- Volume 59 , Number 4
- Page: 569–75
Mycobacterial flora of the skin in leprosy
ABSTRACT
The presence of mycobacteria on the skin of healthy people and in leprosy lesions has been documented previously. The present study observed the mycobacterial flora on the hands (by the hand-washing method) and fingers (by the inoculated culture medium using scraped material obtained during the preparation of slit-skin smears) in 89 untreated leprosy patients. We also evaluated the slit-skin smears from fingers for the diagnosis of leprosy. In 16 patients (17.9%) mycobacteria were cultured from scrapings and hand washings. The frequency of isolates from lepromatous (LL) leprosy cases (52.9%) was significantly higher than from tuberculoid (TT) leprosy cases (5.2%). It was observed that Mycobacterium avium and M. scrofulaceum were the only opportunistic mycobacteria isolated from multi-bacillary patients, and two hypothèses are discussed to explain thèse findings. The slit-skin smears from fingers were as satisfactory as smears from other sites for the diagnosis of leprosy, but they were less satisfactory for estimating the morphological index.RÉSUMÉ
La présence de mycobactéries dans la peau de sujets en bonne santé et dans des lésions lépreuses a été rapportée précédemment. L'étude présente a analysé la flore mycobactérienne présente sur les mains (par la méthode de lavage des mains) et les doigts (en inoculant le milieu de culture avec du matériel de grattage obtenu lors de la préparation de frottis cutanés) de 89 malades de la lèpre non traités. On a aussi examiné les frottis cutanés prélevés aux doigts pour le diagnostic de la lèpre. Chez 16 patients (17, 9%), des mycobactéries ont été cultivées à partir de matériel de grattage et du lavage des mains. La fréquence des isolats était significativement plus élevée chez les patients lépromateux (LL) (52, 9%) que chez les tuberculoïdes (TT) (5.2%). On a observé que Mycobacterium avium et M. scrofulaceum étaient les seules mycobactéries opportunistes isolées à partir de patients multibacillaires, et deux hypothèses sont discutées pour expliquer ces observations. Les frottis cutanés des doigts étaient aussi satisfaisants que ceux prélevés en d'autres endroits pour le diagnostic de la lèpre, mais ils étaient moins satisfaisants pour estimer l'indice morphologique.RESUMEN
Previamente se ha documentado la presencia de micobacterias en la piel de personas sanas y en la piel de las lesiones de la lepra. El presente estudio se refiere a la flora micobacteriana encontrada en las manos (por el método del lavado de las manos) y en los dedos (por cultivo del material de descamación obtenido durante la preparación de extendidos de linfa cutánea) de 89 pacientes con lepra sin tratamiento. También se evaluó la utilidad de los extendidos de linfa cutánea de los dedos para establecer el diagnóstico de la lepra. Se pudieron cultivar micobacterias por descamación y lavado de las manos de 16 pacientes (17.9%). La frecuencia de aislamientos de los casos de lepra lepromatosa (52.9%) fue significativamente más alta que aquella de los casos tubcrculoides (5.2%). Se observó que Mycobacterium avium y M. scrofulaceum fueron las únicas micobacterias oportunistas aisladas de los pacientes multibacilares. Se discuten dos hipótesis para explicar estos resultados. Los extendidos de linfa cutánea de los dedos fueron tan satisfactorios como los extendidos de otros sitios para el diagnóstico de la lepra pero fueron menos satisfactorios para calcular cl indice morfológico.Previously it was shown that mycobacteria were often isolated from cetylpyridinium chloride forearm and hand washings of healthy volunteers residing in Manaus, Amazonas, Brazil (13). The search for mycobacteria contaminating or colonizing healthy skin was performed to gather necessary information for interpreting bacteriological data during investigations on the causes of skin lesions suspected to be of mycobacterial origin (12). In the course of these early studies, biopsies of leprosy lesions were also cultured, and three strains of the Mycobacterium avium complex (MAC) were isolated from the 36 biopsies examined (13). As a follow-up to these studies, we thought it of interest to examine the occurrence of mycobacteria in the skin of patients suffering from leprosy.
