- Volume 58 , Number 4
- Page: 651–9
Prospective immunological follow-up in household contacts of Mexican leprosy patients
ABSTRACT
A 6-year prospective study of 79 household contacts of leprosy cases was made in order to correlate the development of the disease with their specific T-cell immunity, measured by the Mitsuda test, and levels of anti-Mycobacterium leprae antibodies determined in three consecutive observations with the FLA-ABS test. Overall in the contacts, 71.7% were Mitsuda positive and 93.6% showed seropositivity, without regard to their age, sex, or leprosy type of their index case. Households were divided into lower-risk and higher-risk groups according to either the paucibacillary or multibacillary character of their index case. The lower-risk group consisted of 19 contacts of 2 tuberculoid (TT) and 5 indeterminate cases. The higher-risk group was made up of 60 household contacts of 18 active lepromatous (LL) cases. All but two contacts in the former group had a positive Mitsuda reaction; the most common antibody titer was 1:160, with a tendency to stabilize or decrease over time. In the two Mitsuda-negative contacts, increased antibody levels were observed. In the higher-risk group, 61.6% were Mitsuda positive and showed a humoral profile similar to those Mitsuda positive in the lowerrisk group. In most of the Mitsuda-negative LL contacts, the antibody levels remained constant or progressively increased, suggesting a high probability of active subclinical infection. This assumption was partially supported by the finding of a new borderline lepromatous (BL) leprosy case in the Mitsuda-negative LL contact group. Nevertheless, the contribution of the close and extensive contact with a multibacilliferous case as a risk factor was difficult to evalute because of the small size of the sample studiedRÉSUMÉ
Une étude prospective d'une durée de 6 ans fut réalisée parmi 79 contacts domiciliaires de malades de la lèpre afin d'étudier la relation entre le développement de la maladie et l'immunité cellulaire spécifique, mesurée par le test de Mitsuda, et les taux d'anticorps anti-Mycobacterium leprae déterminés par trois observations consécutives avec le test FLA-ABS. Parmi l'ensemble des contacts, 71.7% étaient positifs au Mitsuda, et 93.6% ont montré une séropositivité, sans tenir compte de l'âge, du sexe, ou du type de lèpre du cas index. Les maisonnées furent divisées en groupes à faible risque et à risque élevé en fonction du caractère pauci- ou multibacillaire du cas index. Le groupe à faible risque était constitué de 19 contacts de 2 malades tuberculoides (TT) et de 5 malades indéterminés. Le groupe à risque élevé était constitué de 60 contacts domiciliaires de 18 cas lépromateux actifs (LL). Dans le premier groupe, tous les contacts sauf deux avaient une réaction de Mitsuda positive; le titre d'anticorps le plus fréquent était 1:60, avec une tendance à la stabilisation ou à la diminution au cours du temps. Des taux élevés d'anticorps ont été observés chez les deux contacts négatifs au Mitsuda. Dans le groupe à risque élevé, 61.6% étaient positifs au Mitsuda, et montraient un profil sérologiquc similaire à celui des contacts Mitsuda positifs du groupe à faible risque. Chez la plupart des contacts de LL à Mitsuda négatif, les taux d'anticorps restèrent constants ou augmentèrent progressivement, suggérant une grande probabilité d'infection subclinique active. Cette hypothèse était partiellement étayée par la découverte d'un nouveau cas de lèpre borderline lépromatcusc (BL) dans le groupe des contacts de malades LL à Mitsuda négatif. Néanmoins la contribution comme facteur de risque du contact rapproché et prolongé avec un cas multibacillifère fut difficile à évaluer du fait de la petite taille de l'échantillon étudié.RESUMEN
En un estudio prospectivo de 6 años de 79 contactos convivientes con casos de lepra, se intentó correlacionar el desarrollo de la enfermedad con la respuesta inmune celular específica (reacción de Mitsuda) y con los niveles de anticuerpos anti-Mycobacterium leprae (prueba FLA-ABS, tres observaciones consecutivas). El 71.7% de los contactos fueron Mitsuda positivos y el 93.6% mostraron seropositividad independientemente de la edad, sexo o tipo de lepra del caso índice respectivo. Los convivientes se dividieron en grupos de bajo- y de alto-riesgo de acuerdo al carácter paucibacilar o multibacilar de su caso índice. El grupo de bajo riesgo consistió de 19 contactos de 2 pacientes tuberculoides (TT) y de 5 casos indeterminados. El grupo de alto riesgo estuvo formado por 60 convivientes de 18 casos lepromatosos (LL) activos. Excepto 2, todos los contactos en el primer grupo tuvieron una reacción de Mitsuda positiva; el título más común de anticuerpos fue de 1:160, con tendencia a estabilizarse o a decrecer con el tiempo. En los 2 contactos Mitsudanegativos se observaron niveles aumentados de anticuerpos. En el grupo de mayor riesgo, el 61.6% de los convivientes fueron Mitsuda positivos y mostraron un perfil humoral similar a los Mitsuda positivos en el grupo de menor riesgo. En la mayoría