- Volume 59 , Number 4
- Page: 590–7
Serum antibodies against peripheral nervous system antigens in leprosy
ABSTRACT
Since antibodies against peripheral nervous system (PNS) antigens may play a pathogenetic role in the mechanism of nerve damage in leprosy, sera from leprosy patients and contacts were investigated for anti-PNS antibodies by ELISA and immunoblot. In ELISA, elevated anti-PNS antibody levels were detected in 4 of 98 (4.1%) leprosy patients (4 of 52, 7.7%, lepromatous leprosy patients), in 1 of 28 (3.6%) contacts, and in 1 of 18 (5.6%) normal controls. There was no correlation between anti-PNS antibody levels and the bacterial index or neuropathy in leprosy. Immunoblot with a sample of six leprosy and five control sera showed that the antigenic binding pattern (mainly within the 100-200-kDa region) was very similar in patients and controls. Staining intensity, however, appeared to be higher with the leprosy sera than with the control sera. IgM and IgG were found to contribute to the staining pattern: IgM in the 150-200-kDa range, IgG with multiple bands between 25 kDa and 200 kDa. Thus, the presence and levels of scrum anti-PNS antibodies in leprosy appear to be unrelated to parameters of disease activity, neuropathy in particular, and do not seem to be critically involved in the pathogenesis of nerve damage.RÉSUMÉ
Puisque les anticorps vis-à-vis des antigènes du système nerveux périphérique (SNP) peuvent avoir un rôle pathogène dans le mécanisme des lésions nerveuses de la lèpre, le sérum de patients lépreux et de contacts a été examiné pour la recherche d'anticorps anti-SNP par ELISA et immunoblotting. Des titres élevés d'anticorps anti-SNP ont été détectés par ELISA chez 4 des 98 patients lépreux (4, 1%) (4 parmi 52 patients lépromatcux, ou 7, 7%), chez 1 des 28 contacts (3, 6%) et 1 des 18 témoins normaux (5, 6%). Il n'y avait pas de corrélation entre les titres d'anticorps anti-SNP et l'index bactérien ou la neuropathic de la lèpre. L'immunoblotting réalisé sur le sérum de six lépreux et cinq témoins a montré que le mode de liaison antigénique (principalement dans la région de 100-200 kDa) était très semblable chez les malades et chez les témoins. L'intensité de la coloration était cependant plus forte avec le sérum des lépreux qu'avec celui des témoins. On a observé que des IgM et des IgG contribuaient au mode de coloration: des IgM dans la zone 150-200 kDa et des IgG avec des bandes multiples entre 25 kDa et 200 kDa. En conclusion, la présence et les taux d'anticorps anti-SNP du sérum n'apparaissent pas corrélés à l'activité de la maladie, ni à la neuropathic, et ne semblent pas être impliqués de manière prépondérante dans la pathogénèse des lésions nerveuses.RESUMEN
Puesto que los anticuerpos contra los antígenos del sistema nervioso periférico (SNP) pueden jugar un papel patogenético en el mecanismo del daño a los nervios en la lepra, se analizaron los sueros de pacientes con lepra y de contactos sanos para buscar anticuerpos anti-SNP por ELISA y por inmunoelectrotransferencia ("inmunoblot"). Por ELISA, se encontraron títulos elevados de anticuerpos anti-SNP en 4 de 98 (4.1%) pacientes con lepra (4 de 52, 7.7%, pacientes lcpromatosos), en 1 de 28 (3.6%) contactos, y en 1 de 18 controles sanos. No hubo correlación entre los títulos de anticuerpos anti-SNP y el índice bacteriano o la neuropatía en los pacientes. El "inmunoblot" con los sueros de 6 pacientes con lepra y de 5 individuos sanos, mostró que el patrón de reactividad antigénica en la región de 100-200 kDa fue muy similar en los pacientes y en los controles, sin embargo, la intensidad de la tinción pareció ser mayor con los sueros de los pacientes. Mientras que los anticuerpos de la clase IgM reconocieron antígenos principalmente en la región de 150¬ 200 kDa, los anticuerpos IgG reaccionaron con múltiples antígenos localizados desde los 25 hasta los 200 kDa. Así, la presencia y niveles de anticuerpos anti-SNP en la lepra, no parecen estar relacionados ni con los parámetros de actividad de la enfermedad (en particular la neuropatía), ni con la patogénesis del daño a nervios.Since a