reliable skin-smear services is required. In order to determine the end-point of MDT, skin smears have to be examined at least annually after the first 24 months of the treatment. The quality of the skin-smear services is reported to be poor in many leprosy-endemic countries (7, 8, 18, 25, 36) Further, it is very questionable whether the results of smear examinations are an appropriate parameter for determining the end-point of the treatment. The recommendation to continue MDT until skin-smear negativity suggests a relationship between cure and skin-smear results. The assumption of such a relationship might have been correct when monotherapy with the mainly bacteriostatic drug dapsone was the only antileprosy treatment available for routine use. However, with the introduction of MDT, which includes the very powerful bactericidal drug rifampin, there is a dissociation between rapid killing of Mycobacterium leprae and the slow elimination of dead bacilli from the skin(15, 17).
The finding that the decline in the BI is not faster with MDT than with dapsone monotherapy indicates that this decrease is not related to the quality of chemotherapy(17). The observation by several investigators that the decline in the BI continues and ultimately reaches zero when MDT is stopped after 24 months and skin smears arc still positive (6,14-16) indicates that skin-smear results are not an appropriate parameter to decide when to stop MDT.
Ultimate proof for therapeutical efficacy of 2 years of MDT will be a low relapse rate among patients who have completed the treatment. A few studies have been undertaken in which the relapse rates after 2 years of MDT are being measured. So far the results of such studies are not available. One of these studies is being carried out at ALERT (2). Given a patient intake period of 5 years, which started during 1988, 2 years of MDT to be completed within 3 years and an anticipated period of 5 years of follow-up after release from MDT, the first results of this study will not be available until the late 1990s.
In the THELEP field studies which are carried out in South India, no relapses have occurred among 2241 MB patients during a mean period of follow-up of about 4 years (36). In these studies, new patients and patients treated with dapsone before MDT are included, while the treatment period was at least 2 years and until skin-smear negativity. Therefore, conclusions on the effectiveness of 2 years of MDT cannot be drawn from them.
At present there is no justification for reducing the period of treatment to less than 2 years. The benefit of the extension of MDT beyond 2 years may be the prevention of some relapses. How many is not yet known. The question is whether we should continue to treat patients until skin-smear negativity until results of studies have proved that 2 years of MDT will not give unacceptable relapse rates.
The 2 years of MDT were thought to be the minimum period of treatment to ensure the elimination of drug-resistant mutants, in particular rifampin-resistant mutants, and to reduce the number of drug-sensitive viable organisms to a low level which will not cause unacceptable relapse rates (17, 27). The available knowledge indicates that the 24 doses of rifampin are certainly sufficient to kill the rifampin-susceptible nonpersistent leprosy bacilli (12, 13, 21). In view of the bactericidal activity of single doses of rifampin (19, 20) it is likely that the entire population of these leprosy bacilli, including bacilli resistant to either dapsone or clofazimine, will be eliminated after a few doses of the drug. None of the available drugs, acting either singly or in combination, is likely to affect the persistent bacilli (17, 21-23 ). However, the results of studies suggest that persisting M. leprae, which are probably present in all MB patients, do not carry a high risk of relapses following release from chemotherapy (10, 17, 21, 26)
The elimination of the small population, probably not more than a few thousand, of rifampin-resistant nonpersisting organisms which are expected to be present at the start of treatment has to be done by the mainly bacteriostatic drugs dapsone and clofazimine. This elimination proceeds definitely more slowly than does the killing of rifampin-susceptible nonpersistent organisms and may be incomplete in poorly compliant patients (21). This population of bacilli is considered the most critical population in terms of requirements of chemotherapy (21).
Although it has not been proven yet that the relapse rate among MB patients treated with 2 years of MDT will be low, the available knowledge of the effectiveness of the drugs suggests that 2 years of MDT is sufficient to cure the majority of the patients. The experience in the ALERT leprosy control program shows that when MDT is limited to 26 four-weekly doses of MDT, which should be completed within 3 years, the majority of the patients will complete the treatment. Continuation of MDT until skin-smear negativity is operationally very difficult to maintain, even in a program with a well-established infrastructure. Although continuous progress had been made with the implementation of MDT, the coverage is still low in some parts of the world, notably on the African continent (36). Among the main reasons for the slow implementation of MDT in several African countries are serious operational problems (36). In order to facilitate the implementation and expansion of MDT and to make optimal use of the limited resources, the duration of MDT for MB cases should be fixed at 24 monthly (26 four-weekly) doses of the drugs which should be collected within a period of 3 years.
