- Volume 59 , Number 4
- Page: 576–81
Enzyme immunoassay of phagocytosis stimulating tetrapeptide "Tuftsin" in normal and leprosy sera
ABSTRACT
The serum concentrations of the phagocytosis stimulating the tetrapeptide, tuftsin, were determined by competitive enzyme immunoassay in borderline tuberculoid/tuberculoid (BT/TT, 16 cases), borderline lepromatous/lepromatous (BL/LL, 16 cases), and in healthy controls (20 cases). Using checkerboard titration, 10 ng/well of diphtheria toxoid-p-aminophenylacetyl tuftsin (DTPT) conjugate when incubated with tuftsin antisera at 1:15,000 dilution with a preincubation time of 60 min with the competitor (tuftsin) followed by a further 60min incubation onto the DTPT-coated wells gave consistent results with a sensitivity of 5 ng/well tuftsin. The mean serum tuftsin concentration was significantly lower in BL/LL patients (134.42 ± 48.7 ng/ml,p < 0.01) than in healthy controls (262.86 ± 59.8 ng/ml), while BT/TT sera (210.94 ± 75.5 ng/ml) showed slightly decreased levels than did normals, which was not statistically significant. The mean serum IgG levels in BL/LL and BT/TT patients (37.26 ± 10.99 mg/ml; 28.08 ± 6.57 mg/ml, respectively) showed significantly (p < 0.001) higher concentrations than did healthy controls (12.3 ± 3.6 mg/ml). These observations on the serum concentrations of tuftsin and IgG in leprosy individuals suggest that there is splenic dysfunction in BL/LL patients in terms of the processing of leukokinin to release the free, active molecule tuftsin.RÉSUMÉ
Les concentrations sériques de fuftsine, tétrapeptide stimulant la phagocytose ont été déterminées par un test enzymatique de compétition chez des patients présentant une lèpre borderline tubcrculoïdc/tuberculoïde (BT/TT: 16 cas), borderline lépromateuse/lépromatcuse (BL/LL: 16 cas) et chez des témoins en bonne santé (20 cas). Utilisant la titration sur plaque, 10 ng par puits de p-aminophenylacetyl tuftsine conjugué à la toxoïde diphtérique (DTPT), incubés avec des antiserums vis-à-vis de la tuftsine à une dilution de 1:15.000, avec un temps précédant l'incubation de 60 minutes avec le compétiteur (tuftsine), suivi par 60 minutes supplémentaires d'incubation dans les puits couverts de DTPT, donnaient des résultats cohérents avec une sensibilité de 5 ng de tuftsine par puits. La concentration sérique moyenne de tuftsine était significativement plus basse chez les patients BL/LL ( 134, 42 ± 48,7 ng/ml, p < 0.01 ) que chez les témoins en bonne santé (262.86 ± 59, 8 ng/ml), tandis que les serums des patients BT/TT (210, 94 ± 75, 5 ng/ml) montraient des taux légèrement inférieurs à ceux des personnes normales, mais cette difference n'était pas significative. Les taux sériques moyens d'IgG chez les patients BL/LL et BT/TT (respectivement 37, 26 ± 10, 99 ng/ml et 28,08 ± 6,57 ng/ml) étaient significativement plus élevés (p < 0.001 ) que chez les témoins en bonne santé (12,3 ± 3,6 mg/ml). Ces observations sur les concentrations sériques de tuftsine et d'IgG chez des malades de la lèpre suggèrent un dysfonctionnement splénique chez les patients BL/LL dans la transformation de leukokinine pour libérer les molécules actives de tuftsine.RESUMEN
Usando un inmunoensayo enzimático competitivo se determinó la concentración del tetrapéptido estimulador de la fagocitosis, tufstina, en los sueros de 16 pacientes con lepra tuberculoide o tuberculoide subpolar (BT/TT), 16 casos lepromatosos o lepromatosos subpolares (BL/LL), y 20 controles sanos. Por un procedimiento de titulación en "tablero de ajedrez," utilizando 10 ng por pozo del conjugado toxoide diftérico-p-aminofenilacetil tufstina (DTPT), un suero anti-tufstina diluido 1:15,000, diferentes concentraciones del competidor (tufstina), y periodos de incubación de 60 min, se encontraron resultados consistentes y una sensibilidad del método correspondiente a 5 ng por pozo de tufstina. La concentración media de tufstina fue significativamente menor en los pacientes BL/LL que en los controles sanos (134.42 ± 48.7 ng/ml vs 262.86 ± 59.8 ng/ml, respectivamente, p < 0.01) y estuvo sólo ligeramente disminuida (sin significancia estadística) en los pacientes BT/TT(210.94 ± 75.5 ng/ml). Los niveles promedio de IgG en el suero de los pacientes BL/LL y BT/TT (37.26 ± 10.99 mg/ml y 28.08 ± 6.57 mg/ml, respectivamente) estuvieron significativamente (p < 0.001) mas elevados que en los controles sanos (12.3 ± 3.6 mg/ml). Estas observaciones sobre las concentraciones de tufstina e IgG en el suero de los pacientes con lepra, sugieren que los pacientes BL/LL muestran una disfunción esplénica relacionada con el procesamiento de leucocinina que interfiere con la liberación de la molécula de tufstina activa.Tuftsin (Thr-Lys-Pro-Arg) is an immunologically active tetrapeptide which is derived from the heavy chain of human immunoglobulin (residue 289-292). The release of tuftsin as the free, biologically active form is performed by two enzymes that cleave the peptide bond at each end of the tetrapeptide. One enzyme is present in the spleen and the other, on the outer surface of the phagocytic plasma membrane (1). Tuftsin's main role is to stimulate several functions of phagocytic cells, primarily in the macrophages (2). These functions play an important role in the immune system.
In a recent study, we observed that macrophages derived from normal and borderline tuberculoid/tuberculoid (BT/TT) leprosy patients exhibit a steady increase in tuftsin-stimulated phagocytosis with increasing in vitro culture age and a slow decline in the tuftsin-stimulated microbicidal response in the same chronological scale. On the other hand, macrophages from borderline lepromatous/lepromatous (BL/LL) leprosy cultures were unable to undergo tuftsin-mediated phagocytosis and microbicidal function (3,4).
In view of the aberrant phagocytic and microbicidal functions noticed in BL/LL cultures, it was felt important to determine the endogenous levels of tuftsin in these patients. This paper describes the development of a sensitive and reproducible competitive enzyme immunoassay (EIA) for quantitation of the serum tuftsin levels in leprosy patients.
MATERIALS AND METHODS
Twenty healthy, normal individuals and 16 BT/TT and 16 BL/LL leprosy patients, classified according to their clinical and histopathological findings, were assayed for serum tuftsin levels. The patients were registered at the leprosy clinic, Department of Dermato-Venereology, All India Institute of Medical Sciences, New Delhi, India. Most cases had received anti-Hansen's disease chemotherapy for less than 6 months, while a few were fresh untreated cases.
Trypsinization of serum
Sera from the above individuals were stored at -70ºC. Tuftsin, which is bound to a carrier molecule (subfraction IV of IgG) in plasma in the circulation, was released as the free form by a brief digestion with trypsin (9).
Preparation of papa tuftsin-BSA conjugate for immunization
p-Amino phenyl acetyl tuftsin (10 mg; 13.3 µmol), a synthetic derivative of tuftsin (Sigma Chemical Co., St. Louis, Missouri, U.S.A.) was coupled to bovine serum albumin (BSA) (22.67 mg; 323 µmol) by the azide method (10). It was dialyzed against water, and the dialysate containing unreacted papa tuftsin and the conjugate were lyophilized. The amount of papa tuftsin dialyzed out was estimated (5).
Antibodies were raised in rabbits given intradermal (i.d.) injections of the conjugate (1 ml per rabbit containing 250 µg of the conjugate emulsified with complete Freund's adjuvant) at multiple sites. Five weeks later, intravenous (i.v.) boosters were given with the same amounts of the immunogen in normal saline for 3 consecutive days. Eight days after the third i.v. booster, the rabbits were bled and the sera were separated and stored at - 20ºC. Boosters were repeated every 3 weeks, and the bleeds were collected.
Antibody-titration assay
Papa tuftsin-DT conjugate. Papa tuftsin (2.56 mg; 3.33 µmol) was coupled to diphtheria toxoid (DT) (obtained from Pasteur Institut of India, Coonoor, India, and purified through a Sephacryl-100 column) (5.0 mg; 83.3 µmol) using the azide procedure.