possible role of autoimmunity in the mechanism of nerve damage in leprosy has been suggested (2), the study of serum antibodies directed against peripheral nervous system (PNS) antigens in leprosy has been a subject of interest. By indirect immunofluorescence, Wright, et al. (18) demonstrated an IgG antibody against PNS axons in a high percentage of leprosy patients, but also in polyneuropathy patients and normal controls, and found no obvious relationship between this antibody and neuropathy in leprosy. Using radioimmunoassay, Mshana, et al. (11) demonstrated antibodies against the PNS myelin protein P2 in leprosy patients (13%) without correlation between anti-P2 and neuropathy. Eustis-Turf, et al. (6) found IgG against human nerve in a high percentage of leprosy patients by indirect immunofluorescence; these antibodies were shown to react with neural intermediate filament (IF) proteins by immunoblot. Benjamins, et al. (1) further characterized these anti-IF IgG by immunoblot, and found them to occur in patients with leprosy, tuberculosis, and neurological disease as well as in normal controls. Ghaswala, et al. (7) presented evidence for equal binding of serum IgG from leprosy patients and normal controls to human peripheral nerve by enzyme-linked immunosorbent assay (ELISA). Recently, Parkash, et al. (12) demonstrated antinerve IgG in leprosy patients (22%) and normal controls (6%) using rabbit peripheral nerve as antigen in ELISA. On the other hand, Thomas and Mukherjee(16) reported on an ELISA test for IgG against human peripheral nerve which was clearly discriminative between leprosy patients (of all disease types) and normal controls. Although it is difficult to compare these results on serum antibodies against neural antigens in leprosy because of the variations in the methods and antigen preparations employed, it is obvious that the data are conflicting.
In order to address the question of the role anti-PNS antibodies may play in the pathogenesis of leprosy neuropathy, we studied the occurrence of anti-PNS antibodies in sera from leprosy patients and aimed at a characterization of these antibodies with respect to immunoglobulin (Ig) class and antigen specificity using human peripheral nerve as antigen in ELISA and immunoblot.
MATERIALS AND METHODS
Sera. Sera from 98 leprosy patients, clinically and histologically classified according to the Ridley-Jopling scale (14) as lepromatous (LL, N = 52), borderline lepromatous (BL, N = 8), midborderline (BB, N = 8), borderline tuberculoid (BT, N = 21), and tuberculoid (TT, N = 9) leprosy, as well as sera from 28 household or family contacts (HQ of leprosy patients, were obtained from Bayley Seton Hospital, Staten Island, New York, U.S.A. The bacterial index (BI) was measured on a semilogarithmic scale (0- 6+) approximating that of Ridley (13). Histology, including the BI, from skin biopsies was reported from the Gillis W. Long Hansen's Disease Center, Carville, Louisiana, U.S.A. Neuropathy was assessed by clinical examination and nerve conduction velocity tests (ulnar, radial, median, common peroneal, and tibial nerves) in 86 patients and scored as: grade 0 = nerve conductions within normal limits (N = 8); grade 1 = at least one nerve abnormally responsive in velocity, latency or amplitude (N = 19); and grade 2 = at least one nonresponsivc nerve (N = 59). Sera from 18 healthy subjects (laboratory personnel) were used as normal controls (CO). In addition, serum from a patient with polyneuropathy was used as reference. Serum samples were stored in aliquots below -70ºC.
Quantitation of scrum Ig was kindly performed by Dr. P. Panfili using a nephelometric analyzer (Behring Diagnostics, La Jolla, California, U.S.A.).
Antigen preparation. Nerve fascicles, prepared from normal human femoral nerve (autopsy) as described by Brown, et al. (4), were homogenized in distilled water and lyophilized. The whole PNS homogenate was delipidated (8) by twice extraction with diethyl ether/absolute ethanol (3/2, v/v) followed by once extraction with ether. Whole PNS (wPNS) and delipidated PNS (dPNS) homogenates were stored dry at - 70ºC.
