- Volume 59 , Number 4
- Page: 582–9
M. leprae- and BCG-induced chemiluminescence response of monocytes from leprosy patients and healthy subjects: effects of gamma-interferon and GM-CSF
ABSTRACT
Mycobacterium leprae, in contrast to BCG, failed to trigger any chemiluminescence (CL) response in mononuclear cells from cither leprosy patients or healthy subjects, a deficit not reversed by either interferon-gamma or GM-CSF. Chemiluminescence responses induced without mycobacteria or with BCG were found to be lower in leprosy patients than in controls. M. leprae were also less well phagocytosed than BCG. However, there was a significant difference in phagocytosis between healthy and tuberculoid leprosy subjects. Phagocytosis was not altered by the addition of cither lymphokine, and no major differences between healthy subjects and patients were observed. Preincubating mononuclear cells with anti-mycobactcria antibodies (lepromatous patients' sera) did not increase the CL response nor the phagocytosis of M. leprae or BCG.RÉSUMÉ
Mycobacterium leprae, par opposition au BCG, ne provoqua aucune réponse de chémoluminescence (CL) au niveau des cellules mononucléaires en provenance soit de patients lépreux, soit de sujets sains; cette déficience n'était pas corrigée par l'interféron-gamma ou le GM-CSF. On a observé que les réactions de chémoluminescence induites sans mycobactéric ou avec le BCG étaient plus faibles chez les patients lépreux que chez les témoins. M. leprae était également moins bien phagocyté que le BCG. Cependant, il y avait une différence significative dans la phagocytose entre les sujets sains et les patients présentant une lèpre tuberculide. La phagocytose n'était pas altérée par l'addition de lymphokine, et aucune difference importante ne fut observée entre sujets sains et malades. L'incubation préalable des cellules mononucléaires avec des anticorps anti-mycobactcric (sérums de patients lépromateux) n'a pas augmenté la reaction dc chemoluminescence ni la phagocytosc de M. leprae ou du BCG.RESUMEN
Las células mononuclcares de individuos sanos o de pacientcs con lepra no gencraron quimioluminiscencia (QL) cuando se estimularon con Mycobacterium leprae pero si lo hicieron cuando se estimularon con BCG. El defecto no fue revertido ni por el interferon gamma ni por el GM-CSF. La QL inducida en ausencia de micobacterias o por cl BCG, fue menor en los pacientes con lepra que en los controles sanos. El M. leprae también fue menos fagocitable que el BCG. Entre los sujetos sanos y los pacientes con lepra tuberculoide hubo una significante diferencia en la fagocitosis. La fagocitosis no se alteró por la adición de ninguna de las dos linfocinas y no se observaron diferencias importantes entre los sujetos sanos y los pacientes. La preincubaciôn de las células mononuclcares con anticuerpos antimicobacterianos (sueros de pacientes lepromatosos) no aumentó la QL de las células ni la fagocitosis del M. leprae o del BCG.Most Mycobacterium leprae -infected subjects develop efficient cell-mediated immunity that results either in subclinical self-resolving infection or in overt leprosy of the tuberculoid (T) type, with a limited number of granulomatous inflammatory lesions and a reduced bacterial load. Some infected subjects fail to mount an efficient M. lepraedriven T-cell response; their infection progresses toward lepromatous (L) leprosy with disseminated lesions and a heavy bacterial load (l6). The final outcome of in vivo infections by mycobacteria depends both on T-cell ability to develop acquired specific immunity and on infected macrophages to respond to T-cell-mediated activation signals. It is not known yet whether the primary defect of these subjects is at the T-cell level or in the macrophage population or both.
Phagocytes contain a variety of antimicrobial systems that use toxic oxygen metabolites formed by phagocytosis-induced respiratory bursts (11, 13). Previous studies indicated that M. leprae, in contrast to BCG, was a poor stimulant of this release of toxic oxygen metabolites by normal human monocytes, even in the presence of inter-feron-gamma (IFN-γ) (7, 10). In the present study, we have measured the chemiluminescence (CL) associated with the oxydative phagocyte respiratory burst and the phagocytic activity of human monocytes from either healthy subjects or leprosy patients after stimulation by M. leprae or BCG, alone or with IFN-γ or granulocyte monocyte colony stimulating factor (GM-CSF), both lymphokines known for their macrophage stimulating properties (13, 15, 21) .
