- Volume 59 , Number 4
- Page: 598–604
Effects of cyclosporin A on bacterial growth and immunological responsiveness in BALB/c mice infected with Mycobacterium Leprae
ABSTRACT
When BALB/c mice were infected with Mycobacterium leprae and orally treated 6 times weekly with a dose of 8 mg/kg cyclosporin A (CsA) for 19 months, the number of organisms was slightly higher at 19 months as compared with mice in which the dose of CsA was gradually decreased after 6 months and discontinued at the 8th month (p < 0.01 for the 15th and 19th months). Lymphocyte blast transformation (LBT) showed that spleen cells from CsA-treated mice 4 weeks after infection with M. leprae and 3 weeks after CsA treatment was stopped responded to the sonicated supernatant of M. leprae suspension (SS), M. leprae (Ml), and concanavalin A (ConA) less than those cells from mice not treated with CsA. This response was dose-dependent. At week 15, 14 weeks after CsA administration was stopped, the LBT response to SS and Ml by cells from M. /^rac-infected mice exceeded that of mice without CsA treatment, and the response to ConA in M. leprac-infected mice was less than that in uninfected mice without CsA-treatment. Thus, if CsA was administered, the T-cell functions were suppressed. However, when CsA treatment was discontinued for longer periods, the T-cell function was activated. From these results, we speculate that M. leprae would have the capability of growing more abundantly in mice treated with CsA 100 mg/kg for 1 week every month.RÉSUMÉ
Des souris BALB/c ont été infectées par Mycobacterium leprae et traitées par voie orale par 6 doses de 8 mg/kg de cyclosporinc A (CsA) par semaine pendant 19 mois. Le nombre d'organismes était légèrement plus élevé après 19 mois, par comparaison avec des souris chez lesquelles la dose de CsA fut progressivement réduite après 6 mois, et stoppée au 8èmc mois (p < 0.01 pour les 15ème et 19ème mois). La transformation lymphoblastique des lymphocytes (TBL) a montré que les cellules spléniques de souris traitées à la CsA 4 semaines ares l'infection par M. leprae et 3 semaines après l'arrêt du traitement à la CsA, répondaient moins au surnageant soniqué (SS) d'une suspension de Al. leprae, au Ai. leprae (Ml) et à la concanavalinc A (ConA) que les cellules de souris non traitées à la CsA. Cette réponse était dépendante de la dose. A la semaine 15, c'est-à-dire 14 semaines après l'arrêt de l'administration de CsA, la réponse TBL vis-à-vis de SS et Ml par les cellules de souris infectées par Al. leprae, dépassait celle de souris non traitées à la CsA, et la réponse à la ConA des souris infectées par M. leprae était plus faible que celle des souris non infectées et non traitées à la CsA. Donc si la CsA était administrée, les fonctions des cellules T étaient supprimées. Cependant, quand le traitement à la CsA était arrêté pour des périodes plus longues, la fonction des cellules T était activée. A partir de ces résultats, nous formulons l'hypothèse que Al. leprae aurait la capacité de se reproduire plus rapidement chez des souris traitées à la CsA à la dose de 100 mg/kg pendant 1 semaine chaque mois.RESUMEN
Se infectaron ratones BALB/c con Mycobacterium leprae y se trataran 6 veces a la semana con dosis orales de 8 mg/kg de ciclosporina A (CsA), durante 19 meses. El número de microorganismos fue ligeramente mayor en los ratones tratados durante 19 meses que en aquellos en los cuales la dosis de CsA se disminuyó gradualmente después de 6 meses y se descontinuó al mes octavo (p < 0.01 para los meses 15 y 19). La transformación blastoidc de linfocitos (TBL) a las 3 semanas de haber suspendido el tratamiento con CsA, mostró que las células esplénicas de los ratones tratados con la droga cuatro meses después de la infección con M. leprae, respondieron al sobrenadante sonicado de una suspensión de M. leprae (SS), al M. leprae (ML), y a la concanavalina A (ConA), en menor grado que las células de los ratones no tratados con CsA; esta respuesta fue dependiente de la dosis. A la semana 15, 14 semanas después de haber suspendido la administración de CsA, la respuesta TBL de las células