de los contactos Mitsuda negativos los niveles de anticuerpo permanecieron constantes o aumentaron progresivamente, sugiriendo una alta probabilidad de infección subclínica activa. Esta suposición fue parcialmente apoyada por el hallazgo de un caso nuevo de lepra lepromatosa limítrofe (BL) en el grupo de contactos (de LL) Mitsuda negativos. La contribución del contacto íntimo y prolongado con un caso multibacilífero como un factor de riesgo fue difícil de evaluar debido al pequeño tamaño de la muestra estudiada.Individual susceptibility to Mycobacterium leprae infection and the development of leprosy are not concomitant characteristics, and the disease depends on the concurrence of several host and environmental factors collectively called "risk factors," such as certain genetic traits, a continuous contact with the bacilli, the immune status of the host, the type and magnitude of immune responses elicited, etc. Current knowledge on this subject points out alterations in the immune responsiveness as the major risk factors in the shift from an unnoticed M. leprae infection toward its clinical expression(10,12,19,20,30)
In 1955, Dharmendra and Chatterjee (21) investigated the prognostic value of negative lepromin reactions in persons exposed to M. leprae. Further data have demonstrated the limitations of this parameter when it is the only one taken into consideration, mainly because of crossreactivity with other mycobacteria (6,27) and the fact that such skin reaction is under genetic control (17). The improvement of serological methods for leprosy with better specificity and sensitivity (33) opened a new approach and prompted several epidemiological surveys to be made in order to seek the correlation between the risk of developing the disease and the specific immune status in leprosy households. Most of these surveys have been carried out looking for either lepromin reactivity (21,28,37) or in vitro lymphocyte transformation (25,34,37) in the case of the T-dependent response or, similarly, for the presence of specific antibodies with methods such as the FLA-ABS test (2,7), radioimmunoassay (5,40), or ELISA (3,13,14,16,22,23,35). Few reports have dealt with both cellular and humoral responses evaluated at the same time (5,8,9,38,39) or with more than one observation in the same population (13,14,17,22). According to the strategy of the investigation, method or methods selected and antigen employed, the results were rather different but all of them support the importance of adequate immunological studies if correct identification of individuals at risk is intended. Combined results of skin and serological tests are better indications of risk, as has been shown by Dharmendra (20,21) and Bharadwaj, et al. (9), who recommend the classification of households into four groups where lepromin reactivity is interpreted as a relative resistance marker when specific antibodies are also present.
In our study, the same approach was followed but the paucibacillary or multibacillary character of the index case and changes in antibody levels over time were also considered. After 6 years of observation, a new borderline lepromatous (BL) leprosy case was found in a household with a lepromin unresponsiveness, rising amounts of M. leprae-specific antibodies, and extensive contact with a bacilliferous index case. However, due to the size of the sample studied, more information will be required before any definitive conclusion can be reached regarding the contribution of the multibacillarity of the index cases as an important risk factor.
MATERIALS AND METHODS
Working plan. This study was performed in families living in the neighborhood of the metropolitan area of Guadalajara, State of Jalisco, Mexico. In this area, there are 1685 registered leprosy patients, giving a "cumulative" prevalence rate of 92 per 100,000, almost five times the national prevalence of 20 per 100,000. Epidemiologically, they are under the control of the Health Department of the State of Jalisco, and the majority are treated clinically in the Instituto Dermatológico de Guadalajara.
For purposes of the study, families were randomly chosen on the basis of the identification of an untreated or inadequately treated active leprosy patient (index case) and no less than three household contacts who agreed to participate in the study. In the first interview, a comprehensive clinical, histopathological, and bacteriological study was made of all members of the family. Simultaneously, a skin test with lepromin A was given and a blood sample was taken from each participant. The follow up consisted of at least three home visits in a period of 5 years, at which time another clinical evaluation was made and a new blood sample was obtained from the contacts only. After the classification of the index case in the leprosy spectrum, the families were divided into two groups: a) one group with a lower risk where the index cases were nonbacilliferous (tuberculoid and indeterminate), and b) the other group with a higher risk where the index cases were multibacillary (lepromatous).