MATERIALS AND METHODS
Cell isolation. Peripheral blood was obtained by venipuncture using heparinized "Vacutainer" tubes (Becton-Dickinson, Rutherford, New Jersey, U.S.A.) from 21 untreated leprosy patients attending the Institut de Léprologie Appliquée de Dakar, Senegal. These patients were histologically classified as lepromatous (L) or tuberculoid (T) according to the Ridley-Jopling scale (15). Eighteen healthy subjects served as controls.
Peripheral blood mononuclear cells (PBMC) were isolated over a Ficoll-Hypaque gradient and were suspended in Hanks' solution without phenol red. The percentage of monocytes in the preparation was evaluated on May-Griinwald-Giemsa smears.
Mycobacteria. M. leprae, extracted from armadillo tissues, irradiated, and lyophilized, was provided by the World Health Organization (WHO) (batch CD 56 or CD 128). Viable M. bovis BCG IP Dakar was obtained in lyophilized samples from the "Service du BCG" (Pasteur Institut, Dakar) and was inactivated by heating for 120 min at 70ºC.
Reagents. Luminol (5-amino-2, 3-dihydro-l-4-phtalazinedione) (Sigma Chemical Co., St. Louis, Missouri, U.S.A.) was dissolved in dimethylsulfoxide (DMSO; Sigma) and used at a final concentration of 10-4 M in phosphate buffer. Fluoresceine isothiocyanate (FITC) was purchased from Sigma. Recombinant IFN-γ (r-IFN-γ) was obtained from Amersham (Buckinghamshire, England) and recombinant GM-CSF (r-GM-CSF) from Genzyme (Maidstone, England).
Measurement of CL. We used an automatic photometer and dispenser (1251 Luminometerand 1291 Dispenser; LKB Wallac, Turku, Finland) for measuring chemiluminescence (CL). Briefly, 100 µl of a suspension of mononuclear cells (105/ml) was transferred to a counting tube previously filled with 1 ml of a suspension of bacteria diluted in phosphate buffer to a ratio of 50 per cell. The tube was placed in a dry tube heater at 37ºC under constant agitation, and 200 µl of the luminol solution was subsequently added. The tube was then immediately introduced into a photometer-counting chamber (λ = 425 nm). A measurement mode was available for continuous recording during 150 min. Results of reactive oxygen species production were expressed as light intensity in millivolts at the peak of the course per 105 mononuclear cells.
Mycobacterial phagocytosis assay. Heat-inactivated BCG and irradiated M. leprae were FITC-conjugated by suspending 1 mg of mycobacteria in 1 ml of carbonate-bicarbonate buffer (pH 9.5) with 0.05% Tween 80 and 0.03% FITC for 60 min at room temperature as previously described (8). The bacteria were pelleted by centrifugation, re-suspended in Hanks' solution without phenol red, and then sonicated for 30 sec to disrupt bacteria clumps. Aliquots of 100 µg of bacteria were frozen at - 20ºC until used. Phagocytosis was measured by incubating 105 mononuclear cells in 1 ml of Hanks' solution with bacteria at a ratio of 50 per cell. The percentages of monocytes was determined on May-Gr Unwald-Giemsas mears. After gentle agitation for 4 hr at 37ºC, the tubes were centrifuged twice at 300 x g and the supernatants were discarded. The pellets were resuspended in 200 µl of Hanks' solution and examined with a Leitz fluorescence microscope with a 100 x water immersion lens; 300 to 400 cells were counted and the results were expressed as percentages of monocytes with associated mycobacteria. In some experiments, mycobacterial phagocytosis assays were performed in the presence of a pool of lepromatous patients' sera decomplemented by heating at 56ºC for 30 min.