Enzyme immunoassay (EIA). An EIA was performed in 96-well, flat-bottom plates (Immulon 2; Dynatech Labs., Inc., Alexandria, Virginia, U.S.A.) in triplicate wells. Each well of the plate was coated with 100 µl of diphtheria toxoid-papa tuftsin (DTPT) conjugate (5 ng to 200 ng/well) in carbonate bicarbonate buffer (0.05 M, pH 9.6) at 4ºC for 16-18 hr, and washed three times with phosphate buffered saline (PBS) (0.01 M, pH 7.4) containing 0.5% Tween 20. The wells were blocked with 200 µl of PBS-Tween containing 0.5% casein overnight at room temperature. Following three more washes, 100 µl of tuftsin antisera (1:100 to 1:25,000 dilution) was applied to the wells and incubated for 1 hr at 37ºC. After five washes, 100 µl of peroxidase-conjugated goat anti-rabbit IgG (Tago Inc., Burlingame, California, U.S.A.) at a dilution of 1:2000 in PBS-Tween was added and incubated for another 1 hr at 37ºC. Finally, the plates were washed five times, and 100 yutl of OPD (0.4 mg/ml) in citrate buffer (0.15 M, pH 5.0) containing 0.03% H2O2 was added. The plates were then incubated at 37ºC in the dark. After 15 min the reaction was stopped with 50 µl of 8 N H2S04 and the optical density (OD) was read at 490 nm. Absorbance was calculated for each well, after subtracting the value obtained with wells coated with an equivalent amount of DT, i.e., diphtheria toxoid that had not been reacted with papa tuftsin. The mean absorbance was calculated for each antisera dilution and coated antigen, i.e., DTPT.
Quantitative competitive EIA for tuftsin
Different concentrations of tuftsin or papa tuftsin (5 ng to 640 ng/well) were incubated with tuftsin antisera (1:15,000 final dilution) in 100 µl of PBS-Tween for different time periods (0, 30, 60, and 90 min) at 37ºC. For quantitation of tuftsin in the serum samples, the dried alcohol extracts containing the tuftsin after trypsinization of individual sera were dissolved in 0.5 ml of PBS-Tween, and 50 µl of each were incubated with 50 µl of 1:7500 dilution of the antisera (to give a final dilution of 1:15,000) for 1 hr at 37ºC. At the end of the incubation period, the contents were transferred to wells in microtitration plates which previously had been coated with an optimal concentration of DTPT (10 ng/well). After further incubation for 1 hr at 37ºC, the plates were processed for color development using a second antibody conjugated with horseradish peroxidase (HRPO) and substrate solution.
Quantitative measurement of serum IgG levels
The serum IgG levels in the leprosy patients (16 BT/TT and 16 BL/LL) and normal individuals (20) were measured by solid phase enzyme immunoassay using highly purified human IgG (Calbiochem, San Diego, California, U.S.A.) and goat antihuman IgG coupled to HRPO (Fc specific; Nordic Immunological Laboratories, Tilburg, The Netherlands) for a standard curve. Test sera were coated onto the wells at two dilutions (1:10,000 and 1:20,000), and the color was developed in the presence of a second antibody and substrate as described above. The standard curve was linear from 10 ng to 100 ng/well for human IgG.
Statistical analysis
The results are expressed as the average values with standard deviation (± S.D.). Statistical significance was determined by Student's t test.
RESULTS
Tuftsin was coupled through an amino phenylacetyl derivative to BSA. An average of 25 molecules of tuftsin were linked to each molecule of BSA. During the EIA procedure, papa tuftsin was coupled to DT, a heterologous carrier, to enhance the peptide binding to the wells. Under the experimental conditions described, an average of 45 papa tuftsin moieties were linked to each molecule of DT. When different bleedings of the rabbits were titrated for antituftsin antibodies, all of the animals showed more or less equivalent titers. The first bleed of rabbit 3 was used for all subsequent assays (Fig. 1). Using this bleed, the optimal dilutions of the other reagents viz. DTPT 10 ng/well; the first antibody dilution 1:15,000 and second antibody dilution 1:2000 were determined by checkerboard titration for maximal assay sensitivity and precision. Under these conditions, the sera showed very low nonspecific binding with DT alone (mean absorbance = 0.085 ± 0.005).
