- Volume 59 , Number 4
- Page: 605–12
Inhibition of complement activity in murine leprosy
ABSTRACT
NIH mice infected with Mycobacterium lepraemurium (MLM) show a marked depression in their levels of hemolytic complement that is proportional to the degree of infection. The defect affects more the activation of complement through the classical pathway (CPW) than the activation of complement through the alternative pathway. Although this low activity of CPW-complement may be due to different causes (complement consumption by the infecting microorganism, lack of biosynthesis of complement components, or the presence of complement inhibitory factors), our results seem to support the last possibility. The generation of factors in the infected animals that inhibit the autologous activity of complement as the infection goes on reduces the risk of complement-mediated tissue damage and prolongs the survival time of the host, a wise strategy on the part of the MLM to assure its own survival as a parasite.RÉSUMÉ
Des souris NIH infectées par Mycobactcrium lepraemurium (MLM) montrent une diminution marquée de leur taux de complément hémolytique, et cette diminution est proportionnelle au degré d'infection. Ce défaut affecte plus l'activation du complément par la voie classique (CPW) que par la voie alternative. Bien que cette faible activité du complément par la voie classique puisse être due à différentes causes (consommation du complément par le microorganisme infectant, insuffisance de biosynthèse de composants du complément, ou présence de facteurs inhibiteurs du complément), nos résultats semblent supporter cette dernière possibilité. La production, chez les animaux infectés, de facteurs qui inhibent l'activité autologuc du complément lorsque l'infection se poursuit diminue le risque de lésion tissulaire par l'intermédiaire du complément et prolonge la survie de l'hôte. Ceci est une sage stratégie de la part du MLM pour assurer sa propre survie en tant que parasite.RESUMEN
Los ratones NIH infectados con el Mycobacterium lepraemurium, desarrollan un defecto en sus niveles de complemento hemolítico que es proporcional al grado de infección. El defecto afecta principalmente la vía clásica de activación del complemento y menos marcadamente la vía alterna. Aunque la baja, en algunos casos ausente, actividad de complemento hemolítico podría ohbcdecer a diversas causas: consumo de complemento por el microorganismo infectante, falla en la biosíntesis de algún componente del complemento, o inhibición de la actividad de complemento por factores séricos, nuestros resultados sugieren que esta última posibildad, la gencració de factores séricos inhibitorios como consecuencia de la infección, explica mejor el hallazgo de bajos niveles de complemento hemolítico en los animales infectados. La inactivación de la actividad de complemento mientras transcurre la infección, disminuye la posibilidad del daño mediado por complemento y prolonga la vida del huésped murino; ésta es, sin duda, una eficiente estrategia del microorganismo para prolongar también su supervivencia.The kidney in the mouse with murine leprosy is an almost spared organ. Macroscopically, the kidneys in severely infected animals show, at the most, a moderate increase in size and a variable degree of paleness and edema. When lepromas are found, they are limited to the outer fatty membrane (12, 14) . In the most advanced cases, bacilli can be found isolated, in small clumps, and even in macrophages grouped into discrete granulomas (12). Renal involvement, however, does not reach the dimensions observed in target organs such as the liver, spleen, lymph nodes and skin. Apart from this, little is known of the renal pathology in murine leprosy. From the immunopathological point of view, in a previous communication we reported that the histological examination of kidneys from mice with advanced murine leprosy showed no important alterations. No polymorphonuclear (PMN) infiltration in the renal interstitium nor destructive tissue damage was ever observed despite the fact that histochemically several Mycobacterium lepraemurium (MLM)-infected animals showed a marked glomerular deposition of both IgG and IgM immunogobulins. No complement (C3, C3b, or C3c) deposition was observed (11). The absence of complement deposition, of PMN infiltration, and of tissue damage indicated that the kidney lesions in murine leprosy evolved with little participation (if any) of complement. This nonparticipation of complement was considered to be due possibly to one or several of the following causes: a) the involvement of noncomplement fixing immunoglobulins, b) complement consumption by circulating immune complexes, c) inhibition of the synthesis of a key complement component (CI or C2), or d) inhibition of complement activity by factors present in the serum of the MLM-infected animals.
