- Volume 59 , Number 4
- Page: 613–7
Anti-Mycobacterium leprae activity of several quinolones studied in the mouse
ABSTRACT
The anti-Mycobacterium leprae activity of several fluoroquinolones (A-56619, A-56620, ofloxacin, fleroxacin, lomefloxacin, temafloxacin, tosufloxacin, and PD-117596) was studied in the mouse. In a dosage of 150 mg/kg administered daily, A-56619 is active and A-56620 is inactive against M. leprae. Ofloxacin administered daily for 2 weeks at 300 mg/kg is bactericidal. The minimal effective dose of PD-117596, lomefloxacin and temafloxacin is less than 37.5 mg/kg. When administered at 300 mg/kg at monthly intervals temafloxacin, PD-117596, and ofloxacin are bacteriostatic; while fleroxacin and lomefloxacin arc bactericidal. Tosufloxacin is less active than the other quinolones included in the present study.RÉSUMÉ
L'activité anti-Mycobacterium leprae de diverses fluoroquinolones (A-56619, A-56620, ofloxacine, fleroxacine, lomefioxacine, temafloxacinc, tosufloxacinc, et PD-117596) a été étudiée chez la souris. A la dose de 150 mg/kg administrée quotidiennement, A-56619 est active, et A-56620 est inactive vis-à-vis de M. leprae. L'ofloxacinc est bactéricide à la dose de 300 mg/kg administrée quotidiennement pendant 2 semaines. La dose minimale efficace de PD-117596, lomefioxacine et temafloxacine est inférieure à 37, 5 mg/kg. A la dose de 300 mg/kg administrée mensuellement, la temafloxacine, PD-117596 et l'ofloxacine sont bacteriostatiques, tandis que la fleroxacine et la lomefioxacine sont bactéricides. La tosufloxacinc est moins active que les autres quinolones prises en compte dans l'étude présente.RESUMEN
Se estudió la actividad de varias fluoroquinolonas (A-56619, A-56620, ofloxacina, fleroxacina, lomefloxacina, temafloxacina, y PD-117596) en contra del Mycobacterium leprae en el modelo del ratón. Se encontró que mientras que una dosis diaria de 150 mg/kg de A-56619 es activa contra el M. leprae, una dosis equivalente de A6620 no lo es. La ofloxacina, administrada diariamente durante 2 semanas a una dosis de 300 mg por kg, resultó bactericida. Las dosis efectivas mínimas de PD-117596, lomefloxacina y temafloxacina, fueron menores de 37.5 mg/kg. La temafloxacina, el PD-117596, y la ofloxacina, administrados a 300 mg por kg a intervalos mensuales, resultaron bacteriostáticos, mientras que la fleroxacina y la lomefloxacina fueron bactericidas. La tosufloxacina fue menos activa que las otras quinolonas incluidas en el estudio.Fluoroquinolones are basically drugs active against gram-negative bacteria. Numerous derivatives with a broader spectrum of activity and/or more favorable pharmacokinetics have been synthetized (2). Some quinolones are active against Mycobacterium leprae (5, 7-9, 16), and Pefloxacin and ofloxacin have already been studied in the human disease (8, 13).
We present here results on the activity of several fluoroquinolones against M. leprae in the mouse.
MATERIALS AND METHODS
The anti-M. leprae activity of the drugs was studied in the mouse foot pad by either the continuous or kinetic method (19, 20) or by the proportional bacterial test (3) using M. leprae strains 6348 and 17547 used in previous studies (l6). The drugs were gifts from drug companies and were administered by gavage: ofloxacin (OFLO) from Hoechst, Frankfurt, Germany; A-56619 (= difloxacin), A-56620 (4) and temafloxacin (TEFLO) from Abbott Laboratories, Chicago, Illinois, U.S.A.; fleroxacin (FLERO) from Roche, Basle, Switzerland; lomefloxacin (LOFLO) from Searle & Co., Skokie, Illinois, U.S.A.; tosufloxacin (TOSU); PD117596 from Parke-Davis, Ann Arbor, Michigan, U.S.A.
Statistical calculations were done using Fisher's exact test.