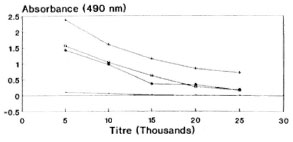
Fig. 1. Antisera dilution curve of rabbit 3, showing absorbance values vs antisera titers of different bleedings by direct EIA (DTPT = 10 ng/well).  = Preimmune bleed; + = 3.1; * = 3.2;
= Preimmune bleed; + = 3.1; * = 3.2;  = 3.3.
= 3.3.
A competitive EIA was carried out using standard tuftsin and papa tuftsin. Both papa tuftsin as well as tuftsin compete with immobolized DTPT conjugate for its antiscra (Fig. 2). Papa tuftsin was found to be a better competitor with a sensitivity ~2.5 ng/well with 50% inhibition of binding achieved at 5 ng/well of papa tuftsin. On the other hand, tuftsin, with a sensitivity of ~ 5 ng/well, gave a 50% inhibition of binding at 10 ng/well. However, the tuftsin levels in the sera of all of the studied individuals were computed from the standard curve of tuftsin.
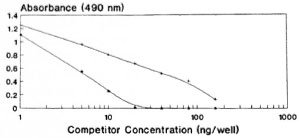
Fig. 2. Competitive EIA using standard tuftsin and papa tuftsin with its antisera following further incubation with DTPT wells. + = Tuftsin; * = papa tuftsin.
In order to standardize the optimal preincubation time at 37ºC for competition of standard tuftsin with its antiscra, different time intervals of 0, 30, 60 and 90 min were chosen. Negligible competition was observed when the competitor and the antisera-without any preincubation-were incubated for 60 min with DTPT wells. A linear curve was obtained at 60 min of preincubation between the competitor and the antiscra (Fig. 3). Preincubation of 90 min also showed a more or less similar curve. Hence, a preincubation time of 60 min at 37ºC was found to be optimal for the interaction between competitor and antiscra, followed by a further 60-min incubation with the coated wells.
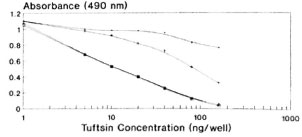
Fig. 3. Standardization of optimal preincubation time for competition of standard tuftsin with its antisera (1:15,000 final dilution) followed by 60-min further incubation with the DTPT wells during competitive EIA.  = No preincubation; + = 30 min.; * =60 min; 111
= No preincubation; + = 30 min.; * =60 min; 111  = 90 min.
= 90 min.
With the help of the antibody titration curve, though 10 ng of DTPT conjugate/well was found to be optimal, it was repeated with 5, 10 and 25 ng of DTPT conjugate during a competitive assay using standard tuftsin with 60 min of preincubation between competitor and antiscra (Fig. 4). Using 5 ng/well of DTPT conjugate, complete inhibition with the competitor was not achievable within the experimental range. Furthermore, the sensitivity of the curve is too low to be of practical use. The curve is linear, and complete inhibition was achieved at 10 ng/well and showed very low nonspecific binding (mean 0.086 ± 0.005). Further, 25 ng/well of the conjugate gave more or less a similar curve but with a higher nonspecific binding (mean 0.14 ± 0.061). Hence, 10 ng/well of the DTPT conjugate was fixed for all future assays.
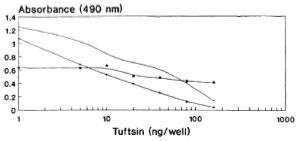
Fig. 4. Standardization of optimal amounts of DTPT conjugate to be coated onto the wells during competitive assay with 60-min preincubation between the competitor (tuftsin) and antisera.  = DTPT 25 ng/well; + = DTPT 10 ng/well; * = DTPT 5 ng/well.
= DTPT 25 ng/well; + = DTPT 10 ng/well; * = DTPT 5 ng/well.