With the purpose of investigating the mycobacterial flora of the skin in leprosy patients, we selected the hands and fingers, taking advantage of a report showing that slit-skin smears from fingers (by the "scraped incision method"11) yielded essentially the same bacteriological results as smears from earlobes or lesions. We thought that the extent of invasion of the underlying tissue by M. leprae might affect the antibacterial properties of the skin, and it should be useful to know about it at the site selected for cultural examinations. This approach also allowed the evaluation of slit-skin smears from the fingers for the diagnosis of leprosy.
MATERIALS AND METHODS
Patients. The 89 patients in this investigation included 38 tuberculoid (TT), 5 borderline tuberculoid (BT), 9 midborderline (BB), 7 borderline lepromatous (BL), 17 lepromatous (LL), and 13 indeterminate (IND) leprosy cases. All were first-time cases, none of whom had received treatment before the samples for bacteriology were collected.
Acid-fast microscopy. The skin smears were stained using the Kinyoun method, except that decolorization was done using 1% instead of 3% hydrochloric acid in ethanol, as described earlier (2, 14) . The sites chosen were the earlobes, elbows, and one or two lesions. In the case of the hands, the material was collected from the middle finger of the left hand for smear and direct inoculation onto the surface of a Lowenstein-Jensen (LJ) slant. Both hands were then washed with 100 ml of a 0.05% cetylpyridinium chloride solution as described earlier (13). After washing, another smear was prepared from the middle finger of the right hand, and the scraped material was also inoculated onto the surface of a LJ slant.
For preparing the smears a No. 5 scalpel (Becton, Dickinson & Co., Rutherford, New Jersey, U.S.A.) was used to make a 5.0-mm long and 2.3-mm deep incision over a fold of skin pinched strongly using the left hand of the operator as recommended (3h). The incised skin was scraped with the scalpel, and the material was smeared as indicated above.
The stained smears were classified in relation to the bacterial (BI) and morphological (MI) indexes (10, 15) which indicate the bacterial load and the percentage of solid-stained bacilli in the smears, respectively.
Culture procedures. The material obtained from the finger scrapings was directly inoculated onto the surface of LJ slants. The left hand material was collected before hand washings; the right hand material was collected after washing to verify if eventual isolates were surface contaminants or were located deep in the skin.
The cetylpyridinium chloride washings were centrifuged, the sediments were treated by the method of Lowenstein (4% v/v sulfuric acid), and were then inoculated onto five LJ slants containing 0.5% pyruvate (LJ Pyr) instead of glycerol and 1 LJ slant supplemented with 1.5% ferric ammonium citrate (LJ FAC). The cultures were incubated at 30ºC and 37ºC as follows: at 30ºC, 2 LJ slants, 1 LJ Pyr slant, and 1 LJ FAC slant; at 37ºC, 3 LJ slants and 1 LJ Pyr slant. All cultures were incubated for 2 months before discarded as negative. The choices of media, temperatures, and times of incubation were chosen to meet the growth conditions of some mycobacterial species, such as M. bovis, M. hemophilum, M. marinum, and M. chelonei.
Culture identification. The mycobacterial isolates were shipped to Paris, and were identified using conventional methods as recommended by the Pasteur Institut, Paris (1).
RESULTS
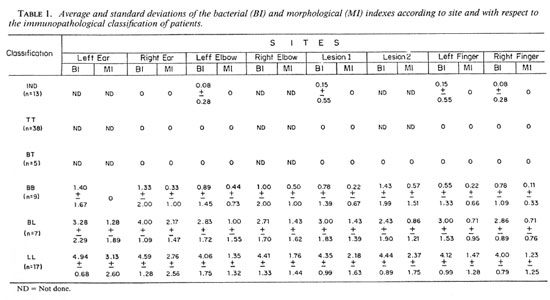
BI and MI. The means and standard deviations of the Bis and Mis obtained from the various sites according to the immunopathological classification of the patients are shown in Table 1. The data on the multibacillary forms (LL, BL and BB) are shown graphically in The Figure. As expected, the Bis were negative in BT and TT leprosy, and increased regularly from BB to BL to LL leprosy. Little variation was found among the examined sites, but the Mis were significantly higher in smears from the earlobes in BL and LL cases. Therefore, we concluded that a slit-skin smear from the fingers was as satisfactory as smears from other sites for diagnosing leprosy, but it was less satisfactory for estimating the MI.