Statistics. The Student's / test was used to compare the different experimental groups.
RESULTS
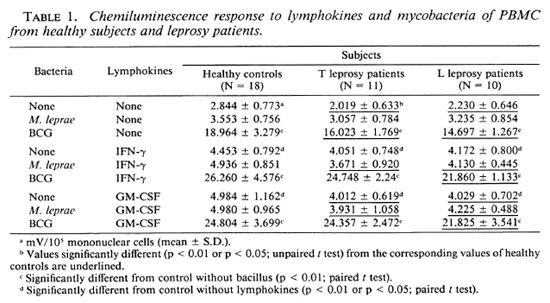
Effects of r-IFN-γ and r-GM-CSF on CL response to BCG and M. leprae. Preliminary experiments were performed to define the optimal conditions for GM-CSF to stimulate a maximal CL response to the mycobacteria. Dose-response curve analysis performed in the presence or absence of BCG and M. leprae with normal PBMC indicated that the maximal response to mycobacteria at a ratio of 50 per cell could be obtained with 5-10 IU/ml of GM-CSF. Preincubation of PBMC (2-1 2 hr) with GM-CSF prior to the addition of mycobacteria and CL assay did not improve the CL response (data not show). Thus, subsequent studies were performed with constant concentrations of 10 IU/ml of GM-CSF or of 500 IU/ml of IFN-γ (as determined from our earlier studies(10), added with a ratio of 50 per cell of either mycobacterial suspension.
As previously described (10), even at high concentrations neither viable nor heat-inactived or irradiated M. leprae triggered any CL increase above that recorded for unstimulated cells from healthy subjects. Thus, we used irradiated M. leprae in all following experiments.
In all three groups of subjects, the kinetics of CL response of unstimulated cells was different from those observed for IFN-γ-and GM-CSF-stimulated cells. Optimal release was obtained 30 min after exposure to BCG and 60 min after exposure to BCG plus either lymphokines tested. No effects of IFN-γ and GM-CSF on the kinetics of the CL response in the presence of M. leprae were shown in any group tested. Mononuclear cells from lepromatous and tuberculoid patients showed the same pattern of response (Figs. 1 and 2). Thus, the CL measurement mode was available for continuous recording during a period of 150 min.
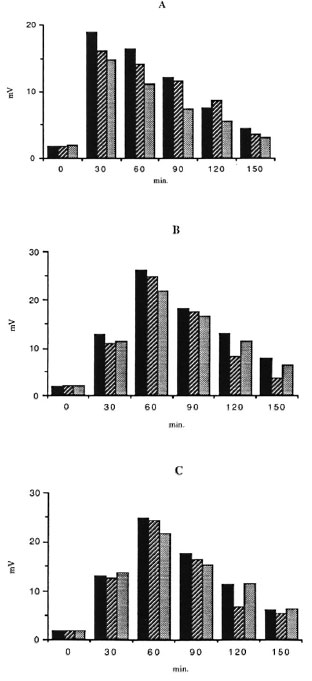
Fig. 1. Kinetics of the effects of r-IFN-γ and r-GM-CSF on the CL response to BCG (ratio 50/1) of mononuclear cells from healthy controls ( ) and lepromatous (
) and lepromatous ( ) and tuberculoid (
) and tuberculoid ( ) leprosy patients. Oxygen-free radical production from not exposed (A), r-IFN-γ (B), and r-GM-CSF (C) stimulated cells was measured.
) leprosy patients. Oxygen-free radical production from not exposed (A), r-IFN-γ (B), and r-GM-CSF (C) stimulated cells was measured.