de los ratones infectados con M. leprae contra el ss y el ML, excedió aquella respuesta de los ratones sin tratamiento con CsA. La respuesta a la ConA de las células de los ratones infectados con M. leprae fue menor que la respuesta de los ratones no infectados y no tratados con CsA. Así, aunque la administración de CsA suprime la función de las células T, ésta se regenera cuando el tratamiento con CsA se descontinúa por periodos largos de tiempo. A partir de estos resultados, especulamos que el Ai. leprae podría multiplicarse más abundantemente en ratones tratados con 100 mg/kg de CsA durante una semana de cada mes.Cyclosporin A (CsA) is a well known immunosuppressive agent, and is clinically used in the transplantation of various organs, such as the kidney, liver, heart and so on. It has suppressive effects on T-ccll-dependent antibody formation and the cellular immune response (2, 3, 8, 9, 27, 28). Several investigators have reported that CsA inhibits the production of lymphokines by T lymphocytes (4, 6, 27, 28) and that it inhibits T-cell growth factor gene expression (12) or impairs the release of interleukin 1 (IL-1) and IL-2 (4). Alberti, et al. (2) have presented the hypothesis that T-helper lymphocytes are the preferential target of CsA immunodepression. Mycobacterium leprae can multiply only in a limited number of experimental animals, one of which is the nude mouse (") which is defective in T-cell function. Therefore, we gave CsA to a normal mouse in order to depress T-lymphocyte function, and injected it with M. leprae to investigate the growth of M. leprae and the immunological responsiveness of CsA-treated mice.
MATERIALS AND METHODS
Microorganism and bacterial suspension. M. leprae were originally obtained from a biopsy of a subcutaneous leproma from a previously untreated lepromatous leprosy patient and serially passed in the foot pads of BALB/c nude mice. The foot pads from infected mice were minced with scissors, ground, and added to phosphate buffered saline (PBS). The suspension was centrifuged at 300 x g to remove debris. The bacterial number was assessed in the supernatant by Shepard's method (23).
Reagents. Cyclosporin A (CsA), a product of Sandoz Pharmaceuticals, Ltd., was dissolved in olive oil for oral administration. Concanavalin A (ConA) was purchased from Sigma Chemical Company, St. Louis, Missouri, U.S.A., and 3H-thymidine was a product of New England Nuclear Research Products, Boston, Massachusetts, U.S.A. Rabbit anti-mouse immunoglobulin, fluorescein conjugated, and rabbit anti-mouse T-cell serum were products of Cedarlane Laboratories, Ltd., Ontario, Canada, and fluorescein-conjugated goat anti-rabbit immunoglobulin was a product of Cooper Biomedical Inc., Malvern, Pennsylvania, U.S.A. Dulbecco's modified Eagle medium (DMEM) was from Flow Laboratories, Inc., Tokyo, Japan.
Administration schedule. Six-week-old, female BALB/c mice (Sizuoka Animal Farm) were separated into several groups. In the first experiment, one group received CsA 12 mg/kg body weight at day -2 and 10 mg at day -I, then 1.5 x 106 M. leprae were injected into the right hind foot pad at day 0. A dose of 8 mg/kg CsA was continously administered six times weekly during the course of the experiments (for 19 months). Another group of mice was treated with CsA in the same manner during the first 6 months, and received 3 mg/kg six times weekly for the next 2 months. At various intervals, the mice were sacrificed and the number of acid-fast bacilli (AFB) in the injection site was assessed by the method of Shepard (23).
In the second experiment, doses of CsA 12 mg/kg and 10 mg/kg body weight were administered to the first group of mice at days -2 and - 1, respectively, followed by subcutaneous injection with 2.0 x 108 M. leprae and doses of oral CsA 8 mg/kg every day for 1 week. The second group of mice was treated with 100 mg/kg daily from day - 2 to day 7. Other groups consisted of mice with or without M. leprae injections which did not receive CsA. M. /<7;rae-infccted mice were treated with or without 100 mg/kg of CsA for the observation of dclayed-type hypersensitivity.