RESULTS
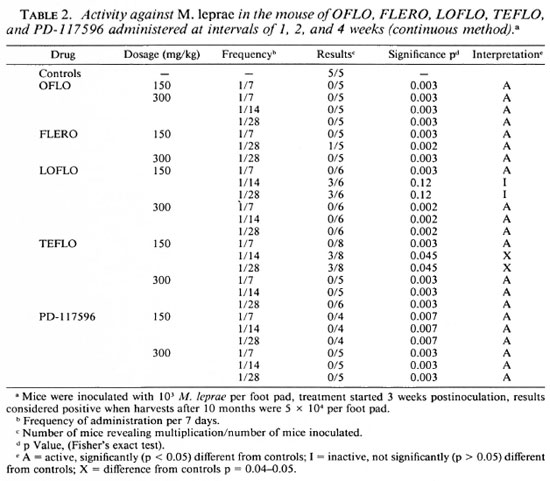
As shown in Table 1, at a dosage of 150 mg/kg A-56620 is inactive and A-56619 is active against M. leprae but only when administered daily, not when administered once weekly.
FLERO at 150 mg/kg is active when administered both daily and once weekly.
TEFLO, LOFLO, and PD-117596 are all active at 150, 75 and 37.5 mg/kg administered daily.
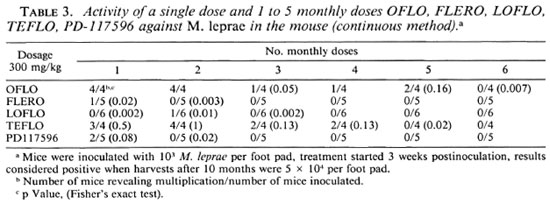
The activity of the drugs when administered intermittently at dosages of 150 or 300 mg/kg with intervals of 1, 2, and 4 weeks studied by the continuous method is shown in Table 2. At the lower dosage of 150 mg/kg LOFLO and TEFLO were less active than FLERO and PD-117596; at the higher dosage of 300 mg/kg there were no differences among these drugs.
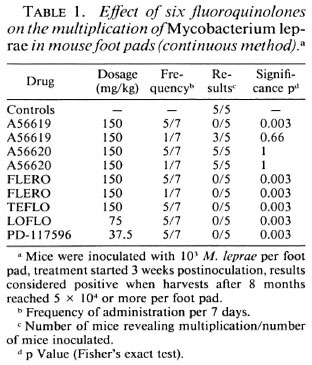
The effects of one to six monthly doses of 300 mg/kg of five quinolones were studied by kinetic tests. Table 3 shows that the most active compounds are FLERO and LOFLO, which are active even after a single dose, followed by PD-117596, active after two monthly doses, while OFLO inhibits the multiplication of M. leprae only after six monthly doses. Since all the mice became positive during the next 3 months, the activities of the drugs in these circumstances are bacteriostatic.
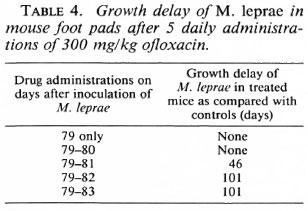
The activity of OFLO was also studied by a kinetic test after a single dose and two to five daily doses of 300 mg/kg. Table 4 shows that three daily doses of 300 mg/kg resulted in a limited growth delay of M. leprae in the treated mice which increased considerably after four and five daily doses.
In a proportional bactericidal test, OFLO administered daily, 5 days a week for two successive weeks, at 300 mg/kg was bactericidal.
TOSU could only be investigated partially since only a small amount of the drug was available. The drug was inactive when administered either 5 days a week for 8 weeks at 37.5 mg/kg or once every 28 days at 150 mg and 300 mg/kg.
DISCUSSION
The discovery of the fluoroquinolones constitutes a major advance in antibacterial therapy, including mycobacteria (10). The potential number of derivatives is enormous, and more than 5000 have already been synthesized (2). Basically, the fluoroquinolones are active against gram-negative organisms, but some of them have more favorable pharmacokinetic properties and some are active also against gram-positive organisms. They also offer great promise for the treatment of leprosy (5, 7-9, 13, 16).
Since the pharmacokinetics of the quinolones is quite different in mice as compared to man and because, in particular, the serum half-life in mice is much shorter, 50-100 mg/kg in the mouse is considered equivalent to 6.6 mg/kg in man (6). The 300 mg/kg administered in mice in some of the experiments described in this paper corresponds to the highest dosage that could be administered in man, e.g., 400 mgand more of the drug per day.