ELISA for anti-PNS antibodies. A suspension (1 mg/ml) of wPNS (or dPNS) in 0.2% w/v sodium dodecylsulfate (SDS; Serva) was prepared by sonication for 5 min on ice. Flat-bottom, polystyrene microliter plates (Nunc) were coated by incubation with 100 µl/well of the wPNS (or dPNS) suspension, diluted to 10 µg/ml in carbonate-bicarbonate buffer (CBC: 15 mM Na2CO3, 35 mM NaHCO3, pH 9.6) as well as with CBC alone as a control coat, for 3 hr at 37ºC and then for 18 hr at 4ºC in a humidified chamber. After washing three times with phosphate-buffered saline (PBS: 8.1 mM Na2HPO4 · 2H2O, 1.5 mM KH2PO4, 2.9 mM KCI, 136.9 mM NaCl, pH 7.4) containing 0.1% v/v Tween 20 (PBST), the plates were incubated for 1 hr at 25ºC with 200 µ/well of 2.5% w/v human serum albumin (HSA; kindly donated by Dr. F. Elsinger, Immuno A.G., Vienna, Austria) in PBS (HSA-PBS). HSA-PBS was then replaced by 100 µl/well of human test serum (in duplicate), diluted 1:100-1:1600 in 2.5% HSA in PBST (HSA-PBST). A positive serum from a patient with polyneuropathy, diluted 1:100, was included as a reference on each plate. The sera were incubated for 1.5 hr at 37ºC. Washing three times with PBST was followed by incubation for 1.5 hr at 37ºC with 100 µl/well of peroxidase-conjugated, goat anti-human IgG plus IgM plus IgA (Zymed) diluted 1:1000 in HSA-PBST. After washing three times with PBST, 100 µl/well of the substrate solution, containing 1.8 mM 2,2-azino-di(3-cthyl-benzthiazoIine-6-sulfonic acid) (ABTS; Boehringer) and 2.9 mM H2O2 in citrate-phosphate buffer (60 mM C6H8O7 · H2O, 80 mM Na2HPO4-2H20, pH 4.0) was added for 1 hr at room temperature (RT) in the dark. The reaction was stopped by the addition of 100 µl/well of 0.32% w/v NaF. Extinction (E) was read with a double wavelength micro-ELISA autoreader MR 580 (Dynatech) at 405 nm (versus 490 nm) against a blank made up of substrate and stop solutions. The results were expressed as ΔE = E (PNS coat) minus E (CBC coat).
In order to minimize the influence of plate-to-plate as well as day-to-day variations on serum screening, ΔE values obtained for test sera were corrected by relating them to the reference serum ΔE on each plate. Serum samples were considered seropositive when their antibody levels exceeded the cut-off point determined as the mean ΔE plus 2 standard deviations (S.D.) of the control group. The relationships between antibody levels as well as between antibody levels and parameters of disease activity (BI, neuropathy) were assessed by the calculation of Pearson correlation coefficients.
SDS-PAGE and Western blot of PNS proteins. A suspension (4 mg/ml) of delipidated PNS homogenate in 2% w/v SDS was prepared by sonication for 5 min on ice. The protein concentration of the suspension was determined by the method of Lowry, et al. (10). Samples for SDS-polyacrylamide gel electrophoresis (SDS-PAGE) were prepared by 1/1 v/v dilution of the 2% SDS suspension with twice concentrated sample buffer (154 mM Tris, 88 mM glycine, 1.6 mM EDTA, 1.6 mM MgCl2, 3% v/v mercaptoethanol, 16% w/v sucrose, 0.04% w/v bromophenol blue, pH 8.0) followed by sonication as above, incubation for 30 min at 37ºC, and centrifugation at 16,000 x g x 2 min. Proteins of PNS homogenate samples (25-80 µg protein/lane) were separated by discontinous SDS-PAGE(9) on 1.5-mm thick vertical slab gels, consisting of a 5% acrylamide stacking gel and a running gel with an 8%-15% linear gradient of acrylamide, in electrode buffer (50 mM Tris, 384 mM glycine, 0.1% w/v SDS, pH 8.3). For the calculation of molecular weights (MW), high and low MW markers from Biorad (2 µg each protein/lane) or from Sigma (3 µg each protein/lane) were used. For comparison, 3 µg/lane of standard human scrum IgG (Sigma) was used. Proteins were electrophoretically transferred (17) from gel to nitrocellulose (NC) sheets with a pore size of 0.45 µm (Hoefer) in transfer buffer (20 mM Tris, 150 mM glycine, pH 8.3, containing 20% v/v methanol). Blotted proteins were visualized by staining NC sheets with Ponceau xylidine, solution (0.05% w/v Ponceau xylidine, 3% w/v trichloroacetic acid), followed by destaining in several changes of PBS (10.4 mM Na2HPO4, 3.2 mM KH2PO4, 116 mM NaCl, pH 7.2).