In this paper we present evidence in support of the last possibility as the most probable explanation of the findings reported above.
MATERIALS AND METHODS
Infection of mice with MLM . Two month-old, white, female NIH mice were inoculated, intravenously (i.v.) or intraperitoneally (i.p.), with 107 MLM Hawaiian strain, and maintained under standard housing conditions until completion of the study. Bacilli were isolated from infected tissue according to Prabhakaran, et al. (8). Four, six, and eight months postinoculation, groups of animals were anesthetized with chloroform, bled by cardiac puncture, and examined to assess the degree of infection. At that time, the kidneys were excised, transversally cut in half, embedded in OCT compound (Miles Laboratories, Naperville, Illinois, U.S.A.), frozen on dry ice, and sectioned to 6-8 um in a microtome cryostat (Tissue-Tek II; Miles). The kidney sections were processed to look for evidence of tissue damage and glomerular deposition of immunoglobulins (IgG and IgM) and complement (C3, C3b and C3c). The results of this immunohistochemical study have already been published (11). Blood from MLM-infected and control animals was allowed to coagulate, and the sera recovered were used to quantitate the activity of complement as described below.
Titration of hemolytic complement through the classical pathway (CPW-complement). The methods of Rosenberg and Tachibana (13) were followed: all reagents used were made up in triethanolamine buffer solution (TBS) with calcium and magnesium (triethanolamine 2.8 ml, NaCl 7.5 g, MgCl2 0.1 g, CaCl2 0.22 g, distilled water 800 ml, 1 N HC1, enough to bring the pH to 7.4 and distilled water to 1000 ml).
Twenty-five µl of each scrum and 75 µl of TBS were mixed with 50 µl of 1% sheep red blood cells (SRBC) sensitized with rabbit hemolysin to SRBC. To sensitize SRBC, one volume of hemolysin diluted 1:10 was mixed with one volume of a 2% SRBS suspension. Higher dilutions of hemolysin (e.g., 1:128, a subagglutinating dose) were ineffective in the hemolytic assay. To avoid agglutination of erythrocytes, the hemolysincoated SRBC were maintained in an ice bath and used within 10 min of preparation. The reaction mixtures contained in 10 x 75-mm test tubes were incubated in a water bath at 37ºC for 30 min with periodic gentle shaking. The reaction was then stopped by transferring the tubes to a bath of melting ice. After the addition of 1 ml of chilled TBS to each tube, they were briefly swirled and centrifuged at 2000 rpm in the cold for 5 min. The variably colored supernates were removed. The pelleted erythrocytes were washed once with TBS by spinning, as before, and the supernate-free remaining erythrocytes lysed with 1.0 ml water. Since most mouse sera were obtained with variable degrees of hemolysis and this interferes with the measurement of hemoglobin released by activated complement, the hemolytic activity of the sera was determined by reading the absorbance (OD 550 nm) of the hemoglobin released from the remaining erythrocytes after their lysis with water. When additives such as zymosan or bacteria had to be tested, they were added in 50 µl volumes, substituting TBS. The results were expressed as percent values after correction by appropriate controls.
Titration of complement through the alternative pathway (APW-complement). This was done following the method of Riches and Stanworth (9) with modifications. Briefly, 50 µl of serum containing added EGTA and MgCl2 at final concentrations of 10 mM and 7 mM, respectively, were mixed in 10 x 75-mm test tubes with 50 µl of calcium-free TBS (with 7 mM MgCl2) and 50 µl of 1% unsensitized rabbit red blood cells (RRBC). The tubes were incubated for 30 min at 37ºC, and the reaction was stopped by adding 15 µl of 0.2 M EDTA to each tube. Blanks contained 50 µl of 1% RRBC and 200 µl of calcium-free TBS. As before, readings of the hemoglobin released after lysis of the remaining erythrocytes were used to calculate the hemolytic activity of the sera.