Index cases. The index cases had a recorded history of clinical leprosy not less than 5 years, had not been treated at all, or their chemotherapy was promptly stopped or followed irregularly. Before the study was initiated, the diagnosis and classification of each case was confirmed by standard established methods. The final selection included 25 patients: 7 (2 tuberculoid and 5 indeterminate) were assigned to the lower-risk group, and 18 lepromatous leprosy cases were selected for the higher-risk group. Conventional treatment was immediately started and carefully watched in all of the index cases.
Household contacts. The household contacts included persons living in a permanent way in the same house with the index case at least 1 year before the patient had initiated regular treatment, if treated at all. The contacts were classified in three degrees: a) type I, the contact lives in the same house as the index case but sleeps in a different room; b) type II, both individuals sleep in the same room; c) type III, they share the same bed. Initially, total household contacts numbered 122 individuals, but only 79 completed 5 years of observation. Individuals were lost to the study mainly because they refused to continue participating or left the area permanently because of marriage or migration. The sixth year of this study was mainly dedicated to locating this group and looking for new leprosy cases among them. The household contacts who completed the survey were in the lower-risk group: 19 individuals (6 males, 13 females) with initial ages between 5 and 38 years (mean, 15 years), the maj ority (17) with type II contact and only 2 with type III. In the higher-risk group, there were 60 household contacts (30 males, 30 females) between the ages of 2 and 68 years (mean, 19 years) who completed the survey; 50 lived in type II contact and 10 in type III.
Mitsuda test. Lepromin A prepared and standardized (4 x 107 bacilli/ml) at the GWL Hansen's Disease Center, Carville, Louisiana, U.S.A., was used throughout the study. The test was given and read by experienced staff using the following scale: 1 + = 5 mm to 7 mm in diameter; 2+ = 8 mm to 10 mm; 3+ = > 11 mm (15).
FLA-ABS test. Specific antibody titers against M. leprae were determined with an indirect immunofluorescence method as proposed by Abe, et al. (2), with slight modifications as described in full detail in a previous report (4). A positive result at any titer was considered enough to qualify the sample as "positive."
Identification of new cases. Minimum criteria for defining a new leprosy case were the presence of delimited depigmentation of the skin, with or without sensory loss, where at least one acid-fast bacillus could be observed after the bacteriological examination of the corresponding skin biopsy. If most typical lesions were found, their classifications in the spectrum of the disease were made following the Ridley and Jopling criteria (38) after bacteriological and histopathological study of the skin biopsies.
Statistics. The Student's t test was employed to compare the means of the results. Regression lines and coefficients of correlation were calculated according by the leastsquare method. Correlation coefficient significances were made using Pearson's test. Values of p < 0.05 were considered to be statistically significant.
RESULTS
Mitsuda test.The results of the Mitsuda test in all of the household contacts included in this study showed that 71.7% were positive to late lepromin reaction. No differences in sex or age were found between positives and negatives. The differential distribution of reactors in the lower- and higher-risk groups are described below.
FLA-ABS test. In order to analyze the humoral immune response of the whole population studied, only the results in the first observation were taken into account because it was the largest of the three samples and, therefore, the most representative of the serological profile of the inhabitants studied. As expected, all 25 index cases showed positive results regardless of their leprosy type. In their contacts, 93.6% were seropositive no matter their sex, age, or type of leprosy in the patient with whom each one had contact. When they were separated in contacts of well-defined bacilliferous cases, on the one hand, and contacts of paucibacillary patients on the other, 95.3% were positive in the former and in the latter 88% were positive, differences which were not striking.
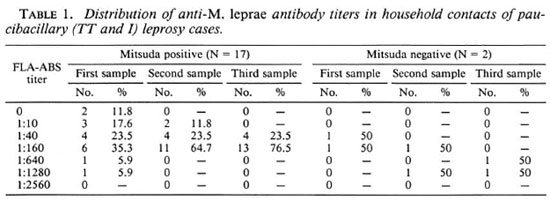
Lower-risk group. From the 19 household contacts of nonbacilliferous cases, 17 showed a positive Mitsuda test (89.5%) with the following distribution: 7 were 1 + reactors, 9 gave 2+ reactions, and only 1 had a 3+ result. The remaining two Mitsudanegative individuals were contacts of different indeterminate index cases. The titers of anti-M. leprae-specific antibodies in all of the cases of this lower-risk group for the three consecutive samplings are shown in Table 1 and Figure 1 A. In the Mitsuda-positive contacts, only two of them showed initial titers of 1:640 or higher which decreased over time. In subsequent observations, none of the rest of them had values higher than 1:160. When the results in the second sample were compared with findings of the first, no statistical differences were observed in the rise or fall of the original titers of specific antibodies. When the third sample was taken into account, only 29% of the sera showed a small increase in titer, always at a low level. The regression line shows a lack of correlation over time (r = 0.25, p > 0.05), which suggests a constant homogeneity in the antibody response in individuals of this subgroup. Conversely, in the two Mitsudanegative individuals the titers showed marked increases (up to eightfold) from the initial value. After 6 years of observation, none of the individuals included in this group manifested any sign suggestive of leprosy.