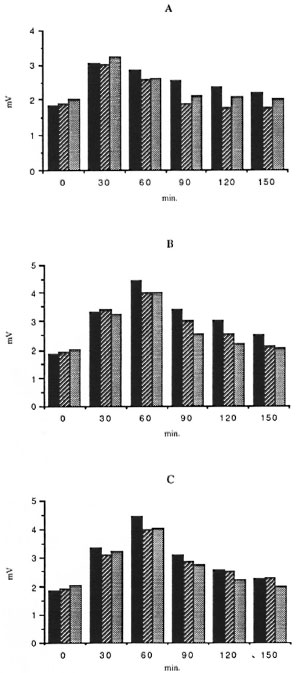
Fig. 2. Kinetics of the effects of r-IFN-γ and r-GM-CSF on the CL response of M. leprae (ratio 50/I) of mononuclear cells from all three groups tested. Forde tails, see Figure 1 legend.
PBMC CL responses to BCG and M. leprae were studied in the presence or absence of IFN-γ or GM-CSF in 18 healthy subjects, 11 T patients, and 10 L patients. In the absence of lymphokines, M, leprae failed to trigger any significant response in any group of subjects. Indeed, CL response to M. leprae was not significantly different from the controls without the bacillus. Conversely, BCG elicited a strong response from all three groups. Both IFN-γ and GM-CSF increased the CL response of PBMC from all subject groups in the absence of mycobacteria. When M. leprae were added, no further increase was noted. Conversely, the CL response to the costimulation by BCG plus either lymphokine was higher than the sum of these responses obtained with each stimulus alone, suggesting that IFN-γ and GM-CSF exerted more than an additive effect upon the BCG-triggered CL production by human PBMC. No difference could be seen between the CL response triggered by IFN-γ and GM-CSF, neither in the absence nor in the presence of mycobacteria (Table 1). Interestingly, CL responses induced with BCG or without mycobacteria were found slightly but significantly higher in healthy subjects than in either group of leprosy patients (especially L patients) whatever the stimulus. Morever, no differences were seen in M. leprae-induced CL responses in all three groups of subjects.
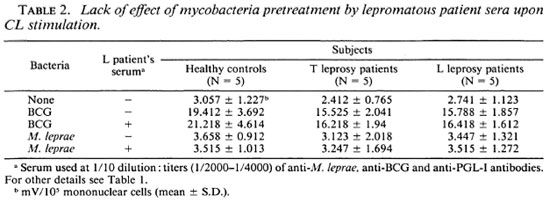
Effects of anti-M. leprae antibodies on M. leprae-triggered cl response of mononuclear cells. The influence of anti-M. leprae antibodies was evaluated using a pool of lepromatous sera containing high titers (1/ 2000-1/4000) of anti-M. leprae, anti-BCG, and anti-phenolic glycolipid-I (PGL-I) antibodies, as measured in an enzyme-linked immunosorbent assay as previously described (1) (data not shown). BCG or M. leprae at a ratio of 50 per cell were preincubated with lepromatous sera at an optimal dilution of 1/10. Two hours later, mononuclear cells were challenged with this mixture. Table 2 shows that pretreatment of either BCG or M. leprae with sera did not enhance the CL responses from mononuclear cells in all three groups.
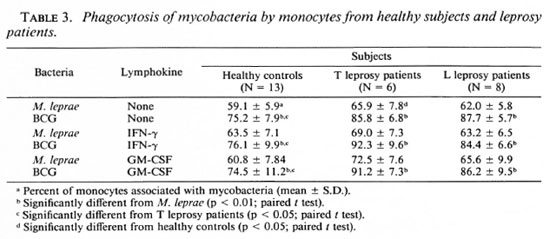
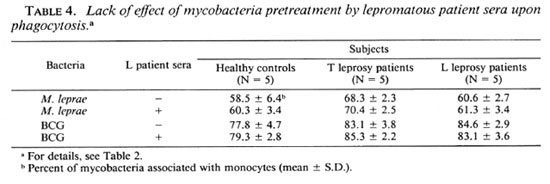
Effects of r-IFN-γ and r-GM-CSF on mycobacteria phagocytosis. The failure of M. leprae to trigger an efficient CL response of PBMC could result from defective phagocytosis of the bacillus. The number of monocytes in the PBMC from all three groups was identical (28.4 ± 3.7, 30.4 ± 2.3, and 27.5 ± 2.6 in healthy controls, L patients, and T patients, respectively). Preliminary results with healthy controls showed that the time course of BCG and M. leprae phagocytosis was maximal between 3 and 4 hours. In Figure 3, a representative experiment is shown. Assuming that only monocytes in the preparation of PBMC contain organisms, the number of FITC-conjugatcd M. leprae associated with monocytes in our phagocytosis assay was indeed significantly less than that of FITC-conjugated BCG in all three groups of patients. Phagocytosis was not altered by the addition of cither lymphokine, but there was a significant difference in phagocytosis between healthy controls and tuberculoid subjects (Table 3). In some experiments, percentiles of cells with 1 to 2 or more mycobacteria associated per cell were determined. No statistical difference in the number of bacteria per cell were demonstrated in the three groups of patients (data not shown). Pretreatment of PBMC with the pool of lepromatous sera described above did not increase the number of FITC-conjugatcd mycobacteria phagocytized (Table 4).