Complement consumption by MLM. Aliquots of pools made of fresh normal mouse sera were preincubated for 30 min at 37ºC with 1.5 x 105 to 1.5 x 109 MLM cells properly washed and suspended to assess the MLM capability to consume complement. The remaining complement was measured both through the classical and the alternative pathways.
Circulating immune complexes (CIC). Soluble immune complexes were looked for in the sera of normal or MLM-infected mice by their reaction with C1q. Mouse C1 q was isolated from a pool of fresh normal sera according to the method proposed by Yonemasu and Stroud (16) for human serum. The reactivity between Clq and the sera was assayed by immunodiffusion in gel as described by Agnello, et al. (1); heat-aggregated mouse gammaglobulin was used as a positive, Clq reactive control (7).
Complement inhibitory activity. To measure the complement inhibitory activity of sera from MLM-infected mice, a pool was made of fresh normal murine sera that individually showed high complement activity. Twenty-five µl of individual sera from MLM-infected animals (25 µl of TBS or 25 µl of normal sera in the control tubes) was added to 25 µl of pooled normal sera and, after a 30-min preincubation at 37ºC, the complement activity was determined as described above for the CPW-complement.
Urinalysis. Since the amount of urine collectable at any time from normal and MLM-infected animals is in the order of tenths of a ml, the protein content in individual samples of urine was measured by means of reactive sticks for urinalysis obtained from Miles Laboratories, Mexico.
Analysis of results. The results were analyzed by the Mann-Whitney U test to assess their statistical significance. In addition, and just to give a graphical idea of the range of variation within groups, the means ± 1 standard deviation (S.D.) are also shown.
RESULTS
Complement levels in normal vs MLM-infected mice. Several lots of animals with well-established murine leprosy were studied to assess their complement levels both by the classical (CPW-complement) and by the alternative (APW-complement) pathways. As expected, complement alterations were more frequent and evident at the more advanced times of infection. Figure 1 shows the levels of CPW- and APW-complement found in three separate experiments with mice having from 4 to 6 months of infection (a total of 30 animals) and 30 corresponding normal counterparts. Compared to the control animals, in all of the experiments the infected mice showed the lowest levels (percent values) of CPW-complement but similar levels of APW-complement. Five out of the 30 MLM-infected animals (and none in the control group) showed a total absence of CPW-complement. None of the differences in APW-complement levels was statistically significant.
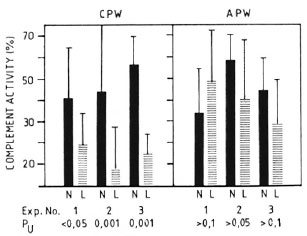
Fig. 1. Complement activity (classical and alternative pathways) in sera of control (N) and MLM-infected (L) mice. Results (mean ± 1 S.D.) from three-series of experiments, each with 10 N and 10 L animals, are illustrated; p values (N) were calculated by the Mann-Whitney U test.
An experiment to compare the levels of CPW-complement with those of APW-complement in individual animals is illustrated in Figure 2. It can be seen that 15 out of 18 MLM-infected animals had levels of CI-complement well below one standard deviation under the mean value found in the control group (59.2 ± 37.2). Twelve out of the same 18 MLM-infected animals, however, showed levels of APW-complement within the normal range (41.9 ± 17.8). The remaining six animals also exhibited diminished levels of APW-complement. It seems that those animals infected with MLM show a more significant diminution in their levels of CPW-complement than in their levels of APW-complement.
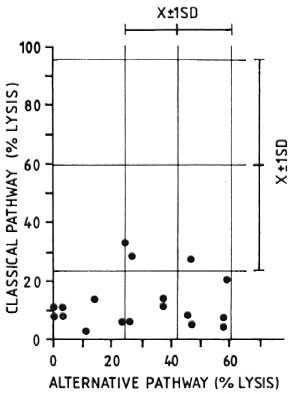
Fig. 2. Complement activity (classical and alternative pathways) in individual sera of 18 MLM-infected mice ( ) compared with average complement activity found in a similar group of healthy animals for which corresponding means and standard deviations (X ± 1 S.D.) are shown.