Stimulants. M. leprae were suspended at a concentration of 5 x 107 per ml of PBS (M. leprae suspension; Ml). After sonication at 100 W for 30 min, the suspension was centrifuged at 1500 x g for 30 min. The supernatant was used as sonicated M. leprae (SS). ConA was used at a concentration of 500 Mg/ml of PBS.
Immunofluorescent antibody method (IFA). To assay T cells, 100 µl of rabbit anti-mouse T-cell antiserum was added to 100 µl DMEM containing 5 x 105 spleen cells from mice infected for 19 months. The mixture was then incubated for 30 min at 37ºC in a humidified atmosphere containing 5% CO2. The cells were washed three times with 0.15 M PBS containing 2% fetal bovine serum (FBS) and 0.02% NaN3, and resuspended in 0.1 ml of 0.15 M PBS. Fluoresccin-conjugated goat anti-rabbit immunoglobulin (100 µl) was added, and the mixture was incubated for 20 min at 4ºC, followed by three washes with PBS. Fluorescein-labeled cells were applied dropwise to glass slides, covered with a glass coverslip, sealed with nail polish, and counted using a fluorescence microscope. To assay B cells, 0.1 ml of fluorescein-conjugated rabbit anti-mouse immunoglobulin was added to spleen cells which were incubated for 20 min at 4ºC and then washed three times. B cells were counted in the same manner as T cells. The number of fluorescein-stained cells per 300 or more cells was assessed in triplicate experiments, and the T/B ratio was calculated.
Lymphocyte blast transformation (LBT). Spleen cells from mice after various infection periods were suspended in DMEM containing 10% FBS. Cells, 1 x 106 in 0.2 ml DMEM per well of a 96-well tissue culture plate, were incubated at 37ºC for 1 hr under 5% CO2 and then stimulated with 20 µl of various mitogens followed by a 48-hr incubation. After labeling with 19 kBq 3H-thymidine for 16 hr, the cells were harvested on glass-fiber filters in a multichannel cell harvester. The radioactivity (counts per minute; cpm) of the 3H-thymidine incorporated into the cells in triplicate experiments was measured by a liquid scintillation counter. The results are shown as the stimulation index (SI) determined by the following formula:

Foot pad swelling reaction. Three weeks after subcutaneous injection on the back with 2 x 108 M. leprae, the mice were challenged with 20 µl of PBS containing 1 x 106 M. leprae into the right hind foot pad and with 20 µl of PBS alone in the left. The difference in thickness between the right and left foot pads was measured with a dial thickness gauge (0.01 mm; Peacock) 2 and 4 days after challenge.
RESULTS
Growth of M. leprae in mouse foot pads (Fig. 1). The number of AFB increased gradually from the 12th to the 19th month in the group of mice to which CsA was administered until the end of the experiment. However, there was a significant decrease in the number of AFB during the same period when the CsA treatment was discontinued by the 8th month (p < 0.01 for the 16th and 19th months).
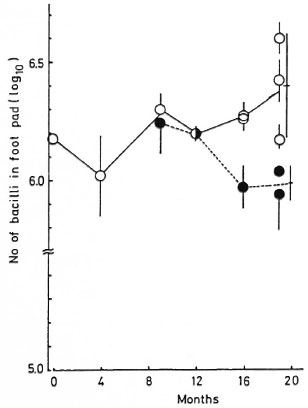
Fig. 1. Growth of M. leprae in the BALB/c mouse foot pad: 8 mg/kg CsA was administered 6 times weekly during the course of experiments ( ). As a control group, the same dose of CsA was administered during the first 6 months, 3 mg/kg 6 times weekly for 2 months thereafter, and then stopped (
). As a control group, the same dose of CsA was administered during the first 6 months, 3 mg/kg 6 times weekly for 2 months thereafter, and then stopped ( ). Each point = mean ± S.D. of the number of bacilli in the foot pad of eachmouse; bar = mean ± S.D. of the total. There is asignificant difference between the two at the 16th and 19th months (p < 0.01).
). Each point = mean ± S.D. of the number of bacilli in the foot pad of eachmouse; bar = mean ± S.D. of the total. There is asignificant difference between the two at the 16th and 19th months (p < 0.01).