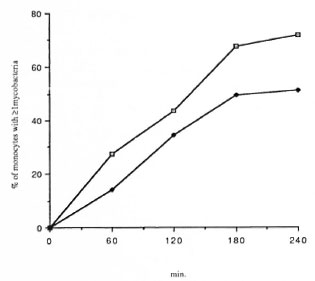
Fig. 3. Time courses of BCG ( ) and M. leprae (
) and M. leprae ( ) phagocytosis in healthy su bjects. Results are expressed as percentile of monocytes with I associated mycobacteria.
) phagocytosis in healthy su bjects. Results are expressed as percentile of monocytes with I associated mycobacteria.
DISCUSSION
The failure of M. leprae, as observed in this study and others (7, 10), to stimulate the phagocyte oxidative burst in human monocytes may be relevant to its pathogenic role in humans. The mechanism of such a failure of M. leprae to trigger an efficient oxydative burst remains unclear. It has been attributed to PGL-I, a unique component of the M. leprae cell wall, which acts as an oxygen species scavenger (3, 14). However, Vachula, et al. (19) have shown that PGL-I specifically suppressed the generation of superoxide anions by mononuclear cells stimulated by M. leprae, but not that triggered by other mycobacteria or by PMA. Thus, an additional specific mechanism for suppressing the metabolic response to M. leprae does exist.
As shown by the present study, the poor phagocytosis of M. leprae by human phagocytes may also contribute to the weak CL phagocyte response. Similar results were reported about M. lepraeniurium, another poor stimulator of phagocyte respiratory bursts (2, 10) which was less efficiently phagocytosed by murine macrophages than a good stimulator such as M. microti (2). PGL-I is a part of the lipid capsule surrounding the mycobacteria that could prevent phagocytosis (5). However, Neil and Klebanoff (14) showed that PGL-I-coated staphylococci were normally ingested -but not killed -by human macrophages, and our previous study could not detect any antiphagocytic function of PGL-I in FITC-conjugated mycobacteria (9). Moreover, treatment of M. leprae and BCG by anti-mycobacterial antibodies, containing anti-PGL-I antibodies, did not modify the capacity of these mycobacteria to stimulate the CL response to M. leprae as previously described by Holzer, et al. (7) and did not increase the phagocytosis of mycobacteria by normal human monocytes. On the other hand, preincubating M. lepraemurium with sera from infected mice enhanced phagocytosis and the oxidative burst (2). All these data show that the mechanism accounting for the low stimulation of the oxidative burst by certain species of mycobacteria is not unique. Interestingly, neither IFN-γ nor GM-CSF could reverse the defective M. leprae-induced CL response; whereas they did synergize with BCG, a mycobacterium of reduced pathogenicity.