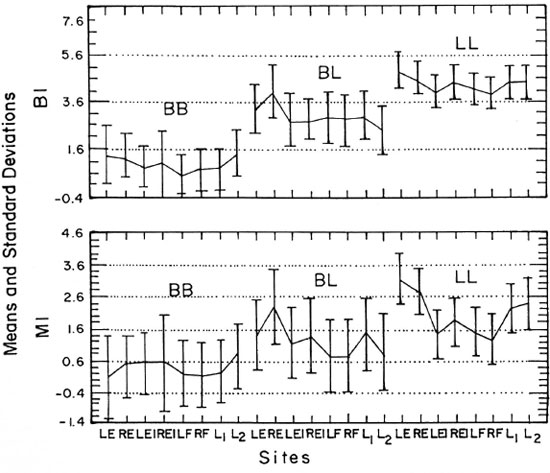
The Figure. Means ± standard deviations,of the bacterial (BI) and morphological (MI) indexes at various sites with respect to the multibacillary forms of leprosy. LE = left ear, RE = right ear, LEI = left elbow, REI = right elbow, LF = left finger, RF = right finger, L1 = lesion 1, L2 = lesion 2.
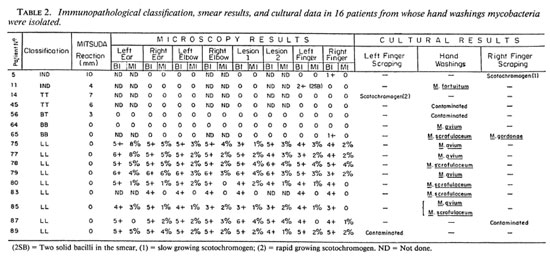
Culture results. As shown in Table 2, mycobacteria were cultured from scrapings and hand washings from 16 patients (17.9% of the patients). In two individuals (case nos. 5 and 65) there was some evidence that the cultured mycobacteria could have false-positive smear results for leprosy because microscopy examinations were negative in all other sites and both cases were paucibacillary (no. 5 classified IND; no. 65, BB). The statistical analysis of the data showed that these findings were not statistically significant, and it was concluded that the occurrence of cultivable mycobacteria on the skin only occasionally may cause false-positive smear results as far as the diagnosis of leprosy is concerned.
The distribution of mycobacterial isolates in respect to the clinical forms of the disease (Table 2) were as follows: 2 isolates from IND cases (N = 13, 15.3%); 2 isolates from TT cases (N = 38, 5.2%); 1 isolate from BT leprosy (N = 5, 20.0%); 2 isolates from BB leprosy (N = 9, 22.2%); 0 isolate from BL cases (N = 7, 0.0%); and 9 isolates from LL cases (N = 17, 52.9%).
The frequency of isolates from LL cases (52.9%) was significantly higher than from TT cases (5.2%). Although the frequency of isolates appeared to increase from TT toward the LL end of the disease spectrum, the number of cases examined was too small to allow any further conclusion about this matter.
Mycobacterial species isolated. The mycobacterial species isolated are shown in Table 2. It was surprising to verify that M. avium and M. scrofulaceum were the only opportunistic mycobacteria isolated from multibacillary leprosy patients, which was not the case from paucibacillary patients.
Considering the number of patients examined (N = 89), it was concluded that these findings were not caused by chance alone, as discussed below.