Not all fluoroquinolones are equally active against M. leprae as illustrated by the first quinolones studied in the mouse- OFLO, pefloxacin and ciprofloxacin, the former being the most active and the latter inactive (1,9 and own unpublished results)- and the present results with A-56619 and A-56620.
The anti-M. leprae activity of quinolones in the mouse can be compared by their minimal effective dose (MED) in the continuous method and their activity after intermittent administration. The MEDs of LOFLO, TEFLO and PD-117596 is less than 37.5 mg/ kg, considerably lower than the MEDs of OFLO and PEFLO which are 50 mg/kg and 150 mg/kg, respectively (6, 8, 15). The limited information obtained with TOSU shows that this drug is less active than the other quinolones included in the present study.
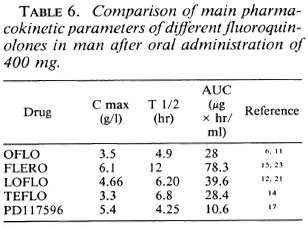
On the basis of their activity after intermittent administration from once weekly to once every 2 weeks and once every 4 weeks in the continuous test, FLERO and LOFLO are the most active followed by PD-117596, TEFLO, and OFLO. However, after monthly administration even those quinolones with a long serum half-life (Table 6) are only bacteriostatic.
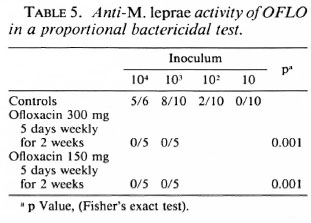
OFLO administered once monthly is inactive but is highly bactericidal when administered daily. Whereas Grosset, et al. (7) showed that OFLO administered daily at 150 mg/kg for 3 months is bactericidal for the mouse foot pad inoculum, it is shown here that the same result is obtained when the drug is administered daily at 300 mg/kg for 2 weeks (Table 5) and even 1 week (Table 4). In the meantime a killing rate of 99%-99.9% of viable M. leprae has been measured in man after 22 daily doses of 400 mg OFLO (8).
The drugs most active in intermittent administration are the two closely related drugs FLERO and LOFLO (2) which have long serum half-lives (Table 6). The greater activity of PD-117596 against M. leprae may be related to its greater activity against gram-positive organisms (17). The important activity of OFLO when administered daily may be due to some accumulation of the drug (6).
It is interesting to compare the present results with those obtained by the in vitro screening of the activity against M. leprae of fluoroquinolones by Franzblau and White (5). In the latter study, PD-117596, OFLO, TEFLO, and FLERO were also found to be among the most active drugs. It would be worthwhile to test the M. leprae activity in mice of several of the fluoroquinolones found by Franzblau and White to be even more active than those investigated in the present study.
It seems at present that for optimal bactericidal effect against M. leprae the fluoroquinolones, when active, have to be administered daily, and that none of. these drugs, even those with longer serum half-lives (FLERO, LOFLO) or with greater intrinsic activity (PD-117596), are suitable for monthly administration. This is important when the eventual addition of a fluoroquinolone to the WHO regimen for the treatment of multibacillary leprosy is considered(22).
Future evaluations of other new fluoroquinolones against M. leprae in mice should be directed to their MEDs in continuous administration and their bactericidal effect after a small number of daily doses.
REFERENCES
1. BANERJEE, D. K. Ciprofloxacin (4-quinolone) and M. leprae. Lepr. Rev. 57(1986)159-162.
2. CHU, D. T. W. AND FERNANDES, P. B. Structure activity relationships of the fluoroquinolones. Antimicrob. Agents Chemother. 33 ( 1989) 131 -135.
3. COLSTON, M. J., HILSON, G. R. F. and BANNERJEE, D. K. The "proportional bactericidal test"; a method for assessing bactericidal activity of drugs against Mycobacterium leprae in mice. Lepr. Rev. 49(1978)7-13.
4. ELIOPOULOS, G. M., MOELLERINO, A. E., MOELLERING, R. C. and REISZNER, E. In vitro activities of the quinolone antimicrobial agents A-56619 and A-56620. Antimicrob. Agents Chemother. 28(1985)514-520.
5. FRANZBLAU, S. G. and WHITE, K. E. Comparative in vitro activities of 20 fluoroquinolones against Mycobacterium leprae. Antimicrob. Agents Chemother. 34(1990)229-231.