In vivo augmentation of the H202 releasing capacity by the lepromatous macrophages after challenge with PMA, when IFN-γ was injected intradermally, was described by Nathan, et al. (12). According to these data, the responses to PMA from monocytes from all three groups tested in this study were increased (data not shown). Using overnight cultures of adherent human mononuclear cells, small but consistent O production with M. leprae stimulation was observed (8, 19). In addition, IFN-γ was able to augment the O
production with M. leprae stimulation was observed (8, 19). In addition, IFN-γ was able to augment the O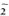 production by M. leprae-adherent monocytes (20). The nature of the effector cells, adherent cells in these studies versus the unseparated mononuclear cells in our work, might explain these conflicting results.
production by M. leprae-adherent monocytes (20). The nature of the effector cells, adherent cells in these studies versus the unseparated mononuclear cells in our work, might explain these conflicting results.
Both GM-CSF and IFN-γ had been shown to activate macrophages and to promote killing of some intracellular pathogens (13, 15, 20). However, the role of IFN-γ in the host defense against mycobacteria has been disputed: r-IFN-γ was shown either to enhance (4, 17, 18) or to reduce (6) the in vitro growth of mycobacteria within monocytes/macrophages. The role of GM-CSF has not yet been explored. In our study, r-GM-CSF increased the CL response of unstimulated human phagocytes as did r-IFN-γ. BCG stimulation further increased the CL response induced by r-GM-CSF; whereas M. leprae stimulation had no detectable effect. These data suggest that the defective activation of the phagocyte oxidative burst by M. leprae could not be reversed by either IFN-γ or r-GM-CSF.
Acknowledgment. The authors acknowledge the skillful assistance of B. Diouf. This work was supported in part by an INSERM grant (no. CRE 863 001).
REFERENCES
1. BACH, M.-A., WALLACH, D., FLAGEUL, B., HOFFENBACH, A. and COTTENOT, F. Antibodies to phenolic glycolipid-I and to whole Mycobacterium leprae in leprosy patients: evolution during therapy. Int. J. Lepr. 54(1986)256-267.
2. BRETT, S. J. and BUTLER, R. Interactions of Mycobacterium lepraemurium with resident peritoneal macrophages; phagocytosis and stimulation of the oxidative burst. Clin. Exp. Immunol. 71(1988)32-38.
3. CHAN, J., FUJIWARA, T., BRENNAN, P., MCNEIL, M., TURCO, S., SIBILLE, J.-C, SNAPPER, M., AISEN, P. and BLOOM, B. R. Microbial glycolipids: possible virulence factors that scavenge oxygen radicals. Proc. Nat. Acad. Sci. U.S.A. 86(1989)2453-2457.
4. DOUVAS, G. S., LOOKER, D. L., VATTER, A. E. and CROWLE, A. F. Gamma interferon activates human macrophages to become tumoricidal and lcishmanicidal but enhances replication of macrophage-associatcd mycobacteria. Infect. Immun. 50(1985)1-8.
5. DRAPER, P. Structure of Mycobacterium leprae. Lepr. Rev. 57 Suppl.2(1986)15-20.
6. FLESCH, I. and KAUFMANN, S. H. E. Mycobacterial growth inhibition by interferon-γ activated bone marrow macrophages and differential susceptibility among strains of Mycobacterium tuberculosis. J. Immunol. 138(1987)4408-4413.
7. HOLZER, T. J., NELSON, K. E., SCHAUF, V., CRISPEN, R. G. and ANDERSEN, B. R. Mycobacterium leprae fails to stimulate phagocytic cell superoxide anion generation. Infect. Immun. 51(1986)514-520.
8. HOLZER, T. J., KIZLAITIS, L., VACHULA, M., WEAVER, C. W. and ANDERSEN, B. R. Human phagocytic cell response to Mycobacterium leprae and Mycobacterium bovis bacillus Calmette-Gucrin; an in vitro comparison of leprosy vaccine components. J. Immunol. 141(1988)1701-1708.
9. LAUNOIS, P., BLUM, L., DIEYE, A., MILLAN, J., SARTHOU, J. L. and BACH, M.-A. Phenolic glycolipid-l from M. leprae inhibits oxygen free radical production by human mononuclear cells. Res. Immunol. 14(1989)847-855.