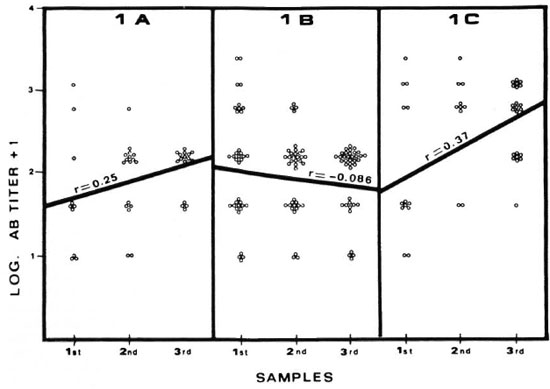
Fig. 1. Correlation between FLA-ABS anti-M. leprae titer and its change over time. 1A = lower-risk group,Mitsuda-positive contacts; 1B = higher-risk group, Mitsuda-positive contacts; 1C = higher-risk group, Mitsuda-negative contacts. Best fitting regression lines are shown as straight lines; r = coefficient of correlation.
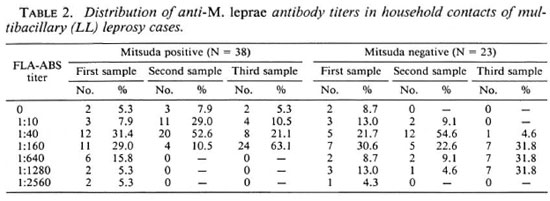
Higher-risk group. In the group of 60 contacts of active multibacillary patients, 38 were positive to the skin reaction with lepromin (61.6%); 21 reached a 1+ reaction, 12 gave a 2+ result, and 4 were 3 + . FLA-ABS test results were analyzed according to Mitsuda responsiveness (Table 2).
In late reactors to lepromin A skin tests, individual titers of antibodies against M. leprae showed some differences in the upper and lower values they reached in each one of the three observations made, but in every one the most frequent titer was 1:160. Regarding individual variations in titer during the study, the majority of the contacts in this category remained constant or even had a significant decrease, 71% from the first to the second sample and 81.5% from the second to the third. Analyzing the data from each time point showed no statistical differences among them and the changes in titer had a negative correlation over time (r = -0.086, p > 0.1; Fig. 1B), quite similar to the Mitsuda-positive contacts in the lower-risk group.
In the Mitsuda-negative contacts of LL patients, the titers of anti-M. leprae anti-bodies reached levels slightly higher than those observed in the Mitsuda-positive contacts. Their distribution showed a flat-type curve without predominance of any specific titer, but contrasting with the results in the group of reactors, individual antibody levels remained constant or increased progressively in almost all of the subjects studied; 95% from the first to the second sample and 82% from the second to the third. The findings were statistically significant (p < 0.02), and when the relationship between changes in titer over time was estimated, a weak but significant correlation was seen (r = 0.37, p < 0.01; Fig. 1C). Thus, this subgroup behaves differently from the Mitsuda responders, either from the lower-risk or higher-risk groups, with a progressive slow exacerbation in the antibody response.
A remarkable finding among those individuals belonging to the Mitsuda-negative contacts of LL patients was the detection of a new leprosy case. This case was a 13-yearold male, son of an irregularly treated, 50year-male LL patient, who maintained a long-term type II household contact. In his initial evaluation a negative lepromin skin test was observed, and a titer of 1:1280 was found in the absence of any clinical sign of leprosy. The skin lesion identified in this new case had features histopathologically and bacteriologically compatible with BL leprosy. Until now, he is the only participant in this study who has developed Hansen's disease.
Lost household contacts. In an attempt to know the rate of new leprosy among the lost household contacts evaluated only once at the beginning of the study, a deliberate attempt to locate them was made 6 years later. This included home visits, the search for new addresses, and a careful review of the official leprosy records from the last 6 years. It was not possible to detect any leprosy cases among those individuals located, but it is important to point out that nearly 30% are known to be migratory workers who spend most of their time traveling through out the country, making difficult their diagnosis and/or their registration in the leprosy surveillance program.