) compared with average complement activity found in a similar group of healthy animals for which corresponding means and standard deviations (X ± 1 S.D.) are shown.
CPW- and APW-complement consumption by MLM. When a pool of fresh normal mouse serum was preincubated with different amounts of purified MLM (see Materials and Methods), up to 1.5 million bacilli did not significantly modify the serum CPW-and APW-complement levels. Beyond this number of bacilli, there was a proportional and comparable diminution in the levels of CPW- and APW-complement (Fig. 3). In a series of three experiments to assess the capability of intact and delipidized 1 x 107 MLM to consume CPW-complement, it was found that delipidized MLM were more anticomplementary than intact bacteria (Fig. 4).
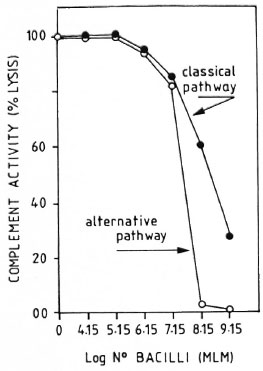
Fig. 3. Consumption of murine complement by variable amounts of purified intact M. lepraenmum. Remaining complement activity was measured throughboth the classical and alternative pathways. Results are normalized in relation to the lysis found in the pool of normal sera free of bacilli (100% lysis).
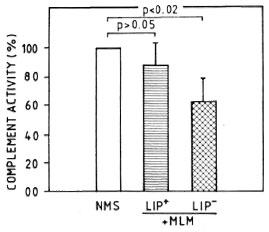
Fig. 4. Complement consumption by 1 x 107 intact (LIP+) or delipidized (LIP-) MLM. Residual complement activity was measured through the classical pathway. Values are shown normalized in reference to hemolytic activity (taken as 100%) found in apool of normal mouse sera (NMS).
Effect of MLM infected sera on complement activity of normal serum. Sera from mice with advanced MLM infection are able to inactive the CPW-complement of normal mouse serum. Figure 5 illustrates the average complement activity found in a group of 10 normal (N) versus 10 MLM-infected mice.
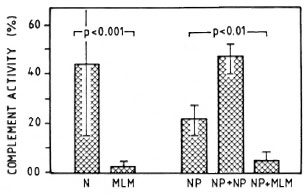
Fig. 5. Average complement activity (classical pathway) in a group of 10 normal (N) and 10 MLM-infected (MLM) mice studied individually. Hemolytic activities of: a) the pool of N sera alone (NP), b) the pool added of pooled normal sera (NP + NP), and c) the pool added of MLM sera (NP + MLM) are illustrated on the right. Mean values and S.D. and the p values calculated by the Mann-Whtney U test ar ealso shown.
Figure 6 shows that, in general terms, the lower the titer of CPW-complement in the individual MLM-mouse sera, the stronger their CPW-complement inhibitory capability (e.g., mice L3, L5, and L9 showed the lowest levels of CPW-complement and the strongest CPW-complement inhibitory capability; mice L8 and L4, with higher CPW-complement levels, were less inhibitory).
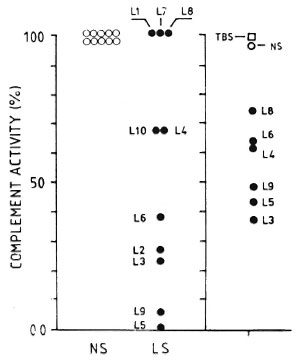
Fig. 6. Normalized (to 100%) individual values of complement activity (classical pathway) in 10 normal sera (NS) and 10 MLM-infected (L I -L I 0) mouse sera (LS) (left panel). Right panel shows effect of adding TBS (complement buffer), a pool of NS, or individual LS (L3-L9) to the pool of normal sera. There was not enough serum from mice LI, L2, L7, and L 10 to evaluate their complement-inhibitory activity.