DISCUSSION
Previous studies (8, 13) have shown that mycobacteria were isolated at high frequencies from the surface of the skin of healthy volunteers (N = 291, 29.2%), from biopsies of leprosy lesions (N = 36, 11.1%), and from sputum specimens from leprosy patients with respiratory symptoms (N = 93, 17.2%). The frequency of these isolates from hand washings of leprosy patients (present study) was 17.9%. Altogether, the statistical analysis showed that there were no statistical differences between the culture results in these studies. Consequently, it was concluded that these findings could be attributed to a high density of mycobacteria in an environment characterized by high temperature and humidity, such as the city of Manaus in Brazil (surface: about 15,000 square kilometers; population about 1,500,000 inhabitants) which was built in the Amazonian forest next to the river (0.3º latitude; 60º longitude). However, it was surprising to verify that the M. avium-intracellulare-scrofulaceum (MAIS) complex was associated with multibacillary forms of leprosy more often than to any other of the above clinical conditions, and this was statistically significant.
Why should MAIS occur in LL leprosy more often than in the general population, and in TT leprosy less frequently? The following hypothesis may explain these findings.
The extensive invasion of the underlying tissues by M. leprae, as evidenced by the BI obtained using the slit-skin smears from fingers, might cause biochemical changes giving a selective advantage to MAIS as opposed to any other less pathogenic mycobacterial species. Although this hypothesis may be considered satisfactory to explore this matter, on these grounds it is difficult to explain the rarity of MAIS on the surface of the skin of TT cases and, therefore, a second hypothesis may be entertained.
MAIS (mainly M. avium complex, MAC) are potentially pathogenic mycobacteria that find a privileged niche in immunosuppressed patients and are frequently associated with AIDS (4, 5, 7, 9, 16) LL leprosy. In LL leprosy they did not cause apparent infection (or disease), indicating that the immunosuppression in these patients was not sufficient to let the bacteria proceed from colonization into disease but was sufficient to let colonization be more extensive than in healthy people. In patients who build up a high degree of cell-mediated immunity, as is the case in TT leprosy, the colonizing bacteria would have been eliminated by the cell-mediated immune processes of the skin. If this interpretation of our findings is correct, then one would expect that tuberculosis-associated leprosy should be encountered more often in LL than in TT leprosy. Therefore, we began investigating tuberculosis-associated leprosy (four cases diagnosed thus far had LL leprosy; unpublished observations).
The unexpected observations described in this report not only raise interesting questions about the possible relationships between susceptibility to mycobacterial diseases in leprosy patients subsequently exposed to mycobacterial pathogens, but also between the susceptibility to leprosy of patients with other mycobacterial infections or diseases. For instance, one may wonder whether infection by MAC first may not contribute to the severity of subsequent leprosy, as suggested by others.
Finally, this study showed that slit-skin smears from fingers for diagnosing leprosy were satisfactory. However, it did not replace the earlobes or lesions as elected sites for use in preparing smears. During this investigation, hand washings were cultured to verify if cultivable mycobacteria might be a significant cause of false-positive results, but this was not the case. However, the use of the sulfuric-acid method for decontamination of the specimens may have reduced the recovery of mycobacteria and, consequently, this matter needs to be re-examined.
Acknowledgment. The authors thank A. Feuillet and V. Lévy-Frébault of the Pasteur Institut (Paris) for their help in identifying the mycobacterial isolates.
REFERENCES
1. DAVID, H. L., LÉVY-FRÉBAULT, V. and THOREL, M. F. Méthodes de Laboratoire pour Micobacteriology Clinique. Paris: Commission dés Laboratoires de Reference et d'Expertise de l'Institut Pasteur, 1989.
2. FANDINHO, F. C. O., ORSI-SOUZA, A. T. and SALEM, J. I. A comparison of the Ziehl-Neelsen and Kinyoun methods in staining smears from leprosy patients. Int. J. Lepr. 58(1990)389-391.
3. GONTIJO-FILHO, P. P., FONSECA, L. S. and SALEM, J. I. Mycobacterium leprae e o diagnóstico laboratorial da hanseníase virchowiana. Rev. Bras. Cent. Ciências Saúde 8(1986)39-41.
4. GREENE, J. B., SIDHU, G. S., LEWIN, S., LEVINE, J. F., MASUR, H., SIMBERKOFF, M. S., NICHOLAS, P., GOOD, R. C, ZOLLAPAZNER, S. B., POLLOCK, A. A., TAPPER, M . L. and HOLZMAN, R. S. Mycobacterium avium-intracellulare: a cause of disseminated life-threatening infection in homosexuals and drug addicts. Ann. Intern. Med. 97(1982)539-546.