DISCUSSION
The long-term prospective investigation of clusters of leprosy bacilli-exposed individuals is the only way to find parameters which separate the disease-prone from protected subjects. The field studies involved cannot be planned unless an adequate infrastructure of specialized health services and, consequently, a high level of operational efficiency is implemented. Guadalajara, the second largest city in Mexico, provides the facilities and conditions for reliable identification and recruitment of index cases and their families, as well as an effective monitoring system.
A comparison of the results in leprosy contacts with other populations for control purposes is not easy because false-positive findings in leprosy tests are common in healthy people. If they come from nonendemic areas probably this positive test result is the effect of cross-sensitization. In populations from leprosy-endemic areas, in addition to possible cross-sensitization, there could be unknown specific exposure to M. leprae (6,11,32,36). For such reasons, in this work healthy families without known contact with leprosy cases were ruled out as a control group, and we decided to separate the contacts of multibacillary (LL or BB) patients from those related to paucibacillary (TT and indeterminate) cases, assuming that there are more new leprosy cases in the former (higher-risk group) than in the latter (lower-risk group) as is generally accepted (12,36) , although some doubts still remain (41). In selecting the methods for evaluating specific immune status, the Mitsuda skin test was chosen, in spite of its inherent operational difficulties in performing and reading, because it remains the best available way to look for specific T-lymphocyte response (15). Concerning anti-M. leprae antibodies, the FLA-ABS test was used due to its high sensitivity throughout the whole spectrum of the disease and its excellent specificity as has been found in all studied populations (2,1,24,26,31), including Mexican Mestizos (4).
Before discussing our findings in both risk groups, it is important to consider the studied population as a whole. The proportion of Mitsuda reactors was similar to that in some other reports (21,28), but not all (3,37). Also, the FLA-ABS test gave results in accordance with other reports (2,7-9,26) except one (39). Disagreements in both cases are probably the consequence of the genetic, cultural, and socioeconomic differences among such populations. The presence of a high number of leprosy contacts with detectable amounts of specific antibodies by the FLA-ABS test was independent of the closeness or extent of the relationship with household contacts with heavy loads of bacilli. This suggests an efficient circulation of M. leprae, an almost universal susceptibility to be colonized with them, a long persistence of antibodies once sensitization has been acquired, and frequent events of restimulation by the bacillus. Thus, with the FLA-ABS test it is easy to demonstrate contact with the bacteria but its positivity is not enough to identify an infection in progress or to estimate the risk of developing the disease.
The lower-risk group studied here was small and definitive conclusions could not be made. Nevertheless, in all but two individual's responses were as expected in an immune situation, that is, a good T-cell reactivity and relatively low stabilized levels of specific antibodies. It is worth pointing out that the remaining two Mitsudanegative household contacts seem to be in an adverse condition if our previous supposition was correct. Both were contacts and close relatives of independent indeterminate cases and their immunological behaviors could be the result of the presence of high-risk factors shared with their index cases and common sources of infection.
The higher-risk group comprised two welldefined populations separated according to Mitsuda test results. The first population are responders, and they display quite a homogeneous pattern in their humoral responses, resembling Mitsuda-positive contacts in the lower-risk group. The second population do not show a late response to the lepromin skin test, which may not necessarily be the result of a leprosy-associated anergy because other causes, such as the effect of certain genetic traits, insufficient sensitization, secondary immunodeficiencies, or some other nonimmunological factors, could account for the same result. As was mentioned, specific as differentiated from nonspecific causes could be assumed if anti-M. leprae antibodies were present simultaneously. Although all of these Mitsuda-negative household contacts had such antibodies, analysis of their titers showed a general tendency to reach greater levels than did Mitsuda-positive contacts and, in most of them, there was an increase over time. It is very likely that such results really reflect the presence of an active subclinical leprosy infection as has been reported (8,9,13,14,17,22), at least in the case of unusual levels of specific antibodies.
In this study, the highest most common FLA-ABS titers in the lower-risk groups (Mitsuda-positive contacts of any leprosy case) were 1:40 and 1:160, hence an eightfold or greater value could arbitrarily be taken as evidence of an active infection. However, we must question the feasibility of this conclusion due to the size of the sample studied. Additional support for the importance of detecting a high titer of antibodies associated with lepromin unresponsiveness is the fact that the new leprosy case reported here fulfills such characteristics. However, it will be necessary to undertake a prospective study of larger groups from other endemic areas before we can reach any definite conclusion.