Immunostaining for anti-PNS antibodies. PNS protein blots on NC strips were incubated with blotto (5% w/v nonfat dry milk in PBS, pH 7.2) in a multichannel chamber overnight at 4ºC. Then, the blots were incubated with 500 µl/channel of human test serum, diluted 1:50 in blotto, for 3 hr at room temperature (RT). Washing five times with blotto was followed by incubation for 1 hr at RT with 500 µl/channel of peroxidase-conjugated, goat anti-human IgG plus IgM plus IgA (Zymed), diluted 1:10.00, or of peroxidase-conjugated, rabbit anti-human IgG (or IgM or IgA) antibody (Dako), diluted 1:500 in blotto. After washing five times with blotto, two times with PBS, and once with the final solution (16.9 mM CH3COONH4, 2.9 mM C6H8O7 · H2O, pH 5.0), color was developed by incubation with substrate solution containing 0.25 mM diaminobenzidine-HCl (DAB; Fluka)and 1.5 mM H2O2 in final solution. The reaction was terminated with distilled water after about 10 min.
Standardization of immunostaining was performed using mouse monoclonal anti-P0 (kindly provided by C. Linington, University of Cardiff, U.K.), diluted 1:400-1:1600, mouse monoclonal antimyelin basic protein (MCA 70; Serotec), diluted 1:2000-1:8000, and rabbit anti-P2 antiserum (kindly provided by J.M. Matthieu, University of Lausanne, Switzerland), diluted 1:600-1:4800 in blotto. Mouse monoclonal anti-birch pollen (kindly provided by D. Kraft, University of Vienna, Austria) and normal rabbit serum were used as controls. Peroxidase-conjugated, goat anti-mouse IgG (Zymed) and peroxidase-conjugated, goat anti-rabbit IgG (Nordic), diluted 1:500 in blotto, were used for the mouse and rabbit systems, respectively.
RESULTS
ELISA for anti-PNS antibodies
Antibody levels in relation to leprosy disease activity. Serum anti-PNS antibody levels in leprosy patients did not appear to relate to the disease types according to the Ridley-Jopling classification, although four LL sera gave high levels (Fig. 1). Of all the sera tested, 4/98 (4.1%) leprosy patients (4/ 52, 7.7%, LL patients), 1/28 (3.6%) contacts, and 1/18 (5.6%) controls were positive. In addition, there was no correlation between the anti-PNS antibody levels and the BI (r = -0.070, N = 95) (not shown). However, the four LL patients who were seropositive had positive BI values ranging from 1 + to 6 + . A statistical analysis of the anti-PNS antibody levels in relation to the presence of neuropathy, as assessed by clinical examination and nerve conduction velocity tests, revealed no correlation (r = 0.160, N = 86).
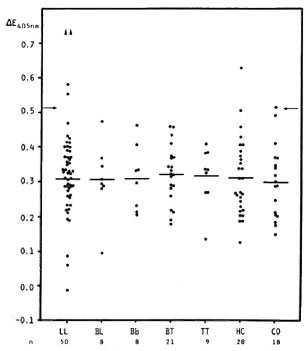
Fig. 1. Serum anti-PNS antibodies (dPNS as antigen) in lepromatous (LL), borderline lepromatous (BL), midborderline (BB), borderline tuberculoid (BT) and tuberculoid (TT) leprosy patients, in contacts (HC) and in controls (CO). Each point = one individual (serum diluted 1:100); horizontal bars = mean  E values; arrows = mean
E values; arrows = mean  E + 2 S.D. of the control group;
E + 2 S.D. of the control group;  = absorbance value beyond the test dilution and not included in mean of the other 50 LL patients.