10. LAUNOIS, P., MAILLERE, B., DIEYE, A., SARTHOU, J. L. and BACH, M.-A. Human phagocyte oxidative burst activation by BCG, M. leprae and atypical mycobacteria: defective activation by M. leprae is not reversed by interferon-γ. Cell. Immunol. 124(1989)168-174.
11. MURRAY, H. W., JUANGBHANICH, C. W., NATHAN, C. F. and COHN, Z. A. Macrophage oxygen-dependent antimicrobial activity. II. The role of oxygen intermediates. J. Exp. Med. 150(1979)950-964.
12. NATHAN, C. F., KAPLAN, G., LEVIS, W. R., NUSRAT, A., WITMER, M. D., SHERWIN, S. A., JOB, C. K, HOROWITZ, C. R., STEINMAN, R. M. and COHN, Z. A. Local and systemic effects of intradermal recombinant interferon-γ in patients with lepromatous leprosy. N. Engl. J. Med. 315(1986)6-15.
13. NATHAN, C. F., MURRAY, H. W., WIEBE, M. E. and RUBIN, B. Y. Identification of interferon-γ as the lymphokine that activates human microbicidal oxidative metabolism and antimicrobial activity. J. Exp. Med. 158(1983)670-683.
14. NEIL, M. A. and KLEBANOFF, S. J. The effect of phenolic glycolipid-1 from Mycobacterium leprae on the antimicrobial activity of human macrophages. J. Exp. Med. 167(1988)30-42.
15. REED, S. G., NATHAN, C. F., PIHL, D. L., RODERICKS, P., SHANEBECK, K., CONLON, P. J. and GRABSTEIN, K. H. Recombinant granulocyte/macrophage colony-stimulating factor activates macrophages to inhibit Trypanosoma cruzi and release hydrogen peroxide; comparison with interferon-γ. J. Exp. Med. 166(1987)1734-1746.
16. RIDLEY, D. S. and JOPLING, W . H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-267.
17. SIBLEY, L. D. and KRAHENBUHL, J. L. Mycobacterium leprae-burdened macrophages are refractory to activation by gamma-interferon. Infect. Immun. 55(1987)446-450.
18. TOBA, H., CRAWFORD, J. T. and ELLNER, J. J. Pathogenicity of Mycobacterium avium for human monocytes: absence of macrophagc-activating factor activity of gamma interferon. Infect. Immun. 57(1989)239-244.
19. VACHULA, M., HOLZER, T. J. and ANDERSEN, B. R. Suppression of monocyte oxidative response by phenolic glycolipid-1 of Mycobacterium leprae. J. Immunol. 142(1989)1696-1701.
20. VACHULA, M., HOLZER, T. J., KIZLAITIS, L. and ANDERSEN, B. R. Effect of Mycobacterium leprae's phenolic glycolipid-I on interferon-gamma augmentation of monocyte oxidative responses. Int. J. Lepr. 58(1990)342-346.
21. WEISER, W. Y., NIEL, A. V., CLARK, S. C, DAVID, J. R. and REMOLD, H. J. Recombinant human granulocyte/macrophage colony-stimulating factor activates intracellular killing of Leishmania donovani by human monocyte-derived macrophages. J. Exp. Med. 166(1987)1436-1446.
1. M.D., Institut Pasteur de Dakar, B. P. 220, Dakar, Senegal.
2. Biological Pharmacist, Institut Pasteur de Dakar, B. P. 220, Dakar, Senegal.
3. Biological Pharmacist, Immunologic Cellulaire, Institut Pasteur de Dakar, B. P. 220, Dakar, Senegal.
4. D.Sc, Immunologie Parasitaire, Institut Pasteur de Dakar, B. P. 220, Dakar, Senegal.
5. M.D., Institut de Léprologie Appliquée de Dakar, Dakar, Senegal.
6. M.D., Director, Institut de Léprologie Appliquée de Dakar, Dakar, Senegal.
7. M.D., Ph.D., Unité de Pathologie de l'Immunité, Faculté Neckcr Enfants Malades, Paris, France.
Received for publication on 30 January 1991.
Accepted for publication in revised form on 3 June 1991.