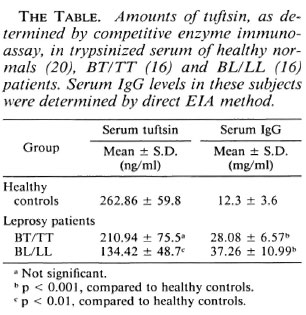
Using the competitive enzyme immunoassay, the amounts of material immunochemically related to tuftsin were quantitatcd in leprosy sera. As shown in The Table, the mean tuftsin concentration in BL/LL sera (16 cases) was 134.42 ± 48.7 ng/ml, BT/TT sera (16 cases) showed 210.94 ± 75.6 ng/ml, while normal healthy sera (20 cases) showed 262.86 ± 59.8 ng/ml. The mean serum IgG levels as determined by the direct EIA method showed 37.26 ± 10.99 mg/ml in BL/LL, 28.08 ± 6.57 mg/ml in BT/TT, and 12.3 ± 3.6 mg/ml in normal healthy controls (The Table).
DISCUSSION
Najjar first described a phagocytic assay that was used to assess patients for functional tuftsin deficiency (8). Although this assay is semiquantitative and reliable, it is not useful for serial monitoring of tuftsin levels. A radioimmunoassay for tuftsin measurement in splenectomized subjects has also been described (10). Recently, an analytical method utilizing reverse phase HPLC and mass spectrometry has been developed for identification of serum tuftsin (6). However, these methods have their limitations in terms of the shelf life of the radiolabel, its disposal, being hazardous to health, or the need for sophisticated equipment and/or trained manpower. The EIA method, however, uses stable reagents and is devoid of the above limitations.
The covalent coupling of an immunogenic peptide, i.e., tuftsin, to a carrier protein by the azide procedure generated high titer antibodies in rabbits. It is pertinent to mention at this juncture that the method of conjugation, the degree of substitution of the peptide, and the selection of carrier are known to affect the immunogenicity of the conjugate. These factors are evident from the antibody dilution curve (Fig. 1) since there is no sharp/steep fall in the absorbance value with the increase in antisera dilution, thereby signifying high affinity antibodies (KD = 4.9 x 10-6 M). It is perhaps possible that the tuftsin moiety is optimally recognized by the immune system by being in proximity to a T-cell epitope expressed by the carrier. During the EIA procedure, tuftsin is linked to DT using the same chemistry, thereby keeping an identical spacer length. The competitive EIA standardization, using tuftsin and papa tuftsin, suggests that because the tuftsin competition curve is lower than that of papa tuftsin, that the antibodies have a lower affinity for the tuftsin when compared to papa tuftsin. The expected levels of tuftsin in the sera arc far above the sensitivity of the tuftsin competition curve, thereby in no way affecting the sensitivity and reliability of the tuftsin assay. Above all, tuftsin is the native form of the molecule released into the sera after trypsinization. From the Figures 2, 3 and 4 it was found that 10 ng/well of DTPT conjugate, when incubated with tuftsin antisera at 1:15,000 dilution with a preincubation time of 60 min with the competitor (tuftsin) followed by 60 min further incubation onto the coated wells, gave consistent results with the limit of sensitivity being 5 ng/well of tuftsin (Fig. 5).
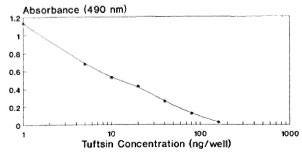
Fig. 5. Tuftsin standard curve at optimally standardized conditions.
From The Table, it is evident that the BL/LL sera were found to have significantly lower levels of tuftsin (p < 0.01) when compared to normals. On the other hand, BT/TT sera showed higher levels than lepromatous sera but slightly lower than those of normal individuals, but the decrease was statistically insignificant. This is in agreement with our earlier observation, that normal and BT/TT macrophages display similar response profiles of stimulated phagocytosis and microbicidal activity exerted by tuftsin at all ages of in vitro culture(2, 3). On the other hand, both tuberculoid and lepromatous sera showed IgG levels that are significantly (p < 0.001) higher than normal values. This is in accordance with the observation made by other workers that IgG levels are usually elevated in both BL/LL and BT/TT patients (4).