= absorbance value beyond the test dilution and not included in mean of the other 50 LL patients.
Characterization of anti-PNS antibodies. The specificity of the anti-PNS antibody ELISA was assessed by relating the anti-PNS levels to total serum Ig concentrations in 20 leprosy sera with a mean of 22.84 ± 4.92 mg Ig/ml (ranging from 14.84 to 33.90 mg/ml). Since the anti-PNS levels did not correlate with serum Ig values (r = 0.256, N = 20), binding of Ig to PNS, not mediated by Fab, appears to be highly improbable.
A comparison of the serum antibody levels against delipidated and whole PNS homogenate in all sera tested showed a very good correlation (r = 0.899, p < 0.001, N = 142) (not shown). However, with delipidated PNS higher levels were obtained than with whole PNS, suggesting that the anti-PNS antibodies are most likely directed against protein antigens.
Immunoblot for anti-PNS antibodies
Standardization of PNS protein electrophoresis, transfer to nitrocellulose, and immunostaining was performed with mouse monoclonal and with rabbit antibodies against individual PNS proteins (Fig. 2).
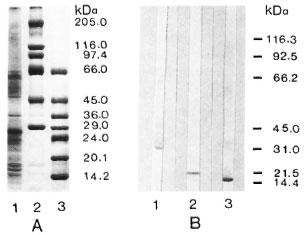
Fig. 2. SDS-PAGE of PNS homogenate (A) and immunoblots showing P0, MBP, and P2 (B). A = SDS-PAGE of PNS homogenate (25 µg protein, lane I) and of Sigma high (lane 2) and low (lane 3) MW markers (3 µg each protein/lane); gel was stained with Coomassie blue R250. B = PNS protein blots (80 µg protein/lane) immunostained with mouse monoclonal anti-P0 diluted 1:400 (lane 1), with mouse monoclonal anti-MBP diluted 1:2000 (lane 2), and with rabbit anti-P2 diluted 1:2400 (lane 3); positions of Biorad MW markers are shown on the right.
Anti-P0, anti-myelin basic protein (MBP), and anti-P2 stained protein bands with molecular weights of 28 kDa, 19kDa and 15kDa, respectively. The PNS homogenate showed multiple protein bands, one of the major bands being the PNS myelin protein P0.
Immunostaining using a conjugate against human IgG plus IgM plus IgA with 6 leprosy sera and 1 contact serum as compared with 5 normal control sera, as well as with the polyneuropathy reference serum, gave several protein bands of varying degrees of intensity and sharpness (Fig. 3). Six bands in the 100-200 kDa MW range were generally found in all of the sera. Furthermore, faint bands were detected in the lower MW region. Three additional bands in the 55-65 kDa MW range were found with the polyneuropathy serum. Although the band patterns were similar in both leprosy patients and controls, the bands generally appeared stronger in the patients and in the contacts than they did in the controls. In particular, among the leprosy and contact sera tested, all ELISA-positive sera (Fig. 3, lanes 4, 5, 6, 7), except one (lane 2), gave stronger bands as compared to ELISA-negative sera (Fig. 3, lanes 1, 3). However, among the controls tested, immunostaining did not seem to differentiate between ELISA-positive sera (lane 9) and ELISA negative (below the mean ΔE of the control group) sera (Fig. 3, lanes 10, 11, 12, 13).
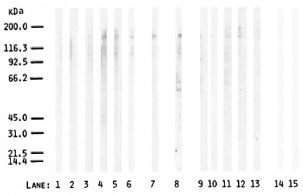
Fig. 3. Immunostaining of PNS protein blots (80 µg protein/lane) with leprosy sera (lanes 1-6), contact serum (lane 7), polyneuropathy (reference) serum (lane 8), and control sera (lanes 9-13). Blanks without serum are shown in lanes 14 and 15; peroxidase-conjugated, goat anti-human IgG plus IgM plus IgA was used at 1:1000 dilution; positions of Biorad MW markers are shown on the left.