Circulating immune complexes. By using the combined methods of Yonemasu and Stroud (16) and Agnello, et al. (1), we could not demonstrate the presence of CIC in the serum of any of the MLM-infected animals. However, a neat precipitation band appeared when the mouse Clq preparation interacted with heat-aggregated mouse gammaglobulin. Although our negative results are probably due to the low sensitivity of the method used, it is interesting that other authors have also had problems in demonstrating the presence of CIC in the sera of mice infected with MLM, despite the higher sensitivity of the (radioactive) method used (15). Previous attempts to demonstrate CIC in the sera of MLM-infected mice by the polyethylene glycol precipitation-complement consumption test (3) were also unsuccessful. (This test, however, has been successfully applied to human tuberculosis in our laboratory10.)
Proteinuria. Mice with a 4-month infection with MLM showed no proteinuria beyond the levels found in their normal counterparts (Fig. 7). At 8 months, however, most of the infected animals showed marked increases in their urinary levels of protein. The lack of PMN infiltration (and tissue destruction) continued to be a remarkable characteristic of the renal pathology in the MLM-infected mouse, even at this advanced time of infection. Other urinary parameters (pH, glucose, ketone, bilirubin, urobilinogen, and blood) were always within normal limits.
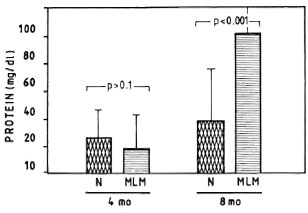
Fig. 7. Average amount of urinary protein found in a group of 30 mice bearing a 4-month infection with MLM and again at 8 months postinfection (MLM). Proteinuria in control animals (N) is also shown. p Values were calculated according to the Mann-Whitney U test.
DISCUSSION
As in other mycobacterial diseases, the kidneys in murine leprosy show great resistance to the infection; the reasons for this are ill understood. Once it was thought that the kidneys were particularly resistant to mycobacterial infection because of the high levels of spermine in this organ. This tetraamine isolated from bovine kidney by Hirsch and Dubos in 1952 (6), in the presence of a specific amine oxidase (a serum alphaglobulin), completely inhibited the growth of M. tuberculosis in low concentrations in vitro (4, 5). Since this system was not studied further, its participation in the host resistance to pathogenic mycobacteria is, at present, difficult to assess. We favor the idea that kidneys owe their resistance to their lack of a significant amount of reticuloendothelial system.
Murine leprosy is a chronic disease in which large amounts of bacilli co-exist with high levels of antimycobacterial antibodies, so that immune complexes are very likely to be formed (15). Accordingly, the immunohistochemical study of the kidney shows a heavy glomerular deposition of IgG and IgM immunoglobulins but, surprisingly, negligible deposition (if any) of complement (C3). Histologically, kidneys in MLM-infected mice do not show evidence of major tissue damage; this correlates well with the lack of glomerular PMN infiltration (11). The lack of complement participation in the renal pathology of MLM-infected mice was considered to be due to one or more of the following causes: a) deposition of noncomplement-fixing immunoglobulins; b) suppression of the synthesis of complement components (at least of a key component, such as C1 or C3); c) complement consumption by microbial components; and d) generation of complement-inhibitory factors. The first possibility is very unlikely since the immunoglobulins deposited in glomeruli belong to the IgG and IgM isotypes.
To explore the other possibilities, we measured the levels of complement activated through both the classical (CPW-complement) and the alternative (APW-complemenf) pathways. Despite the variations within the groups, the CPW-complement activity in the several experiments performed was always low or absent in the MLM-infected animals. On the other hand, the activity of APW-complement in the infected animals was not very different from that found in the controls. Since C3 is the first complement component activated in the alternative route, and this route is not significantly altered in the infected animals, the possibility of a defect in the synthesis of C3 or the subsequent complement components (C5-C9) can be reasonably ruled out. The possibility remains, however, of a defect in the synthesis of any of the first three components (CI, C4, C2) since this could explain the low levels of CPW-complement found in the infected animals.