The increment of antibody titers found in part of the Mitsuda-negative contacts, their weak but significant correlation over time, could be interpreted as being the result of a constant antigenic stimulation and, consequently, the existence of subclinical leprosy. This is an approach which routinely has been used in the serodiagnosis of several acute viral and microbial infections and seldom used in chronic infectious diseases. The preliminary data presented here suggest its application in the case of leprosy. Surely the interval between each serological determination must be in terms of years apart, and this involves serious operational difficulties which can limit its practice on a wider scale in many endemic areas. When possible, we feel this procedure could be worthwhile if it could eventually be adequately validated.
In conclusion, our results clearly support the role of the immune response as a risk factor in the development of leprosy, but not the close and extensive contact with an active multibacilliferous case. Moreover, our lower- and higher-risk groups seem to be very similar in their immunological behavior, regardless of the bateriologic nature of their index cases. Such a situation fits very well with the proposition made and developed by Dharmendra (20,21) and Bharadwaj, et al. (8,9) as already mentioned. Therefore, it will be necessary to continue the surveillance of this study group for a longer time in order to find conclusive answers. This and other similar studies should provide useful data for the identification of persons at risk of developing leprosy.
Acknowledgments. This work was partially supported by grants nos. PCSABNA-21649 and PCSACNA-050625 from Conscjo Nacional de Cicncia y Tecnologia (CONACyt), Mexico. We arc most grateful to Dr. Eleanor Storrs for supplying the armadillo liver infected with M. leprae. We arc also grateful to Dr. Victor Manuel Ruiz Godoy for his helpful advice, support and guidance throughout this work. We are thankful to Mrs. Socorro Sandoval for her cooperation. Our thanks also go to Dr. Ethel Garcia for her helpful comments on and revision of the manuscript, and to Mrs. Silvia Diaz for her excellent secretarial assistance.
REFERENCES
1. ABE, M., I ZUMI, S., S AITO, T. and M ATHUR, S. K. Early serodiagnosis of leprosy by indirect immu nofluorescence. Lepr. India 48 (1976) 272-276.
2. ABE, M., MINAGAWA, F., Y OSHINO , Y., O ZAWA, T., S AIKAWA , K. and S AITO , T. Fluorescent leprosy antibody absorption (FLA-ABS) test for detecting subclinical infection with Mycobacterium leprae. Int. J. Lepr. 48(1980)109-119.
3. AGIS, F., S CHLICH , P., C ARTEL, J.-L., GUIDI, C. and B ACH , M.-A. Use of anti-M. leprae phenolic glycolipid-I antibody; detection for early diagnosis and prognosis of leprosy. Int. J. Lepr. 56(1988)527-536.
4. A MEZCUA, M. E., E SCOBAR -G UTIERREZ, A., M AYEN, E. and C AZARES , J. V. Sensitivity and specificity of the FLA-ABS test in Mexican populations. Int. J. Lepr. 55(1987)286-292.
5. A SHWORTH, M., S INHA, S., P ATIL, S. A., R AMU, G. and S ENGUPTA , U. The detection of subclinical leprosy using a monoclonal antibody based radioimmunoassay. Lepr. Rev. 57(1986)237-242.
6. BECHELLI, L. M., Q UAGLIATO, R., N AKAMURA, S. and L IMA -F ILHO , E. C. Lepromin test in contacts (mainly 1-19 years old) living in an area of low leprosy endemicity. Int. J. Lepr. 39(1971)136-145.
7. BHARADWAJ, V. P., R AMU, G. and D ESIKAN , K. V. Fluorescent leprosy antibody absorption (FLA-ABS) test for early serodiagnosis of leprosy. Lepr. India 53(1981)518-524.
8. BHARADWAJ, V. P., R AMU, G. and D ESIKAN , K. V. A preliminary report on subclinical infection in leprosy. Lepr. India 54(1982)220-227.
9. BHARADWAJ, V. P., R AMU, G., D ESIKAN , K. V. and KTOCH , K. Extended studies on subclinical infection in leprosy. Indian J. Lepr. 56(1984)807-812.
10. BLOOM , B. R. Learning from leprosy: a perspective on immunology and the Third World. J. Immunol. 137(1986)i-x.
11. BRETT, S., D RAPER, P., P AYNE, S. N. and REES, R. J. W. Serological activity of a characteristic phenolic glycolipid from Mycobacterium leprae in sera from patients with leprosy and tuberculosis. Clin. Exp. Immunol. 52(1983)271-179.