LBT and T/B ratios at 19th month. As shown in Table 1, lymphocytes from mice continuously treated with CsA had a diminished response to SS and ConA compared with those from CsA-discontinued mice. This is due to the continuous administration of CsA. However, the LBT reaction in CsA-treated and M. leprae-infected mice was almost the same as that of uninfected normal mice without CsA. It may be that the 8 mg/kg dose of CsA was too small to suppress the T-cell function. The T/B ratio of mice continuously treated with CsA was significantly decreased compared with other groups (Fig. 2). Therefore, it is reasonable to consider that decreased total T-cell functions are due to the decreased T-cell number. In order to determine how long CsA administration can be discontinued while retaining immune function suppression, we investigated the LBT reaction in the early stages of M. leprae infection.
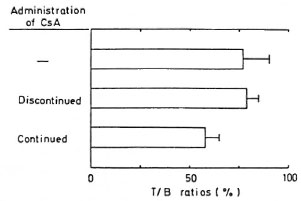
Fig. 2. T/B cell ratios of lymphocytes at 19th month: BALB/c mice were treated as shown in Figure 1; control mice were not infected and not treated with CsA. Total spleen cells were 1.1 x 108 in control mice, 0.8 x 108 in discontinued group, and 1.2 x 108 in continued group.
LBT reaction after 9 days of CsA administration. The lymphocytes from CsA-untreated mice inoculated with M. leprae responded to SS, Ml, and ConA more than those from uninfected control mice (Table 2). When a 100 mg/kg dose of CsA was administered to mice for 9 days, lymphocytes stimulated with SS and Ml incorporated as much 3H-thymidine as did those of mice without CsA. However, responses to ConA in lymphocytes from mice treated with CsA decreased remarkably as compared with those from infected mice without CsA. Thus, CsA only slightly suppressed the immune response to the M. leprae antigens, SS and MI, but did so distinctly to ConA after being given to M. leprae-infected mice for 9 days (Table 2).
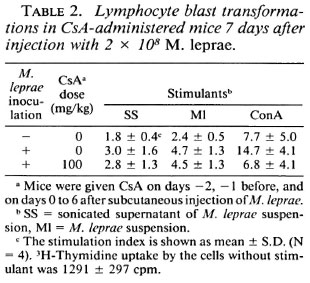
LBT reaction at week 4. Incorporation 3H-thymidine into lymphocytes in response to all three stimulants was greater in mice inoculated with M. leprae than in uninfected mice, and decreased in proportion to the dose of CsA administered to the infected mice (Table 3). These results indicate that T-cell functions were activated in M. leprae-infected mice but that CsA suppressed these activities. It is noteworthy that the LBT response to ConA in mice treated with 100 mg/kg of CsA was remarkably decreased compared with control mice.
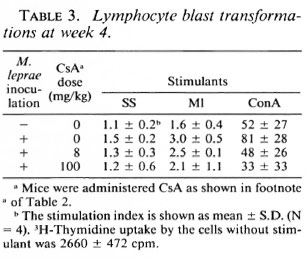
LBT reaction at week 15. LBT responses to SS and Ml in the cells from M. leprae-infected mice exceeded that of control mice (Table 4). However, the response to ConA in M. leprae-infected mice was lower than that of normal mice. In contrast with the LBT at week 4 (Table 3), spleen cells from CsA-treated mice incorporated slightly (but not significantly) more 3H-thymidine than those from M. leprae-infected mice without CsA when stimulated with all three stimulants. When ConA and Ml were used to stimulate simultaneously, the stimulation index (SI) showed an intermediate value between that of Ml and ConA (Table 4). This indicates that stimulation by ConA was partially suppressed with M. leprae in all three groups of mice in vitro.
Delayed-type hypersensitivity reaction. Foot-pad swelling was measured as the difference between the thickness of the right foot pad which was challenged with M. leprae and that of the left which received PBS. CsA-treated mice had slightly less swelling than untreated mice (Fig. 3).