The proposition that complement might be consumed by microbial components was tested by measuring the activity of CPW-and APW-complement before and after preincubation of normal mouse serum with several amounts of purified bacilli. The normalized results from these experiments (Fig. 3) indicate that MLM modify in a similar fashion the activity of CPW- and APW-complement. However, because the anticomplementary effect of MLM is observed in both the CPW-complement (in the absence of anti-MLM antibody) and the APW-complement routes, and only in the presence of very large numbers of bacilli, this anticomplementary effect of MLM does not seem to be relevant in vivo. In addition, the fact that the infected animals exhibit a remarkable specific diminution in their levels of CPW- (but not of APW-) complement makes very improbable that such a diminution might be due to a "physiological" consumption of complement by the microorganism. In situ consumption of complement by the highly bacilliferous granulomas is also unlikely since PMN, although attracted to the lesion sites, maintain a peripheral distribution, and the necrosis that eventually occurs starts at the center of the granulomas. This central necrosis (in tuberculosis) has been proposed to be due to the infarction of the blood vessels around the granulomas that lead to anoxia of the lesion (2).
Another interesting result was the moderate, yet reproducible, increment in the capability of delipidized-MLM over intact-MLM to consume complement, suggesting that the lipidic coat might play a certain protective role in vivo for this and other mycobacteria (for instance, M. tuberculosis and M. leprae) since intact, extracellular (spreading) bacilli would be rather resistant to the potentially harmful effects of complement. However, since the lipids were extracted under rather drastic conditions (shaking the bacterial suspension with a mixture of acetone:methanol [1:1] for 24 hr), this finding deserves further investigation.
Finally, the possibility that factors able to inhibit the activity of complement might be generated as a result of the infection with MLM was analyzed, measuring the levels of CPW-complement in a pool or normal sera incubated in the presence or absence of sera from normal or MLM-infected mice. As shown in Figures 5 and 6, this seemed to be the case. While the addition of a buffer or normal sera did not inhibit the CPW-complement activity of the pool of normal sera, the addition of sera from MLM-infected animals caused a diminution of its hemolytic activity proportional to the level of CPW-complement found in the individual sera. The lower the level of CPW-complement in the sera of MLM-infected animals, the stronger their inhibitory capability.
Thus, the presence of complement inhibitory factors in the serum of MLM-infected mice seems to explain best the low (in some cases, absent) levels of CPW-complement, the lack of significant glomerular complement deposition, the absence of glomerular PMN infiltration, and the lack of tissue damage in those animals. The nature of such inhibitory factors is currently under investigation in our laboratory.
Acknowledgment. The authors appreciate the continuous encouragement received from Dr. Sergio Estrada-Parra, and the periodic supply of pathogen-frec, NIH mice by Drs. J. Ruiz Puente and F. Ruiz Cabrera from the Instituto Nacional de Higiene, México. This work received financial support from the Dirección de Estudios Profesionales c Investigación del I.P.N. (Proyectos 892467: Participación de los complejos inmunes en la patología renal de la lepra de los ratones, and 880345: Lepra lepromatosa de las ratas y ratones: el papel de los complejos inmunes en la patogenia de la enfermedad). O. Rojas-Espinosa holds fellowships from COFAA, IPN, and the Sistema Nacional de Investigadores (SNI), México.
REFERENCES
1. AGNELLO, V., WINCHESTER, R. J. and KUNKEL, H. G. Precipitin reactions of the Clq component of complement with aggregated gamma-globulin and immune complexes in gel diffusion. Immunology 19(1970)909-916.
2. COURTADE, E. T., TSUDA, T., THOMAS, C. R. and DANNENBERG, A. M. Capillary density in developing and healing tuberculous lesions produced by BCG in rabbits; a quantitative study. Am. J. Pathol. 78(1975)243-260.