Acknowledgment. I wish to express my thanks to the ALERT leprosy control supervisors who recorded and reported the data, to Mr. W. 't Mannetje and Mr. Tesfaye Fekade who computerized part of the data, and to the ALERT/AHRI Research Committee for the approval of my study on the operational aspects of MDT. I also thank Prof. A. S. Muller, Prof. H. A. Valkenburg, and Dr. P. Feenstra for their comments and suggestions. This study was made possible through financial support by the Armauer Hansen Research Institute. This Institute is supported by the Norwegian and Swedish Agencies for International Development, NORAD and SIDA, respectively.
REFERENCES
1. ALL AFRICA LEPROSY AND REHABILITATION CENTER. ALERT Manual for the Implementation of Multiple Drug Therapy (MDT). 2nd rev. Addis Ababa: ALERT, 1987.
2. ALERT MDT FIELD EVALUATION STUDIES (AMFES). Research protocol, April 1988.
3. BECX-BLEUMINK, M. Operational aspects of multidrug therapy. Int. J. Lepr. 57(1989)540-551.
4. CHATTOPADHYAY, S. P., GUPTA, C. M., BHATE, R. D., BHATE, R. P. and SREEVATSA. Evaluation of two multidrug regimens in hospitalised multibacillary cases. Indian J. Lepr. 61(1989)96-205.
5. GANAPATI, R., REVANKAR, C. R. and PAI, R. R. Three years assessment of multidrug therapy in multibacillary leprosy cases. Indian J. Lepr. 59(1987)44-49.
6. GANAPATI, R., PAI, R., GANDEWAR, K. L. and THRESSIA, X. J. For how long should a multibacillary' leprosy patient be treated? Indian J. Lepr. 61(1989)467-471.
7. GEORGIEV, G. D. and MCDOUGALL, A. C. Skin smears and the bacterial index (BI) in multiple drug therapy leprosy control programs; an unsatisfactory and potentially hazardous state of affairs. (Utter) Int. J. Lepr. 56(1988)101-103.
8. GEORGIEV, G. D. and MCDOUGALL, A. C. A reappraisal of clinical and bacteriological criteria in the implementation of multidrug therapy for leprosy control programmes and proposals for their better use. Lepr. Rev. 61(1990)64-72.
9. GROSSET, J. Criteria for determining the exact end of MDT in leprosy. In: Criteria to Determine the Exact End of Multidrug Therapy in Leprosy: Workshop. Pritzc, S., ed. Wiirzburg: Armauer Hansen Institut, 1988, pp. 11-14.
10. JOPLING, W. H., RIDLEY, M. J., BONNICI, E. and DEPASQUALE, G. A. A follow-up investigation of the Malta-project. Lepr. Rev. 55(1984)247-253.
11. LECHAT, M. F., DECLERCQ, E. E., MISSON, C. B., PERANI, E. G. and N'DELI, L. N. Selection of MDT strategies through epidemiomctric modeling. Int. J. Lepr. 58(1990)296-301.
12. LEIKER, D. L. Duration of treatment in multibacillary leprosy with MDT regimens containing rifampicin. In: Criteria to Determine the Exact End of Multidrug Therapy in Leprosy; Workshop. Pritze, S., ed. Wiirzburg: Armauer Hansen Institut, 1988, pp. 15-19.
13. LEVY, L., SHEPARD, C. C. and FASAL, P. The bactericidal effect of rifampicin on M. leprae in man: a) single doses of 600, 900 and 1200 mg; and b) daily doses of 300 mg. Int. J. Lepr. 44(1976)183-187.
14. Li, H.-Y. Technical and operational problems in implementing multidrug therapy at different levels. Paper presented at WHO Consultation of Technical and Operational Aspects of Multidrug Therapy in Leprosy, Male, Maldives, 11-15 June 1990.
15. PATTYN, S. R., BOURLAND, J., GRILLONE, S.. GROENEN, G. and GHYS, P. Combined regimens of one year duration in the treatment of multibacillary leprosy -I. Combined regimens with rifampicin administered during one year. Lepr. Rev. 60(1989)1 18-117.
16. PATTYN, S. R., GROENEN, G., JANSSENS, L., DEVERCHIN, J . and GHYS, P. Combined regimens of one year duration in the treatment of multibacillary leprosy -II. Combined regimens with rifampicin administered during 6 months. Lepr. Rev. 60(1989)118-123.
17. PRITZE, S., ed. Criteria to Determine the Exact End of Multidrug Therapy in Leprosy; Workshop. Würzburg: Armauer Hansen Institut, 1988.
18. DERIJK, A.J., NILSSON, T. and CHONDE, M. Quality control in leprosy programmes: preliminary experience with inter-observer comparison in routine services. Lepr. Rev. 56(1985)177-191.