12. BROWNE, S. G. Leprosy. In: Adams and Maegraith: Clinical Tropical Diseases. 7th cd. Macgraith, B., cd. Oxford: Blackwell Scientific Publications, 1980, pp. 196-228.
13. BUCHANAN, T. M., D ISSANAYAKE, S., Y OUNG , D. B., M ILLER, R. A., A CEDO, J. R., H ARNISCH, J. P., K HANOLKAR, S. R. and E STRADA -P ARRA, S. Evaluation of the significance of antibodies to phenolic glycolipid of Mycobacterium leprae in leprosy patients and their contacts. Int. J. Lepr. 51(1983)658-659.
14. CHANTEAU, S., CARTEL, J.-L., G UIDI, C, PLICH-ART , R. and BACH , M.-A. Seroepidemiology study on 724 household contacts of leprosy patients in French Polynesia using disaccharide-octyl-BSA as antigen. Int. J. Lepr. 55(1987)626-632.
15. CONVIT, J., P INARDI, M. E., A RIAS -R OJAS, F., GONZALEZ, I., C OREY, G., A RVELO, J. J. and MONZÓN , H. Test with three antigens in leprosy-endemic and non-endemic areas. Bull WHO 52(1975)193-198.
16. CREE, I. A., SMITH, W. C. and BECK, J. S. Serum antibody responses to mycobacteria in leprosy patients and their contacts. Lepr. Rev. 59(1988)317-327.
17. DE VRIES, R. R. P., S ERJEANTSON, S. W. and LAYRISSE, Z. Leprosy. In: Histocompatibility Testing 1984. Albert, E. D., Baur, M. P. and Mayr, W. R. Berlin: Springer-Verlag, 1984, pp. 362-367.
18. DESFORGES, S., BOBIN, P., BRETHES, B., HUERRE, M., MOREAU, J. P. and BACH , M.-A. Specific anti-M. leprae PGL-I antibodies and Mitsuda reactions in the management of household contacts in New Caledonia. Int. J. Lepr. 57(1989)794-800.
19. DHARMENDRA. Detection of subclinical infectionin leprosy. Lepr. India 54(1982)193-207.
20. DHARMENDRA. Prognostic value of the leprominand serological tests combined. Indian J. Lepr. 61(1989)176-178.
21. DHARMENDRA and CHATTERJEE, K. R. Prognostic value of the lepromin test in contacts of leprosycases. Lepr. India 27 (1955) 149-158.
22. DOUGLAS, J. T. and WORTH, R. M. Field evaluation of an ELISA to detect antibody in leprosypatients and their contacts. Int. J. Lepr. 52 (1984)26-33.
23. FINE, P. E. M., PONNINGHAUS, J. M., BURGESS, P.,CLARKSON J. A. and DRAPER, C. C. Seroepide-miological studies of leprosy in northern Malawibased on an enzyme-linked immunosorbent assayusing synthetic glycoconjugate antigen. Int. J. Lepr.56(1988)243-254.
24. GARRAUD, O., RIBIERRE, O. and BACH, M.-A. A follow up of T-cell subsets and of anti-M. leprae antibody titer as measured by the FLA-ABS testin Melanesian leprosy patients under polyche-motherapy. Int. J. Lepr. 54(1986)38-45.
25. GODAL, T., LOFGREN, M. and NEGASSI, K. Immune response to Al. leprae of healthy leprosycontacts. Int. J. Lepr. 40(1972)243-250.
26. GONZALEZ-ABREU, E., GONZALEZ-SEGREDO, A. and DELA CRUZ, F. Anti-Mycobacterium leprae antibodies induced by lepromin injection as dem-onstrated by indirect immunofluorescence. Lepr.Rev. 55(1984)337-340.
27. GOREN, M. B. Immunoreactive substances of mycobacteria. Am. Rev. Respir. Dis. 125(1982)50-69.
28. GUINTO, R. S., DouLL, J. A. and MABALAY, E. B.The Mitsuda reactions in persons with and with-out household exposure to leprosy. Int. J. Lepr.23(1955)135-138.
29. HARBOE, M., CLOSS, 0., BJUNE, G., KRONVALL, G.and AXELSEN, N. H. Mycobacterium leprae specific antibodies detected by radioimmunoassay.Scand. J. Immunol. 7(1978)111-120.
30. HASTINGS, R. C., GILLIS, T. P., KRAHENBUHL, J. L. and FRANZBLAU, S. G. Leprosy. Clin. Microbiol. Rev. 1(1988)330-348.