6. GROSSET, J. H. Pharmacokinetics in drug screening. Int. J. Lepr. 55(1987)852-856.
7. GROSSET, J. H., GUELPA-LAURAS, C. C, PERANI, E. G. and BEOLETTO, G. Activity of ofloxacin against Mycobacterium leprae in the mouse. Int. J. Lepr. 56(1988)259-264.
8. GROSSET, J. H., Ji, B., GUELPA-LAURAS, C. C, PERANI, E. G. and N'DELI, L. Clinical trial of Pefloxacin and ofloxacin in the treatment of lepromatous leprosy. Int. J. Lepr. 58(1990)281 -295.
9. GUELPA-LAURAS, C. C, PERANI, E. G., GIROIR, A. M. and GROSSET, J. H. Activities of Pefloxacin and ciprofloxacin against Mycobacterium leprae in the mouse. Int. J. Lepr. 55(1987)70-77.
10. LEYSEN, C. D., HAEMERS, A. and PATTYN, S. R. Mycobacteria and the new quinolones. Antimicrob. Agents Neurother. 33(1989)1-5.
11. Lode, H., Höflken, G., Olschewski, P., Sievers, B., Kirsch, A., Borner, K. and KOEPPE, P. Pharmacokinetics of ofloxacin after parenteral and oral administration. Antimicrob. Agents Chemother. 31(1987)1338-1342.
12. MORRISON, P. J., KANT, T. G., ROBINSON, J., and KUNKA, R. L. Pharmacokinetics and tolerance of lomefloxacin after sequentially increasing oral doses. Antimicrob. Agents Chemother. 32(1988)1503-1507.
13. N'DELI, L., GUELPA-LAURAS, C. C, PERANI, E. G. and GROSSET, J. H. Effectiveness of Pefloxacin in the treatment of lepromatous leprosy. Int. J. Lepr. 58(1990)12-18.
14. NYE, K., SHI, Y. G., ANDREWS, J. M., ASHBY, J. P. and WISE, R. The in vitro activity, pharmacokinetics and tissue penetration of temafloxacin. J. Antimicrob. Chemother. 24(1989)415-424.
15. PANNETON, A. C, BERGERON, M. C. and LE BEL, M. Pharmacokinetics and tissue penetration of fleroxacin after single and multiple 400 and 800 mg dose regimens. Antimicrob. Agents Chemother. 32(1988)1515-1520.
16. PATTYN, S. R. Activity of ofloxacin and pefloxacin against Mycobacterium leprae in the mouse. Antimicrob. Agents Chemother. 31(1987)671-672.
17. ROLSTON, K. V. I., LE BLANC, B., HO, D. H. and BODEY, G. P. In vitro activity of PD-117596, a new quinolone, against isolates from cancer patients. J Antimicrob. Chemother. 26(1990)39-44.
18. SAITO, H., TOMIOKA, H. and NAGASHIMA, K. In vitro and in vivo activities of ofloxacin against Mycobacterium leprae infection induced in mice. Int. J. Lepr. 54(1986)560-562.
19. SHEPARD, C. C. A kinetic method for the study of the activity of drugs against Mycobacterium leprae in mice. Int. J. Lepr. 35(1976)429-435.
20. SHEPARD, C. C. and CHANG, Y. T. Effect of several anti-leprosy drugs on multiplication of human leprosy bacilli in foot pads of mice. Proc. Soc. Exp. Biol. Med. 109(1962)636-638.
21. STONE, J. W., ANDREWS, J. M., ASHBY, J. P., GRIGGS, D. and WISE, R. Pharmacokinetics and tissue penetration of orally administered lomefloxacin. Antimicrob. Agents Chemother. 32(1988)1508-1510.
22. WHO EXPERT COMMITTEE ON LEPROSY. Sixth Report. Geneva: World Health Organization, 1988. Tech. Rep. Ser. 768.
23. WISE, R., KIRKPATRICK, B., ASHBY, J. and GRIGGS, D. J. Pharmacokinetics and tissue penetration of RO 23-6240, a new trifluoroquinolone. Antimicrob. Agents Chemother. 31(1987)161-163.
M.D., Professor, Medical Microbiology, Institute for Tropical Medicine and University of Antwerp, Nationalestraat 155, B-2000 Antwerp, Belgium.
Received for publication on 22 October 1990.
Accepted for publication in revised form on 25 June 1991.