19. SHEPARD, C. C, LEVY, L. and FASAL, P. Rapid bactericidal effect of rifampicin on Mycobacterium leprae. Am. J . Trop. Med . Hyg. 21(1972)446-449.
20. SHEPARD, C. C, LEVY, L. and FASAL, P. Further experience with the rapid bactericidal effect on Mycobacterium leprae. Am. J. Trop. Med . Hyg. 23(1974)1120-1124.
21. SUBCOMMITTEE ON CLINICAL TRIALS OF THE CHEMOTHERAPY OF LEPROSY (THELEP) SCIENTIFIC WORKING GROUP OF THE UNDP/WORLD BANK/WHO SPECIAL PROGRAM ME FOR RESEARCH AND TRAINING IN TROPICAL DISEASES. Persisting Mycobacterium leprae among THELEP trial patients in Bamako and Chingleput. Lepr. Rev. 58(1987)325-337.
22. UNDP/WORLD BANK/WHO SPECIAL PROGRAMME FOR RESEARCH AND TRAINING IN TROPICAL DISEASES. Tropical Disease Research: a Global Partnership; Eighth Program Report. Maurice, J. and Pearce, A. M., eds. Geneva: World Health Organization, 1987.
23. UNDP/WORLD BANK/WHO SPECIAL PROGRAMME FOR RESEARCH AND TRAINING INTROPICAL DISEASES. Tropical Diseases; Progress in International Research, 1987-1988; Ninth Progress Report. Geneva: World Health Organization, 1989.
24. UNDP/WORLD BANK/WHO SPECIAL PROGRAMME FOR RESEARCH AND TRAINING IN TROPICAL DISEASES. Report ofthe Fifth Meeting of the Scientific Working Group on the Chemotherapy of Leprosy. Geneva: World Health Organization, 1986. TDR/THELEP-SWG(5)/86.3.
25. WATERS, M. F. R., RIDLEY, D. S. and RIDLEY, M. J. Clinical problems in the initiation and assessment of multidrug therapy. Lepr. Rev. 57 Suppl.3(1986)92-100.
26. WATERS, M. F. R., REES, R. J. W., LAING, A. B. G., FAH, K. F., MEADE , T. W., PARIKSHAK, N. and NORTH, W. R. S. The rate of relapse in lepromatous leprosy following completion of twenty years of supervised sulphonc therapy. Lepr. Rev. 57(1986)101-109.
27. WHO STUDY GROUP. Chemotherapy of Leprosy for Control Programmes. Geneva: World Health Organization, 1982. Tech. Rep. Ser. 675.
28. WHO STUDY GROUP. Epidemiology of Leprosy in Relation to Control. Geneva: World Health Organization, 1985. Tech. Rep. Ser. 716.
29. WHO EXPERT COMMITTEE ON LEPROSY. Sixth Report. Geneva: World Health Organization, 1988. Tech. Rep. Scr. 768.
30. WORLD HEALTH ORGANIZATION. A Guide to Leprosy Control. 2nd ed. Geneva: World Health Organization, 1988.
31. WORLD HEALTH ORGANIZATION. Report of a Coordinating Meeting on Implementation of Multidrug Therapy in Leprosy Control, New Delhi, 1984. WHO/LEP/84.1.
32. WORLD HEALTH ORGANIZATION. Report ofa Consultation on Implementation of Multidrug Therapy for Leprosy Control, Geneva, 1985. WHO/ LEP/85.1.
33. WORLD HEALTH ORGANIZATION. Report of the Second Coordinating Meeting on Implementation of Multidrug Therapy in Leprosy Control Programmes, Geneva, 1986. WHO/CDS/LEP/87.2.
34. WORLD HEALTH ORGANIZATION. Report of the Third Coordinating Meeting on Implementation of Multidrug therapy (MDT) in Leprosy Control Programmes, The Hague, 1988. WHO/CDS/LEP/ 88.4.
35. WORLD HEALTH ORGANIZATION. Report of the Interregional Conference on Leprosy Control in Africa, Brazzaville, 1989. WHO/CDS/LEP/89.1.
36. WORLD HEALTH ORGANIZATION. Report of the Consultation on Technical and Operational Aspects of Leprosy, Male, Maldives, 1990. WHO/ CTD/LEP/90.3.
M.D., D.T.P.H., All Africa Leprosy and Rehabilitation Training Center (ALERT) and Armauer Hansen Research Institute (AHRI), Addis Ababa, Ethiopia.
Present address; Plaswcg 15, 3768 AK Soest, The Netherlands.
Received for publication on 7 February 1991.
Accepted for publication in revised form on 11 June 1991.