31. JI, B., TANG, Q., Li, Y., CHEN, J., ZHANG, J., DONG, L., WANG, C., MA, J. and Ye, D. The sensitivity and specificity of fluorescent leprosy antibody ab-sorption (FLA-ABS) test for detecting subclinicalinfection with Mycobacterium leprae. Lepr. Rev. 55(1984)327-335.
32. KAZDA, J. Occurrence of non-cultivable acid-fastbacilli in the environment and their relationshipto Al. leprae. Lepr. Rev. 52 Suppl. 1(1981)85-91.
33. MELSOM, R. Serodiagnosis of leprosy: the past,the present and some prospects for the future. Int.J. Lepr. 51(1983)235-252.
43. MENDEZ-SAMPERIO, P., LAMB, J., BOTHAMLEY, G.,STANLEY, P., ELLIS, C. and IVANYI, J. Molecular study of the T-cell repertoire in family contactsand patients with leprosy. J. Immunol. 142(1989)3599-3604.
35. MENZEL, S., HARBOE, M., BERGSVIK, H. and BREN- NAN, P. J. Antibodies to a synthetic analog ofphenolic glycolipid-I of Mycobacterium leprae inhealthy household contacts of patients with leprosy. Int. J. Lepr. 55(1987)617-625.
36. NEWELL, K. W. An epidemiologist's view of leprosy. Bull. WHO 34(1966)827-857.
37. PRICE, M. A., ANDERS, E. M., ANDERS, R. F.,RUSSELL, D. A. and DENNIS, E. S. Cell-mediated immunologic status of healthy members of families with a history of leprosy. Int. J. Lepr 43(1975)307-313.
38. RIDLEY, D. S. and JOPLING, W. H. Classificationof leprosy according to immunity; a five-groupsystem. Int. J. Lepr. 34(1966)255-273.
39. SAMPATTANAVICH, S., SAMPOONACHOT, R.,KONGSUEBCHART, K., RAMASOOTA, T., PINRAT, U., MONGKOLWONGROI, P., OZAWA, T., SASAKI, N. and ABE, M. Immunoepidemiological studies on sub-clinical infection among leprosy household contact in Thailand. Int. J. Lepr. 57(1989)752-765.
40. SINHA, S., SENGUPTA, U., RAMU, G. and IVANYI, J. Serological survey of leprosy and control subjects by a monoclonal antibody-based immuno-assay. Int. J. Lepr. 53(1985)33-38.
41. TAYLOR, C. E., ELLISTON, E. P. and GIDEON, H.Asymptomatic infections in leprosy. Int. J. Lepr.33(1965)716-727.
42. YOUNG, D. B. and BUCHANAN, T. M. A serologicaltest for leprosy with a glycolipid specific for Mycobacterium leprae. Science 221(1983)1057-1059.
1. M.Sc, Head, Laboratory for Mycobacterial Research. Departamento de Investigaciones Inmunológicas, Instituto Nacional de Diagnóstico y Referencia Epidemiológicos, Secretaría de Salud México, Carpió 470, México, D. F. 11340, México.
2. Ph.D., Head of Department, Departamento de Investigaciones Inmunológicas, Instituto Nacional de Diagnóstico y Referencia Epidemiológicos, Secretaría de Salud México, Carpió 470, México, D. F. 11340, México.
3. Biol. Tech., Departamento de Investigaciones Inmunológicas, Instituto Nacional de Diagnóstico y Referencia Epidemiológicos, Secretaría de Salud México, Carpió 470, México, D. F. 11340, México.
4. B.Sc; Departamento de Investigaciones Inmunológicas, Instituto Nacional de Diagnóstico y Referencia Epidemiológicos, Secretaría de Salud México, Carpió 470, México, D. F. 11340, México.
5. M.D., Director, Instituto Dermatológico de Guadalajara, Secretaría de Salud México, Federalismo Norte 202, Atemajac, Zapopan, Jalisco 45190, México.
6. M.D., M.S.P., Epidemiologist, Instituto Dermatológico de Guadalajara, Secretaría de Salud México, Federalismo Norte 202, Atemajac, Zapopan, Jalisco 45190, México.
7. M.D., Dermatologist, Instituto Dermatológico de Guadalajara, Secretaría de Salud México, Federalismo Norte 202, Atemajac, Zapopan, Jalisco 45190, México.
Reprint requests to Dr. Escobar-Gutiérrez.
Received for publication on 17 July 1989.
Accepted for publication in revised form on 20 April 1990.