5. KLATT, E. C, JENSEN, D. V. and MEYER, P. R. Pathology of Mycobacterium avium-intracellulare infection in acquired immunodeficiency syndrome. Human Pathol. 18(1987)700-714.
6. LEIKER, D. L. and MCDOUGALL, A. C. Technical Guide for Smear Examination for Leprosy by Direct Microscopy. Amsterdam: Leprosy Documentation Service (INFOLEP), 1983.
7. MACHER, A. M., KOVACS, J. A., GILL, V., ROBERTS, G. D., AMES, J., PARK, C. H., STRAUS, S., LANE, H. C, PARRILLO, J. E., FAUCI, A. S. and MASUR, H. Bacteremia due to Mycobacterium avium-intraccllulare in the acquired immunodeficiency syndrome. Ann. Intern. Med. 99(1983)782-785.
8. MARÓJA, M. F., SALEM, J. I., IHARA, L. T., BARBOSA, R. M. and DAVID, H. L. Micobacterias isoladas em amostras de escarro de pacientes dc hanseniase. 44º Congresso Brasileiro de Dermatologia, Porto Alegre (RS), Brasil, 02 a 06 de setembro de 1989.
9. MASUR, H. Mycobacterium avium-intracellulare: another scourge for individuals with the acquired immunodeficiency syndrome. (Editorial) JAMA 248(1982)3013.
10. RIDLEY, D. S. Bacterial indices. In: Leprosy in Theory and Practice. Cochrane, R. G. and Davey, T. F., cds. Bristol: John Wright and Sons, Ltd, 1964, pp. 620-622.
11. RIDLEY, M., JOPLING, W. H. and RIDLEY, D. S. Acid-fast bacilli in the fingers of long-treated lepromatous patients. Lepr. Rev. 47(1976)93-96.
12. SALEM, J. I., GADELHA, A. R., MAROJA, F. and DAVID, H. L. Non-cultivable mycobacteria in the ulcers of the skin. Acta Leprol. (Geneve) 7 Suppl.1(1989)10-15.
13. SALEM, J. I., GONTIJO-FILHO, P., LEVY-FREBAULT, V. and DAVID, H. L. Isolation and characterization of mycobacteria colonizing the healthy skin. Acta Leprol. (Geneve) 7 Suppl.1(1989)18-20.
14. VESTAL, A. L. Procedures for the Isolation and Identification of Mycobacteria. Atlanta: Centers for Disease Control, U.S. Public Health Service, Department of Health, Education and Welfare, 1981.
15. WATERS, M. F. R. and REES, R. J. W. Changes in the morphology of M. leprae in patients under treatment. Int. J. Lepr. 30(1962)266-277.
16. YOUNG, L. S., INDERLIED, C. B., BERLIN, O. G. and GOTTLIEB, M. S. Mycobacterial infections in AIDS patients, with an emphasis on the Mycobacterium avium complex. Rev. Infect. Dis. 8(1986)1024 -1033.
1. M.Sc. (Microbiology), Investigator, Laboratory of Mycobacteriology, National Research Institute of Amazonia (INPA), Caixa Postal 478, 69083 Manaus, AM, Brazil.
2. M.D., Ph.D., Chief, Laboratory of Mycobacteriology, National Research Institute of Amazonia (INPA), Caixa Postal 478, 69083 Manaus, AM, Brazil.
3. M.D., M.Sc, Investigator, Laboratory of Mycobacteriology, National Research Institute of Amazonia (INPA), Caixa Postal 478, 69083 Manaus, AM, Brazil.
4. M.D., Ph.D., Chief, Laboratory of Mycobacteriology, Federal University of Rio de Janeiro (UFRJ), Rio de Janeiro, Brazil.
5. M.D., Ph.D., Chief, Unite de la Tuberculose et des Mycobacteries, Institut Pasteur, 25 Rue du Dr. Roux, 75724 Paris 15, France.
Reprint requests to Professor David.
Received for publication on 5 December 1990
Accepted for publication in revised form on 11 June 1991.