In light of the observed IgG levels, it can be concluded that no correlation exists between the serum IgG levels and serum tuftsin levels in these patients. The tuftsin extraction procedure from serum involves trypsin treatment that cleaves the peptide bond at the amino terminal, i.e., between Lys 288 and Thr 289 on the 7-chain of immunoglobulin molecule. This reaction is similar to the one catalyzed by the enzyme leukokininase S which is present on the outer phagocytic membrane. This procedure therefore bypasses any aberration in the release of tuftsin as far as the macrophage or phagocyte are concerned. The other possibility arises out of the defective processing of tuftsin release from the 7-chain of immunoglobulin with a nick between Arg 292 and Glu 293 by splenic endocarboxypeptidase at the carboxy end of the sequence. It has been established that the endocarboxypeptidase is the first step in the two-step enzymatic cleavage of tuftsin, followed by the phagocytic leukokininase S action (7).
It can, therefore, be concluded that in the case of leprosy patients, and more so in lepromatous than in the tuberculoid patients, there is a defect at splenic processing that may account for the decreased release of tuftsin from the IgG molecule during trypsinization. This interpretation is in agreement with the observation seen in patients who have undergone splenectomy (l0). This indication of a defective splenic function is of significance in leprosy patients. It is likely that tuftsin deficiency, in addition to its importance as an indicator of splenic hypo-function, also impairs the phagocytic function of the blood polymorphonuclear leukocytes and of macrophages in these patients.
Acknowledgment. We are thankful and grateful to CSIR, New Delhi, for financial assistance. Special thanks to the Lady Tata Memorial Trust, Bombay, for awarding a Junior Fellowship to Jagdeep Kaur.
REFERENCES
1. FRIDKIN, M. and GOTTLIEB, P. Tuftsin. Thr-Lys-Pro-Arg. Anatomy of an immunologically active peptide. Mol. Cell. Biochem. 41(1981)73-97.
2. IYER, R. R., PRASAD, H. K, BHUTANI, L. K. and RAO, D. N. Modulation of human lepromatous monocyte-macrophage functions in vitro by tuftsin. Int. J. Immunopharmac. 12(1990)847-858.
3. IYER, R. R., PRASAD, H. K., BHUTANI, L. K. and RAO, D. N. Effect of tuftsin stimulation on the microbicidal activity exerted by blood monocytes-macrophages of leprosy patients. Int. J. Immunopharmac. 12(1990)859-869.
4. KELKAR, S. S., MONDKAR, A. D. and WARAWDEKAR, W. Scrum immunoglobulins in leprosy. Lepr. India 51(1979)189-193.
5. MOORE, S. and STEIN, W. H. A modified ninhydrin reagent for the photometric determination of amino acids and related compounds. J. Biol. Chem. 211(1954)907-913.
6. NAIM, J. O., DESIDERIO, D. M., TRIMBLE, J. and HINSHAW, J. R. The identification of serum tuftsin by reverse-phase high-performance liquid chromatography and mass spectrometry. Anal. Biochem. 164(1987)221-226(published erratum appears in Anal. Biochem. 166(1987)449).
7. NAJJAR, V. A. Biological and biochemical characteristics of the tetrapeptide tuftsin, Thy-Lys-Pro-Arg. In: Macrophages and Lymphocytes; Part A. Escobar, M. R. and Friedman, H. eds. New York: Plenum Publishing Corp., 1980.
8. NAJJAR, V. A. and CONSTANTOPOULOS, A. A new phagocytosis stimulating tetrapeptide and its role in disease. J. Reticuloendothel. Soc. 12(1972)197-215.
9. NISHIOKA, K., CONSTANTOPOULOS, A., SATO, P..S., MITCHELL, W. M. and NAJJAR, V. A. Characteristics and isolation of the phagocytosis-stimulating peptide, tuftsin. Biochim. Biophys. Acta 310(1973)217-229.
10. SPIRER, Z., ZAKUTH, V., BOGAIR, N. and FRIDKIN, M. Radioimmunoassay of the phagocytosis-stimulating peptide tuftsin in normal and splenerctomized subjects. Eur. J. Immunol. 7(1977)69-74.
1. M.Sc, All India Institute of Medical Sciences, New Delhi 110029, India.
2. M.Sc., All India Institute of Medical Sciences, New Delhi 110029, India.
3. Ph.D., Department of Biochemistry, All India Institute of Medical Sciences, New Delhi 110029, India.
4. M.D., Department of Dermato-Venereology, All India Institute of Medical Sciences, New Delhi 110029, India.
Reprint requests to Dr. Rao.
Received for publication on 2 January 1991.
Accepted for publication in revised form on 16 July 1991.