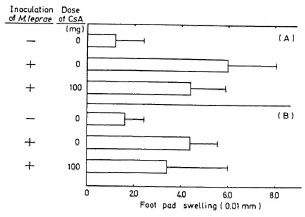
Fig. 3. Foot-pad swelling of mice 3 weeks after infection with 2 x 108 M. leprae: BALB/c mice were challenged with 1 x 106 M. leprae; 2 (A) and 4 (B) days later, foot-pad swelling was assayed by a dial thickness gauge. Each column shows mean ± S.D. of 5 mice.
DISCUSSION
There have been several reports that infections are complicated by CsA treatment in experimental animals inoculated with various microorganisms. Most are exacerbations of infectious lesions due to the inhibition of development of protective immunity (1, 5, 10, 13, 17, 18, 26). However, there are contradictions. CsA inhibited both the in vitro and in vivo growth of Cryptococcus neoformans (16). Survival of CsA-treated mice infected with Trypanosoma musculi was somewhat prolonged (20). Cutaneous lesions of Leishmania tropica-infected mice were suppressed with CsA (25). Grau, et al. (7) showed that CsA displayed a strong protective effect on neurological complications when low doses were given to Plasmodium berghei ANKA-infected CBA/Ca mice, and that protection was not seen when parasitocidal doses were used. In this study, we investigated the growth capability of M. leprae in mice treated with CsA.
When CsA was administered throughout the experiments, a small enhancement of the growth of M. leprae was recognized (Fig. 1). The mice were orally treated with 12 mg/kg and 10 mg/kg CsA at days -2 and - 1, respectively, and with 8 mg/kg daily six times weekly thereafter. This is a standard dosage finally proposed for human transplantation. Other authors have reported that effective dosages of immune suppression in bacterial infection experiments were 50, 75, 80, or 100 mg/kg (1, 3, 18, 26, 27) If CsA was administered at 100 mg/kg, M. leprae would likely flourish since the LBT reaction in mice treated with 100 mg/kg CsA was less evident than that in mice treated with 8 mg/ kg CsA (Table 3), and the number of M. leprae in the CsA-treated mouse foot pads showed an increase compared with untreated mice after 8 months (3.7 [± 1.9] x io5 bacilli in mice treated with CsA for 1 week per month compared with 1.8 [± 0.4] x 10s bacilli in CsA-untreated mice).
We tried to inoculate CsA-treated mice with 106 M. leprae, since if CsA suppressed T-cell-dependent immunity, the number of M. leprae would increase to over 106, as shown in nude mice (11). Shepard, et al. (22) reported that 5 x 103 M. leprae increased to approximately 106, which was the ceiling of multiplication in normal mice. Accordingly, we should have inoculated with 103 M. leprae for confirmation of bacterial multiplication. However, growth of M. leprae did not exceed 106.
When 100 mg/kg CsA was administered to normal mice for a week after infection with M. leprae, their lymphocyte responses to stimulation were lower than those of untreated mice at weeks 1 and 4, but not at week 15. This suggests that CsA can suppress T-cell-dependent immunity for at least 4 weeks, but that after 15 weeks a rebound phenomenon specific to CsA appears. Takashima, et al. (27) suggested this phenomenon in their delayed-typc hypersensitivity experiments. Therefore, in order to suppress the T-cell functions of normal mice, CsA should be continuously given for at least 1 of every 4 weeks.
In the delayed-type hypersensitivity experiment, foot-pad swelling in CsA-treated and M. leprae-infected mice was smaller than that in untreated and infected mice. Nomaguchi, et al. observed delayed-type hypersensitivity by challenging 4 months after immunization (unpublished data), and Matsuoka did so on the 20th week (unpublished data). A weak delayed-type hypersensitivity reaction was detected in untreated mice 3 weeks after infection with M. leprae. The reaction was distinctly decreased in CsA-treated mice.
We conjecture that immunization with large numbers of M. leprae without CsA treatment causes a specific immune response or immune tolerance. In our experiments, lymphocytes from mice subcutaneously infected with 2 x 108 M. leprae showed an increased LBT response to Ml which was higher than that of the uninfected control mice (Table 4). However, in the same mice, Ml partially suppressed the LBT response to ConA on week 15 in all three groups of mice, and the highest suppression was in the normal control group. Shepard, et al. (24) reported that mice intravenously infected with 107 or 108 heat-killed M. leprae developed immune tolerance. Lepromin and phenolic glycolipid-I (PGL-I) suppressed the ConA response of peripheral mononuclear cells of lepromatous leprosy patients (14, 15, 21). Nomaguchi, et al. (19) reported that a similar suppression occurred in mouse lymphocytes, and that it could be restored by the addition of mouse macrophages. The conditions which induce immune response or immune tolerance should now become clear.