Characterization of anti-PNS antibodies with respect to immunoglobulin classes (Fig. 4) indicates that these antibodies are mainly of the IgG and IgM classes in both leprosy patients and controls. The protein bands with IgA appeared very faint in the 100-200 kDa MW region. While the bands with IgM were confined mainly to the 150-200 kDa MW range, multiple bands extending from 25 kDa to 200 kDa were obtained with IgG. Staining of four strong bands in the 150-200 kDa MW region appeared to be identical with IgG and IgM. In the IgG class the leprosy serum gave two faint bands (at 26 kDa and 50 kDa) which were not seen with the control serum.
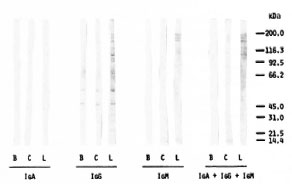
Fig. 4. PNS protein blots (80 µg protein/lane) immunostained with a leprosy serum (L = same as lane 5 in Fig. 3) and a normal control serum (C = same as lane 10 in Fig. 3) for IgA, IgG, IgM, as well as total Ig. B is blank without serum; positions of Biorad MW markers are shown on the right.
A high background, i.e., binding of conjugate to PNS proteins in the absence of serum, was observed with IgG conjugate in contrast to IgM and IgA (Fig. 4). That the PNS homogenate most likely contains residual serum IgG responsible for the background effect was confirmed by a very similar pattern obtained when running standard human serum IgG and the human PNS preparation in parallel and staining with anti-human IgG conjugate (Fig. 5).
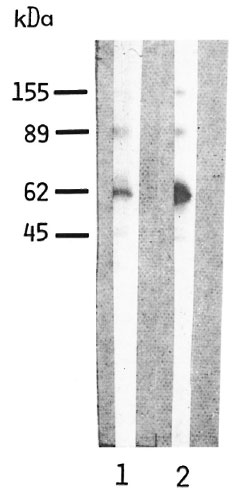
Fig. 5. Reaction of peroxidase-conjugated, rabbit antihuman IgG with blots of human PNS homogenate (80 µg protein, lane I) and of standard human serum IgG (3 µg, lane 2). Blots were immunostained with peroxidase-conjugated, rabbit antihuman IgG diluted 1:500; calculated molecular weights are shown on the left.
DISCUSSION
The occurrence of serum antibodies against PNS antigens in leprosy patients, contacts, and controls was investigated by ELISA and immunoblot. In the ELISA, anti-PNS antibodies were detected in 4/98 (4.1%) leprosy patients with the LL disease form (4/52, 7.7%). Anti-PNS activity appeared not to be due to unspecific Ig binding to PNS and seemed to be directed mainly against protein antigens. These antibodies statistically were not related to the leprosy disease type or the bacterial index (BI), although the four seropositive LL patients had positive BI values. This is in accordance with the reports of other authors (1, 6, 7, 11, 18) and clearly contradicts the report from Thomas and Mukherjee (16). Furthermore, these anti-PNS antibodies appeared not to be related to leprosy neuropathy, again confirming earlier reports (1, 11, 18).
Of the contacts and controls, 1/28 (3.6%) and 1/18 (5.6%) were seropositive for anti-PNS antibodies, respectively. The occurrence of autoantibodies against PNS antigens in sera from normal control individuals has been reported (1, 6, 7, 12, 18). Interestingly, the only leprosy contact positive for anti-PNS antibodies was from a family with more than one case of multi-bacillary leprosy, had presented with BI-negative skin lesions, and also had an elevated titer of serum IgA, against phenolic glycolipid-I (15). On the other hand, the normal individual positive for anti-PNS antibodies had once lived in a malaria-endemic area. It has been reported that malaria patients have high levels of circulating autoantibodies (3).
Although these results suggest that anti-PNS antibodies are related neither to leprosy nor to leprosy neuropathy, the sera positive in the anti-PNS ELISA were further investigated for binding to individual PNS proteins by immunoblot. Immunostaining with an antihuman total Ig conjugate revealed binding of all sera tested to unidentified PNS proteins with no obvious differences in band patterns, although the staining appeared to be stronger in leprosy patients than in controls. The finding that the staining intensity did not parallel the ELISA values, especially among controls, may be due to unspecific Ig binding to blotted proteins or to differences in antigen presentation by the two methods.