3. HARKIS, G. D. and BROWN, D. L. Detection of immune complexes by a new assay: the polyethylene glycol precipitation-complement consumption test (PEG-CC). Clin. Exp. Immunol. 36(1979)117-129.
4. HIRSCH, J. G. The essential participation of an enzyme in the inhibition of growth of tubercle bacilli by spermine. J. Exp. Med. 97(1953)327-344.
5. HIRSCH, J. G. Spermine oxidase; amine oxidase with specificity for spermine and spermidine. J. Exp. Med. 97(1953)345-355.
6. HIRSCH, J. G. and DUBOS, R. J. The effect of spermine on tubercle bacilli. J. Exp. Med. 95(1952)191-208.
7. MULLER-EBERHARD, H. J. and KUNKEL, H. G. Isolation of a thermolabile scrum protein which precipitates γ-globulin aggregates and participates in immune hemolysis. Proc. Soc. Exp. Biol. Med. 106(1961)291-295.
8. PRABHAKARAN, K, HARRIS, E. B. and KIRCHHEIMER, W. F. Binding of l4C-labeled DOPA by Mycobacterium leprae in vivo. Int. J. Lepr. 44(1976)58-64.
9. RICHES, D. W. H. and STANWORTH, D . R. A simple new method of measuring the capacity to activate the alternative complement pathway. Immunol. Lett. 1(1980)363-366.
10. ROJAS-ESPINOSA, O., OLTRA, R. A. and ARCE, P. P. Circulating immune complexes in patients with advanced pulmonary tuberculosis detected by a polyethylene glycol precipitation-complement consuming test (PEG-CC test). Rev. Latinoam. Microbiol. 30 (1988) 25-29.
11. ROJAS-ESPINOSA, O., OLTRA, R. A., MENDEZ, A. P., ARCE, P. P. and GONZALEZ, M. A. Glomerular immunoglobulin deposition in the absence of tissue damage in murine leprosy. Int. J. Lepr. 57(1989)879-882.
12. ROJAS-ESPINOSA, O. and REYES, M . E. Renal alterations in murine leprosy. (Letter) Int. J. Lepr. 59(1991)652-655.
13. ROSENBERG, L. T. and TACHIBANA, D. K. Activity of mouse complement. J. Immunol. 89(1962)861-867.
14. TANIMURA, T. and NISHIMURA, S. Studies on pathology of murine leprosy. Int. J. Lepr. 20(1952)83-84.
15. TAVERNE, J., REICHLIN, M., TURK, J. L. and REES, R. J. W. Detection of immune complexes in mice infected with Mycobacterium lepraemurium. Clin. Exp. Immunol. 24(1976)157-167.
16. YONEMASU, K. and STROUD, R. M . Clq: rapid purification method for preparation of monospecific antisera and for biochemical studies. J. Immunol. 106(1971)304-313.
1. Q.B.P., Sc.Dr., Escuela Nacional de Ciencias Biológicas, Instituto Politécnico Nacional, Carpió y Plan de Ayala, Colonia Santo Tomás, 11340 México, D.F., México.
2. Q.B.P., Escuela Nacional de Ciencias Biológicas, Instituto Politécnico Nacional, Carpió y Plan de Ayala, Colonia Santo Tomás, 11340 México, D.F., México.
3. Q.B.P., Departamento de Inmunología, Escuela Nacional de Ciencias Biológicas, Instituto Politécnico Nacional, Carpió y Plan de Ayala, Colonia Santo Tomás, 11340 México, D.F., México.
4. I.B.Q., Departmento de Ingeniería Bioquímica, Escuela Nacional de Ciencias Biológicas, Instituto Politécnico Nacional, Carpió y Plan de Ayala, Colonia Santo Tomás, 11340 México, D.F., México.
Received for publication on 18 January 1991.
Accepted for publication in revised form on 3 June 1991.