In this study, there was less growth of M. leprae in CsA-treated mice than in nude mice. However, the enhanced capability of M. leprae to proliferate was suggested in the foot pads of mice which had received CsA at least 1 week in every 4 weeks. We hope in the future that larger amounts of live M. leprae can be obtained more easily in animals by using immunosuppressive agents or by inducing immunological tolerance.
Acknowledgment. The authors arc grateful to Sandoz Pharmaccuticals Ltd. for the supply of cyclosporin A. This work was supportcd by a grant from the U.S.-Japan Coopérative Medical Science Program.
REFERENCES
1. ADINOLFI, L. E. and BONVENTRE, P. F. Cyclosporin A treatment converts Leishmania donovani-infected C57BL/10 (curing) mice to a noncuring phenotype. Infect. Immun. 58(1990)3151-3153.
2. ALBERTI, S., BORASCHI, D., LUINI, W. and TAGLIABUE, A. Effects of in vivo treatment with cyclosporin-A on mouse cell-mediated immune responses. Int. J. Immunopharmacol. 3(1981)357-364.
3. BRAIDA, M. and KNOP, J. Effect of cyclosporin A on the T-effector and T-suppressor cell response in contact sensitivity. Immunology 59(1986)503-507.
4. BUNJES, D., HARDT, C, ROLLINGHOFF, M. and WAGNER, H. Cyclosporin A mediates immunosuppression of primary cytotoxic T cell responses by impairing the release of interlcukin 1 and interleukin 2. Eur. J. Immunol. 11(1981)657-661.
5. COX, J., KNIGHT, B. C. and IVANYI, J. Mechanisms of recrudescence of Mycobacterium bovis BCG infection in mice. Infect. Immun. 57(1989)1719-1724.
6. ESPEVIK, T., FIGARI, I. S., SHALABY, M. R., LACKIDES, G. A., LEWIS, G. D., SHEPARD, H. M. and PALLADINO, M. A., JR. Inhibition of cytokine production by cyclosporin A and transforming growth factor β. J. Exp. Med. 166(1987)571-576.
7. GRAU, G. E., GRETENER, D. and LAMBERT, P .-H. Prevention of murine cerebral malaria by low-dose 21.cyclosporin A. Immunology 61(1986)521-525.
8. HARRIS, D. T., KOZUMBO, W., CERUTTI, P. A. and CEROTTINI, J. C. Mechanism of cyclosporin A-induced immunosuppression. Cell. Immun. 109(1987)104-114.
9. HAUG, C. E., GILL, R. G., BABCOCK, S. K., LAFFERTY, K. J., BELLGRAU, D. and WEIL, R., III. Cyclosporin-induced tolerance requires antigens capable of initiating an immune response. J. Immunol. 139(1987)2947-2949.
10. HOF, H. and STRAUS, R. Suppression of acquired immunity to infection with Salmonella typhimurium by cyclosporin A. Curr. Microbiol. 13(1986)177-178.
11. KOHSAKA, K., MORI, T. and ITO, T. Lepromatoid lesion developed in nude mouse inoculated with Mycobacterium leprae. L Lepro 45(1976)177-187.
12. KRONKE, M., LEONARD, W. J., DEPPER, J. M., ARYA, S. K., WONG-STAAL, F., GALLO, R. C, WALDMANN, T. A. and GREENE, W . C. Cyclosporin A inhibits T-cell growth factor gene expression at the level of mRNA transcription. Proc. Natl. Acad. Sci. U.S.A. 81(1984)5214-5218.
13. MACGREGOR, M. P., LUCIA, H. L. VINE. W., FITZGERALD, P. and BIA, F. J. Effects of cyclosporin and cortisone on the pathogenesis of primary infection with cytomegalovirus in the guinea pig. J. Infect. Dis. 153(1986)503-510.