Characterization of anti-PNS antibodies in a leprosy an d a control serum with respect to Ig class by immunoblot showed that the main contribution to the staining with antihuman Ig conjugate came from IgG and IgM. Staining for IgG gave multiple bands between 25 kDa and 200 kDa; staining for IgM was mainly found in the high (150-200 kDa) MW region and included four bands, which were also detected with IgG. Antibodies against nerve antigens in leprosy sera have thus far been reported to be of the IgG Class(1, 6, 7, 12, 16, 18).
In the IgG class, a faint band was seen at 25 kDa with the leprosy scrum but not with the control serum. This staining might reflect binding to P0 protein, although the M W for P0 obtained with the mouse monoclonal anti-P0 antibody was 28 kDa. Eustis-Turf, et al. (6) reported reactivity of 2/17 leprosy sera with a 30 kDa band considered to be P0, but only when Western blots of PNS myelin were used. Furthermore, another faint band at 50 kDa was detected in the IgG class with the leprosy serum as opposed to the control serum. This may correspond to binding to neural intermediate filament proteins, as has been described by Eustis-Turf, et al. (6) in the 45-55 kDa MW region. Again, our results on immunostaining of PNS proteins with leprosy sera are in contrast to those of Thomas and Mukherjee (16), who observed prominent IgG staining with leprosy but not control sera. This may possibly be due to a difference in the PNS preparation used: Upon electrophoresis, two proteins (around 40 and 70 kDa MW) predominated in their preparation, a supernatant of a PNS sonicate (16), while one of the major proteins of our PNS homogenate was the PNS myelin protein P0 with 28 kDa MW.
Immunostaining for IgG was hampered by a strong background binding of antihuman IgG conjugate to human PNS blots. This observation compares well to the background binding seen by Benjamins, et al. (1) and considered nonspecific. In our study, the anti-IgG binding pattern of human PNS was demonstrated to be very similar to that of human serum IgG, suggesting that this binding is most probably caused by a contamination of the PNS preparation with serum IgG. This difficulty may be circumvented using highly purified antigen (e.g., PNS myelin) or antigen from other than human species.
In conclusion, this study could not establish any relationship of anti-PNS antibody levels to the parameters of disease activity, such as classification, bacterial index, and neuropathy in leprosy. The presence of anti-PNS autoantibodies in leprosy patients may be attributed to a variety of factors including a response to nerve destruction, a cross-reactivity between M. leprae antigens and host proteins, or an unspecific polyclonal B-cell activation (5, 19). The question remains open whether and how even low levels of antibodies against PNS antigens may contribute to the pathogenetic mechanisms of nerve damage in the context of leprosy neuropathy.
Acknowledgment. Our work was supported by the Austrian Science Research Fund, Projects P 6438M and P 7740-M ED. We are grateful to Prof. Dr. H. Lassmann for kindly forwarding some of the monoclonal antibodies and Dr. G. F. Suchanek for useful discussion.
REFERENCES
1. BENJAMINS, J. A., CALLAHAN, R. E., RUNFT, D., GERRAS, G. and LEFFORD, M. J. Anti-neural antibodies in leprosy sera: further characterization of the antigens. J. Neuroimmunol. 21(1989) 125-135.
2. BODDINGIUS, J. Mechanisms of nerve damage in leprosy. In: Immunobiologic Aspects of Leprosy, Tuberculosis and Leishmaniasis. Humber, D.P., ed. Oxford: Exccrpta Medica, 1981, pp. 64-73.
3. BONFA, E., LLOVET, R., SCHEINBERG, M., DE SOUZA, J. M. and ELKON, K. B. Comparison between autoantibodies in malaria and leprosy with lupus. Clin. Exp. Immunol. 70(1987)529-537.
4. BROWN, M. J., PLEASURE, D. E. and ASBURV, A. K. Microdissection of peripheral nerve: collagen and lipid distribution with morphological correlation. J. Neurol. Sci. 29(1976)361-369.