14. MEHRA, V., MASON, L. H., FIELDS, J. P . and BLOOM, B. R. Lepromin-induced suppressor cells in patients with leprosy. J. Immunol. 123 (1979) 1813-1817.
15. MEHRA, V., BRENNAN, P. J., RADA, E., CONVIT, J. and BLOOM, B. R. Lymphocyte suppression in leprosy induced by unique M. leprae glycolipid. Nature 308 (1984) 194-196.
16. MODY, C. H., TOEWS, G. B. and LIPSCOMB, M. F. Cyclosporin A inhibits the growth of Cryptococcus neoformans in a murine model. Infect. Immun. 56(1988)7-12.
17. MUTHUKKUMAR, S. and MUTHUKJCARUPPAN, V. EfTect of cyclosporin A on murine experimental salmonellosis. Comp. Immunol. Microbiol. Infect. Dis. 11(1988)153-161.
18. NAKANE, A., MINAGAWA, T., YASUDA, I., Yu, C. and KATO, K. Prevention by gamma interferon of fatal infection with Listeria monocytogenes in mice treated with cyclosporin A. Infect. Immun. 56(1988)2011-2015.
19. NOMAGUCHI, H., DOHI, Y., OHNO, N., FUJIWARA, T. and ITO, T. Suppression of Con A response of mouse lymphocytes with unique M. leprae glycolipid. Jpn. J. Lepr. 58(1989)191-196.
20. ROGER, M., VIGEANT, P. and VIENS, P. The effect of cyclosporin A on Trypanosoma musculi infection of mice. Can. J. Microbiol. 34(1987)92-94.
21. SASIAIN, M. DEL C, DE LA BARRERA, S., VALDEZ, R. and BALINA, L. M. Reduced suppressor cell response to Mycobacterium leprae in lepromatous leprosy. Infect. Immun. 57(1989)951-956.
22. SHEPARD, C. C. and MCRAE, D. H. Mycobacterium leprae in mice: minimal infectious dose, relationship between staining quality and infectivity, and effect of cortisone. J. Bacteriol. 89(1965)365-372.
23. SHEPARD, C. C. and MCRAE, D. H. A method for counting acid fast bacteria. Int. J. Lepr. 36(1968)78-82.
24. SHEPARD, C. C, WALKER, L. L., VAN LANDINGHAM, R. M. and YE, S. Z. Sensitization or tolerance to Mycobacterium leprae antigen by route of injection. Infect. Immun. 38(1982)673-680.
25. SOLBACH, W., FORBERG, K., KAMMERER, E., BOGDAN, C. and ROLLINGHOFF, M. Suppressive effect of cyclosporin A on the development of Leishmania tropica-induced lesions in genetically susceptible BALB/c mice. J. Immunol. 137(1986)702-707.
26. STRAUSS, R., HEYMER, B. and HOF, H. Effects of cyclosporin A on experimental infection with Listeria monocytogenes. Clin. Exp. Immunol. 62(1985)491-498.
27. TAKASHIMA, T. and COLLINS, F. M. Immunosuppressive effect of cyclosporin A on Mycobacterium bovis BCG infections in mice. Infect. Immun. 55(1987)1701-1706.
28. WIESINGER, D . and BOREL, J. F. Studies on the mechanism of action of cyclosporin A. Immunobiology 156(1979)454-463.
1. B.Sc, Research Fellow, Department of Bacteriology, Faculty of Medicine, Tottori University, 86 Nishi-machi, Yonagoshi, Tottori 683, Japan.
2. M.D., D.Med.Sc., Professor, Department of Bacteriology, Faculty of Medicine, Tottori University, 86 Nishi-machi, Yonagoshi, Tottori 683, Japan.
3. D.V.Sc, D.Med.Sc., Director, Department of Microbiology, National Institute for Leprosy Research, 4-2-1 Aoba-cho, Higashimurayama-shi, Tokyo 189, Japan.
Reprint requests to Prof. Y. Tanaka.
Received for publication on 12 December 1990.
Accepted for publication in revised form on 21 June 1991.