5. DUGGAN , D. B., MACKWORTH-YOUNG , C, KARI-LEFVERT, A., ANDRE-SCHWARTZ, J., MUDD , D., MCADAM, K. P. W. J. and SCHWARTZ, R. S. Polyspecificity of human monoclonal antibodies reactive with Mycobacterium leprae, mitochondria, ssDNA, cytoskclctal proteins, and the acetylcholine receptor. Clin. Immunol. Immunopathol. 49(1988)327-340.
6. EUSTIS-TURF, E. P., BENJAMINS, J. A . and LEFFORD, M. J. Characterization of the anti-neural antibodies in the sera of leprosy patients. J. Neuroimmunol. 10(1986)313-330.
7. GHASWALA, P. S., MISTRY, N. F. and ANTIA, N. H. Scrum antibodies of normals and leprosy patients show equal binding to peripheral nerve. (Letter) Int. J. Lepr. 57(1989)690-692.
8. GREENFIELD, S., NORTON, W. T. and MORELL, P. Quaking mouse: isolation and characterization of myelin protein. J. Neurochem. 18(1971)2119-2128.
9. LAEMMLI, U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 227(1970)680-685.
10. LOWRY, O. H., ROSEBROUOH, N. J., FARR, A. L. and RANDALL, R. J. Protein measurement with the Folin-phenol reagent. J. Biol. Chem. 193(1951)265-275.
11. MSHANA, R. N., HARBOE, M., STONER, G. L., HUGHES, R. A. C, KADLUBOWSKI, M. and BELEHU, A. Immune responses to bovine neural antigens in leprosy patients. I. Absence of antibodies to an isolated myelin protein. Int. J. Lepr. 51(1983)33-40.
12. PARKASH, O., SREEVATSA and SENGUPTA, U. Anattempt to demonstrate antinerve antibodies in leprosy sera using rabbit nerve as an antigen. (Letter) Int. J. Lepr. 58(1990)129-130.
13. RIDLEY, D. S. Bacterial indices. In: Leprosy in Theory and Practice. Cochrane, R. G. and Davey, T. F., eds. Baltimore: Williams and Wilkins, 1964, pp. 620-622.
14. RIDLEY, D. S. and JOPLING, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
15. SCHWERER, B., CHUJOR, C. S. N., BERNHEIMER, H., RADL, J., HAAIJMAN, J. J., MEEKER, H. C, SERSEN, G. and LEVIS, W. R. IgA antibodies against phenolic glycolipid I from Mycobacterium leprae in serum of leprosy patients and contacts: subclass distribution and relation to disease activity. Clin. Immunol. Immunopathol. 53(1989)202-211.
16. THOMAS, B. M. and MUKHERJEE, R. Antineural antibodies in sera of leprosy patients. Clin. Immunol. Immunopathol. 57(1990)420-429.
17. TOWBIN, H., STAEHELIN, T. and GORDON, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. U.S.A. 76(1979)4350-4354.
18. WRIGHT, D. J. M., HIRST, R. A. and WATERS, M. F. R. Neural autoantibodies in leprosy. Lepr. Rev. 46(1975)157-169.
19. YOUNG, D. B. Stress-induced proteins and the immune response to leprosy. Microbiol. Sci. 5(1988)143-146.
1. Ph.D., Neurological Institute, University of Vienna, Schwarzspanierstrasse 17, A-1090, Vienna, Austria.
2. M.D., Neurological Institute, University of Vienna, Schwarzspanierstrasse 17, A-1090, Vienna, Austria.
3. M.D., Neurological Institute, University of Vienna, Schwarzspanierstrasse 17, A-1090, Vienna, Austria.
4. Ph.D., Neurological Institute, University of Vienna, Schwarzspanierstrasse 17, A-1090, Vienna, Austria.
5. M.S., New York State Institute for Basic Research in Mental Retardation, Staten Island, New York, U.S.A.
6. M.D., New York State Institute for Basic Research in Mental Retardation, Staten Island, New York, U.S.A.
Reprint requests to Dr. Schwerer.
Received for publication on 23 April 1991.
Accepted for publication in revised form on 29 July 1991.